Abstract
Background: During the last two decades, it has become obvious that 3,5-diiodothyronine (3,5-T2), a well-known endogenous metabolite of the thyroid hormones thyroxine (T4) or triiodothyronine (T3), not only represents a simple degradation intermediate of the former but also exhibits specific metabolic activities. Administration of 3,5-T2 to hypothyroid rodents rapidly stimulated their basal metabolic rate, prevented high-fat diet-induced obesity as well as steatosis, and increased oxidation of long-chain fatty acids.
Objective: The aim of the present study was to analyze associations between circulating 3,5-T2 in human serum and different epidemiological parameters, including age, sex, or smoking, as well as measures of anthropometry, glucose, and lipid metabolism.
Methods: 3,5-T2 concentrations were measured by a recently developed immunoassay in sera of 761 euthyroid participants of the population-based Study of Health in Pomerania. Subsequently, analysis of variance and multivariate linear regression analysis were performed.
Results: Serum 3,5-T2 concentrations exhibited a right-skewed distribution, resulting in a median serum concentration of 0.24 nM (1st quartile: 0.20 nM; 3rd quartile: 0.37 nM). Significant associations between 3,5-T2 and serum fasting glucose, thyrotropin (TSH), as well as leptin concentrations were detected (p<0.05). Interestingly, the association to leptin concentrations seemed to be mediated by TSH. Age, sex, smoking, and blood lipid profile parameters did not show significant associations with circulating 3,5-T2.
Conclusion: Our findings from a healthy euthyroid population may point toward a physiological link between circulating 3,5-T2 and glucose metabolism.
Introduction
Despite first descriptions of the metabolic effects of 3,5-diiodothyronine (3,5-T2) (1,2), it was considered as a physiologically inactive metabolite of the two major thyroid hormones (TH) thyroxine (T4) and triiodothyronine (T3). Recent pharmacological applications of 3,5-T2 mainly in hypothyroid rodent models (3,4) challenged this hypothesis because they revealed rapid and chronic metabolic changes, partly independent of the classical T3 receptors (TR). 3,5-T2 rapidly enhanced hepatic oxygen consumption (5,6), and hepatic mitochondria were identified as specific targets (7,8). Moreover, 3,5-T2 was suggested to influence glucose metabolism by stimulating glucose-6-phosphate dehydrogenase (G6PD) activity (9) or by modulating gluconeogenesis via sirtuin 1 (10,11). Moreno et al. (6) reported a complementary effect of 3,5-T2 to T3 on stimulating the resting metabolic rate in rats. Furthermore, administered in pharmacological doses, 3,5-T2 prevented rodents from weight gain from a high-fat diet (12) and restrained the development of insulin resistance (10). Possible discussed underlying mechanisms included the activation of thermogenesis (13), the prevention of fat accumulation in skeletal muscle as well as in the liver (10,14), and the enhancement of lipolysis (10,12,13), together resulting in remarkable antisteatotic effects under high-fat diet conditions.
In comparison, knowledge about the role of 3,5-T2 in human metabolism is sparse. Following the development of 3,5-T2 radioimmunoassays (RIAs), age- and sex-specific differences in serum 3,5-T2 concentrations (15,16) as well as associations with chronic renal diseases, liver cirrhosis, and sepsis were reported (17–19). Nevertheless, a validation of many of those associations in larger studies is lacking.
Expanding these initial findings and questioning former results that reported a wide spectrum of serum 3,5-T2 concentrations over almost two orders of magnitude in rather small groups of analyzed individuals, we now used a newly developed assay (20) to determine serum 3,5-T2 concentrations in a larger healthy study population. The aim of the present study was to analyze associations between serum 3,5-T2 concentrations and metabolic parameters from glucose and lipid metabolism as well as anthropometric measures in a euthyroid population.
Materials and Methods
Study population
The Study of Health in Pomerania (TREND) (SHIP-TREND) is a second cohort of a population-based research project in West Pomerania, a rural region in northeast Germany (21). A stratified random sample of 8826 adults aged 20–79 years was drawn from population registries. Sample selection was facilitated by centralization of local population registries in the Federal State of Mecklenburg-West Pomerania. Stratification variables were age, sex, and city/county of residence. Baseline examinations were conducted between 2008 and 2012. Out of all invitations, 4420 choose to participate (50.1% response). All participants gave written informed consent. The study was approved by the local ethics committee and conformed to the principles of the declaration of Helsinki. SHIP data are publically available for scientific and quality control purposes. Data usage can be applied for via www.community-medicine.de.
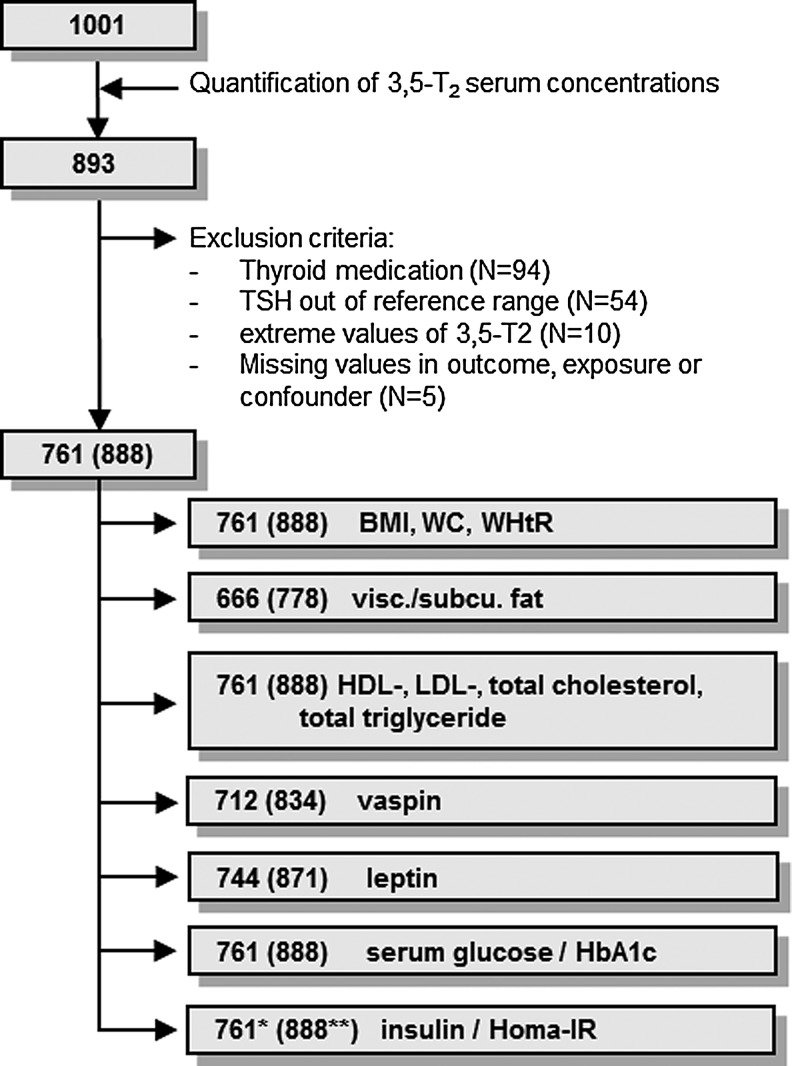
The present study population comprises a subsample of 1001 subjects without self-reported diabetes. Of the 1001 participants, 108 subjects with missing data for serum 3,5-T2 concentrations were excluded. Furthermore, 132 subjects with at least one of the following conditions (overlap exists) were excluded: use of thyroid medications (ATC code H03A, n=94), serum thyrotropin (TSH) levels outside the reference range (0.30–3.59 mIU/L, n=54), missing values in investigated parameters (n=5), or serum 3,5-T2 concentrations differing more than two times the standard deviation from the mean concentration (>2.13 nM, n=10). The final study population (euthyroid sample) available for the present analysis comprised 761 subjects (Fig. 1). For 666 subjects, whole-body magnetic resonance imaging (MRI) data were available, whereas vaspin and leptin serum levels were determined for 712 and 744 participants respectively.
FIG. 1.
Flowchart representing the steps for study compilation. Numbers in parentheses indicate the respective data for the whole sample. *n=423 and **n=492 subjects for continuous analysis.
Measurements
Each SHIP-TREND participant underwent standardized medical examinations, blood sampling, and an extensive computer-aided personal interview. Data on sociodemographic characteristics and medical histories were collected. During the physical examination, standardized measurements of body weight and height were performed with calibrated scales. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Waist circumference (WC) was measured using an inelastic tape while the participant stood securely with weight equally distributed on both feet. The measurement relies on the circumference between the lower rib margin and the iliac crest in the horizontal plane. Based on these measurements, the waist-to-height ratio (WHtR) was calculated as WC (cm)/height (cm).
Whole body magnetic resonance imaging (MRI) was performed on a commercial 1.5-Tesla system (Magnetom Avanto; Siemens Healthcare AG, Erlangen, Germany; software version Syngo MR B15) using a body phased array coil. The quantification of subcutaneous and visceral fat was done using the automatic tissue and labeling analysis software ATLAS and in-house developed software from the University of Ulm (22). Afterwards, manual correction by certified students was applied.
Fasting blood samples (fasting ≥8 h) were drawn between 6:00am and 7:00pm from the cubital vein of subjects in the supine position and analyzed immediately or stored at −80°C. Serum TSH concentrations were measured using an immunoassay (Dimension VISTA; Siemens Healthcare Diagnostics, Eschborn, Germany) with a functional sensitivity of 0.005 mIU/L. Total cholesterol, total triglyceride, and serum glucose concentrations were measured by photometry (Dimension VISTA; Siemens Healthcare Diagnostics). High-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were selectively precipitated and then determined by homogenous assays (Dimension VISTA, Siemens Healthcare Diagnostics). Serum vaspin (AdipoGen, Liestal, Switzerland) and leptin (Mediagnost, Reutlingen, Germany) concentrations were measured using the enzyme-linked immunosorbent assay (ELISA) technique. Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (Bio-Rad, Munich, Germany).
Insulin serum concentrations were measured using a chemiluminescent immunometric assay (Immulite 200 XPi; Siemens Healthcare Diagnostics) with a functional sensitivity of 2 μIU/mL. The homeostatic model of insulin resistance (HOMA-IR) was calculated as insulin (μIU/mL)×glucose (mmol/L)/22.5 according to Matthews et al. (23).
Serum 3,5-T2 concentrations were measured with a recently developed monoclonal antibody-based chemiluminescence immunoassay (20). Blood samples were stored for about five years prior to analysis. In the course of assay validation (20), different storage conditions, that is, room temperature, 4°C and −20°C, as well as several freeze–thaw cycles did not disturb 3,5-T2 stability, leading to the assumption that even prolonged storage would not affect the results. The functional sensitivity of the assay was specified as 0.2 nM. The interassay variation was between 5.6% and 12.9%. The working range was declared as 0.2–10 nM 3,5-T2.
Statistical analysis
Continuous data are expressed as median (1st, 3rd quartile); nominal data are expressed as percentages. For bivariate statistics, the Kruskal–Wallis test (continuous data) or the chi-square test (nominal data) were used to compare men and women. In a pre-analysis step, we compared participants below (n=274) and above (n=487) 0.2 nM, with respect to age, sex, and smoking. As there were no significant differences (data not shown), we decided to continue without stratification regarding 3,5-T2 as outcome. In a first step, we tested the influence of age using 10-year age groups, sex, and smoking on serum 3,5-T2 concentrations. Boxplots were used to visualize the distribution of serum 3,5-T2 concentrations. Values below the detection limit (0.2 nM) were stratified as follows: (a) values distinct from zero but <0.1 nM were set to 0.05 nM as the lowest point of the standard curve, or (b) values >0.1 nM were set to 0.2 nM. Kruskal–Wallis tests were used to detect overall significance and Wilcoxon rank-sum tests to test for group differences. In a second step, two types of linear regression analysis were performed. First, mean values of the considered markers for anthropometry, glucose, or lipid metabolism with confidence intervals (CI) depending on 3,5-T2 concentration were calculated by age- and sex-adjusted analysis of variance (ANOVA). For this purpose, three distinct groups of participants were defined according to their serum 3,5-T2 concentrations: <0.2 nM (n=274) but distinct from zero, 0.2–0.33 nM (n=243), and >0.33 nM (n=244). Second, multivariate linear regression analysis was performed with 3,5-T2 as continuous independent variable, including age and sex as covariates, thereby excluding all participants with values below the detection limit of the assay (n=487). As ANOVA results indicated possible nonlinear trends, restricted cubic splines with three equidistant knots (5th, 50th, and 95th percentile) were used to detect possible nonlinear associations (24). The additional spline variable was only included if it led to a significant increase in model fitness assessed by a likelihood ratio test. Dependent variables were log-transformed to achieve a normal distribution if necessary. The number of subjects depended on the used outcome variable as shown in Figure 1.
To observe whether an altered thyroid state would affect the presented results, all analyses were also performed on the whole sample, only excluding participants with missing values in variables under investigation (n=888; n=315 with values <0.2 nM but distinct from zero).
A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS v9.3 (SAS Institute, Inc., Cary, NC) and R v3.0.1 (25).
Results
General characteristics for men and women are displayed in Table 1. Women had lower values of BMI, WC, WHtR, and visceral fat, whereas the amount of subcutaneous fat as well as vaspin and leptin concentrations were higher compared to men. Furthermore, women exhibited more favorable lipid profiles (higher HDL, lower LDL, and lower total triglycerides) as well as more advantageous glycemic profiles (lower serum glucose, HbA1c, and HOMA-IR) and smoking behavior.
Table 1.
General Characteristics of the Study Population
| Characteristics | Male (n=358) | Female (n=403) | p* |
|---|---|---|---|
| Age, years | 50 (38; 60) | 49 (40; 59) | 0.79 |
| Smoking, % | <0.01 | ||
| Never smoker | 32.0 | 52.7 | |
| Former smoker | 43.4 | 27.1 | |
| Current smoker | 24.6 | 20.2 | |
| TSH, mIU/L | 1.13 (0.80; 1.47) | 1.27 (0.90; 1.75) | <0.01 |
| Body mass index, kg/m2 | 27.7 (25.0; 30.3) | 26.2 (23.1; 30.0) | <0.01 |
| Waist circumference, cm | 94.0 (86.0; 102.0) | 81.5 (73.5; 90.1) | <0.01 |
| Waist-to-height ratio | 0.53 (0.48; 0.58) | 0.50 (0.45; 0.55) | <0.01 |
| Visceral fat, L | 4.70 (2.75; 7.02) | 2.40 (1.14; 3.66) | <0.01 |
| Subcutaneous fat, L | 6.13 (4.58; 8.06) | 7.99 (6.10; 11.11) | <0.01 |
| HDL cholesterol, mmol/L | 1.28 (1.10; 1.47) | 1.59 (1.34; 1.87) | <0.01 |
| LDL cholesterol, mmol/L | 3.41 (2.78; 4.00) | 3.33 (2.70; 3.95) | 0.44 |
| Total cholesterol, mmol/L | 5.3 (4.6; 6.1) | 5.5 (4.9; 6.2) | 0.01 |
| Total triglycerides, mmol/L | 1.28 (0.91; 1.95) | 1.12 (0.84; 1.56) | <0.01 |
| Vaspin, ng/mL | 0.47 (0.26; 0.76) | 0.76 (0.47; 1.46) | <0.01 |
| Leptin, ng/mL | 6.1 (3.5; 9.4) | 18.1 (11.3; 29.5) | <0.01 |
| Serum glucose, mmol/L | 5.4 (5.1; 5.8) | 5.2 (4.9; 5.6) | <0.01 |
| HbA1c, % | 5.2 (4.9; 5.6) | 5.1 (4.8; 5.5) | <0.01 |
| HOMA-IR | 0.64 (0.47; 1.37) | 0.54 (0.45; 1.26) | <0.01 |
| Insulin, μIU/mL | 2.6 (2.0; 5.3) | 2.3 (2.0; 5.1) | 0.27 |
| 3,5-Diiodothyronine, nM | |||
| 20–29 years (m=39, f=31) | 0.20 (0.20; 0.31) | 0.22 (0.20; 0.40) | 0.40 |
| 30–39 years (m=57, f=64) | 0.21 (0.20; 0.41) | 0.25 (0.20; 0.39) | 0.35 |
| 40–49 years (m=81, f=111) | 0.27 (0.20; 0.40) | 0.25 (0.20; 0.35) | 0.67 |
| 50–59 years (m=83, f=99) | 0.30 (0.20; 0.43) | 0.23 (0.20; 0.32) | 0.07 |
| 60–69 years (m=64, f=69) | 0.26 (0.20; 0.35) | 0.23 (0.20; 0.36) | 0.55 |
| 70+years (m=34, f=29) | 0.22 (0.20; 0.34) | 0.29 (0.21; 0.40) | 0.08 |
| Overall | 0.24 (0.20; 0.38) | 0.24 (0.20; 0.37) | 0.94 |
Continuous data are expressed as median (25th; 75th); nominal data are given as percentages.
Chi-square test (nominal data) or Kruskal–Wallis test (interval data) were used for comparison.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; TSH, thyrotropin; HOMA-IR, homeostatic model assessment of insulin resistance; HbA1c, glycosylated hemoglobin.
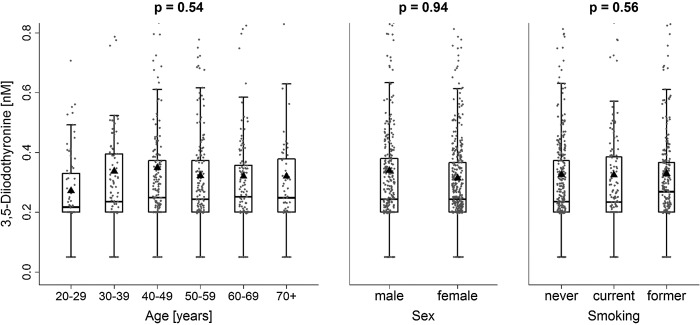
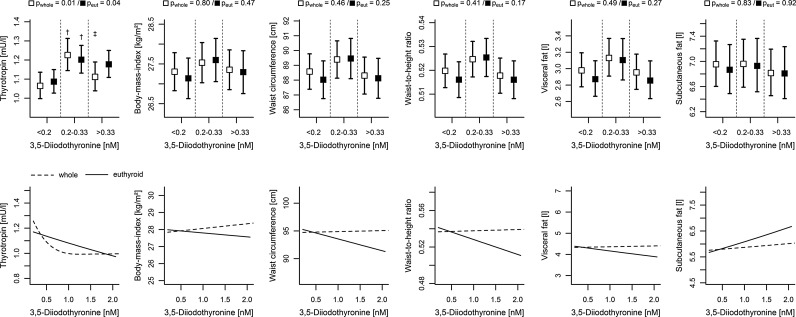
Concerning serum 3,5-T2 concentrations, no sex-specific differences were apparent (Table 1). The median serum 3,5-T2 concentration amounted to 0.24 nM (1st quartile: 0.20 nM; 3rd quartile: 0.37 nM). In addition, neither age nor smoking were significantly associated with serum 3,5-T2 concentrations (Fig. 2). Adjusted ANOVA revealed a partly positive association between TSH and 3,5-T2, where subjects with serum 3,5-T2 concentrations <0.2 nM exhibited lower adjusted mean TSH concentrations (1.09 [CI 1.03–1.15]) compared to subjects with intermediate serum 3,5-T2 concentrations (1.20 [CI 1.13–1.28]; Fig. 3 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy). In contrast, further increasing serum 3,5-T2 concentrations were inversely associated with TSH concentrations as indicated by linear regression analysis (Fig. 3 and Table 2).
FIG. 2.
Boxplots combined with jitter plots of serum 3,5-diiodothyronine concentrations by age, sex, and smoking. The black triangles indicate the group means.
FIG. 3.
Comparison between the whole and the euthyroid subsample. Top: Estimated mean levels of thyrotropin (TSH) and anthropometric markers with 95% confidence intervals by serum 3,5-diiodothyronine concentrations calculated by analysis of variance adjusted for age and sex. pwhole and peut, p-values from analysis of variance (ANOVA) about an overall difference between groups in the whole and euthyroid sample, respectively. Significant differences between groups were marked for comparison with the †lowest and ‡intermediate (p<0.05). Bottom: Predicted mean values of the same parameters conditioned on 3,5-diiodothyronine serum concentrations >0.2 nM for a 50-year-old man from linear regression analysis. Corresponding p-values are listed in Table 2.
Table 2.
Comparison in Linear Regression Analyses Between All Participants with 3,5-T2 Serum Concentrations >0.2 nM (Whole) and a Euthyroid Subpopulation
| Whole | Euthyroid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Log-scale | N | βlin [95% CI]* | p | βspline [95% CI]** | p | N | βlin [95% CI]* | p | βspline [95% CI]** | p |
| TSH, mIU/L | Yes | 573 | −0.56 [−1.02, −0.11] | 0.02 | 1.30 [0.11, 2.49] | 0.03 | 487 | −0.10 [−0.26, 0.06] | 0.22 | — | — |
| Body mass index, kg/m2 | No | 573 | 0.28 [−0.32, 0.88] | 0.37 | — | — | 487 | −0.23 [−1.65, 1.19] | 0.75 | — | — |
| Waist circumference, cm | No | 573 | 0.18 [−1.29, 1.65] | 0.81 | — | — | 487 | −2.14 [−5.58, 1.31] | 0.22 | — | — |
| Waist-to-height ratio | No | 573 | 0.001 [−0.007, 0.010] | 0.75 | — | — | 487 | −0.016 [−0.037, 0.004] | 0.12 | — | — |
| Visceral fat, L | Yes | 502 | 0.01 [−0.07, 0.09] | 0.83 | — | — | 423 | −0.07 [−0.26, 0.13] | 0.52 | — | — |
| Subcutaneous fat, L | Yes | 502 | 0.02 [−0.04, 0.09] | 0.44 | — | — | 423 | 0.09 [−0.06, 0.23] | 0.26 | — | — |
| HDL cholesterol, mmol/L | No | 573 | −0.02 [−0.07, 0.03] | 0.45 | — | — | 487 | −0.01 [−0.12, 0.10] | 0.84 | — | — |
| LDL cholesterol, mmol/L | No | 573 | −0.07 [−0.20, 0.05] | 0.26 | — | — | 487 | −0.13 [−0.42, 0.16] | 0.38 | — | — |
| Total cholesterol, mmol/L | No | 573 | −0.12 [−0.26, 0.03] | 0.12 | — | — | 487 | −0.20 [−0.54, 0.13] | 0.24 | — | — |
| Total triglycerides, mmol/L | Yes | 573 | −0.04 [−0.11, 0.03] | 0.26 | — | — | 487 | −0.08 [−0.24, 0.07] | 0.31 | — | — |
| Vaspin, ng/dL | Yes | 536 | 0.08 [−0.06, 0.23] | 0.27 | — | — | 454 | 0.28 [−0.06, 0.62] | 0.10 | — | — |
| Leptin, ng/dL | Yes | 561 | 0.02 [−0.07, 0.12] | 0.66 | — | — | 475 | −0.05 [−0.28, 0.17] | 0.64 | — | — |
| Serum glucose, mmol/L | No | 573 | 0.46 [0.01, 0.91] | 0.05 | −1.40 [−2.56, −0.23] | 0.02 | 487 | 0.82 [0.08, 1.57] | 0.03 | −4.10 [−7.89, −0.31] | 0.03 |
| HbA1c, % | No | 573 | 0.004 [−0.077, 0.086] | 0.92 | — | — | 487 | −0.12 [−0.31, 0.08] | 0.23 | — | — |
| HOMA-IR | Yes | 573 | 0.001 [−0.098, 0.100] | 0.98 | — | — | 487 | 0.02 [−0.21, 0.25] | 0.85 | — | — |
| Insulin, μIU/mL | Yes | 313 | 0.10 [−0.06, 0.27] | 0.22 | — | — | 269 | −0.03 [−0.29, 0.23] | 0.81 | — | — |
Log-scale indicates whether the dependent variable was transformed by natural logarithm before analysis.
Multivariate linear regression model adjusted for age and sex; **additional variable if 3,5-T2 concentrations were modeled by means of restricted cubic splines (appears only when the usage of splines represented a gain in model quality assessed by a likelihood ratio test).
Subsequently, we analyzed 3,5-T2 concentrations in relation to anthropometric measures, including BMI, WC, and WHtR, as well as the amount of visceral and subcutaneous fat (Fig. 3). All measures except subcutaneous fat demonstrated similar-shaped variations in the means between the three defined 3,5-T2 groups, indicating a nonlinear trend. However, none of them displayed a significant nonlinear behavior in linear regression analyses on the subsample with serum 3,5-T2 concentrations >0.2 nM.
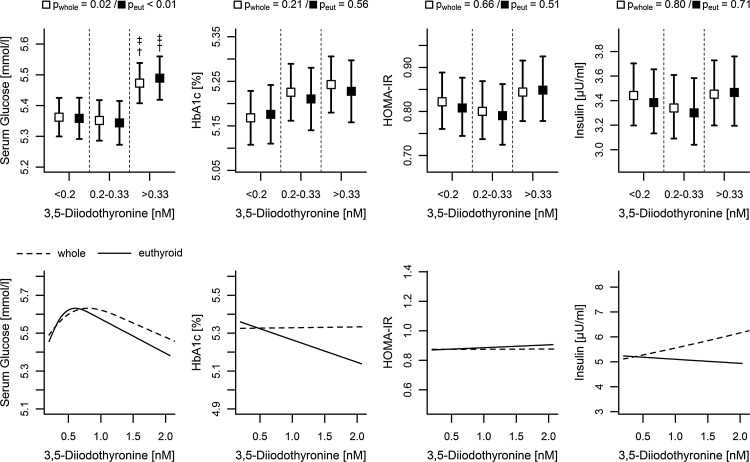
Association analyses by adjusted ANOVA with several parameters of glucose metabolism revealed a significant positive correlation between fasting glucose and 3,5-T2, while HbA1c, insulin concentrations, and HOMA-IR were also slightly positively correlated but did not reach statistical significance (Fig. 4). For glucose, an estimated mean value of 5.49 mmol/L [CI 5.42–5.56] was found for the group with high serum 3,5-T2 concentrations, while the remaining groups exhibited values of about 5.35 mmol/L (Fig. 4 and Supplementary Table S2). Interestingly, linear regression analyses on the subsample with 3,5-T2 serum concentrations >0.2 nM revealed a significant bell-shaped association, confirming ANOVA results up to a peak at 0.5 nM (Fig. 4 and Table 2).
FIG. 4.
Comparison between the whole and the euthyroid subsample. Top: Estimated mean levels of serum glucose, HbA1c, HOMA-IR, and insulin with 95% confidence intervals by serum 3,5-diiodothyronine concentrations calculated by ANOVA adjusted for age and sex. pwhole and peut, p-values from ANOVA about an overall difference between groups in the whole and euthyroid sample, respectively. Significant differences between groups were marked for comparison with the †lowest and ‡intermediate (p<0.05). Bottom: Predicted mean level of the same parameters conditioned on 3,5-diiodothyronine serum concentrations >0.2 nM for a 50-year-old man from linear regression analysis. Corresponding p-values are listed in Table 2. HOMA-IR, homeostatic model of insulin resistance; HbA1c, glycosylated hemoglobin.
Adjusted mean concentrations of parameters for lipid metabolism remained stable along the 3,5-T2 groups. The exception were slightly lower HDL cholesterol concentrations in subjects with low serum 3,5-T2 concentrations but without reaching statistical significance (Fig. 5 and Table 2). Finally, regarding adipokines, adjusted ANOVA revealed that subjects with intermediate serum 3,5-T2 concentrations exhibited significantly higher concentrations of leptin compared to those with low serum 3,5-T2 concentrations, indicating a nonlinear association. As discussed below, this relation was probably mediated by TSH, since further adjustment for serum TSH concentrations leads to a loss of association between leptin and 3,5-T2.
FIG. 5.
Comparison between the whole and the euthyroid subsample. Top: Estimated mean levels for LDL, HDL, total cholesterol, total triglyceride, leptin, and vaspin with 95% confidence intervals by serum 3,5-diiodothyronine concentrations calculated by ANOVA adjusted for age and sex. pwhole and peut, p-values from ANOVA about an overall difference between groups in the whole and euthyroid sample, respectively. Significant differences between groups were marked for comparison with the †lowest and ‡intermediate (p<0.05). Bottom: Predicted mean level of the same parameters conditioned on 3,5-diiodothyronine serum concentrations >0.2 nM for a 50-year-old man from linear regression analysis. Corresponding p-values are listed in Table 2. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Neither adjusted ANOVA nor linear regression analysis revealed sustainable differences in association patterns between the whole or euthyroid sample. The association between 3,5-T2 and TSH was, however, more pronounced in the whole sample, as can be seen from linear regression analysis (Fig. 3 and Table 2), which further indicates an exponential decline in mean TSH concentrations for serum 3,5-T2 concentrations >0.2 nM.
Discussion
To the best of our knowledge, this is the first focused analysis of a large euthyroid study population that specifically investigates associations between serum 3,5-T2 concentrations and several metabolic characteristics, including anthropometric and glucose and lipid metabolism measurements. For this purpose, we used a newly developed immunoassay (20), representing the only currently available method to determine serum 3,5-T2 concentrations in humans (26–28). Our data revealed significant bell-shaped associations of serum 3,5-T2 concentrations with serum glucose concentration and TSH concentrations in the fasting state, pointing toward a versatile role of circulating 3,5-T2 in human metabolism. In contrast, markers for blood lipid profile and anthropometry seemed to be effected by TSH itself or unaffected respectively.
Compared with results reported in previous studies on euthyroid humans, we measured markedly higher median serum 3,5-T2 concentration (15–19,29–33). One possible explanation might be the brief incubation time of almost one hour in most polyclonal antibody-based RIAs compared with the overnight incubation performed in our monoclonal antibody immunoassay. Furthermore, previously reported age- (16) or sex-related (15) differences in serum 3,5-T2 concentrations were not confirmed by the present analyses. The small sample sizes of the former studies including 81 (16) or 52 (15) subjects might be the most likely reason. With respect to TSH, a bell-shaped trend along the 3,5-T2 groups was observed, which was more pronounced when participants with subclinical thyroid disease were included (Fig. 3 and Table 2). In particular, this link is in accordance with animal studies showing that 3,5-T2 was only a third as potent as T3 in suppressing TSH concentrations, and rather high pharmacological 3,5-T2 concentrations are required for TSH suppression (34–36). However, several animal studies in rodents and other species, for example killifish, indicated that 3,5-T2 interferes with the hypothalamus–pituitary–thyroid axis at several levels such as pituitary, thyroid, and peripheral action, thereby displaying a broad spectrum of mechanisms involved such as classical interaction with TR and also rapid effects at the cell membrane and mitochondria (10,12,14,34–40) (for a review, see (3)). Action of 3,5-T2 via these different targets and molecular mechanisms might occur at different 3,5-T2 concentrations and depend on acute or chronic exposure (35,37,40), thus possibly explaining the unexpected bell-shaped relationship with serum TSH.
Adjusted ANOVA revealed a positive correlation between fasting glucose and low to intermediate serum 3,5-T2 concentrations. Of note, several other relevant parameters including serum insulin, HOMA-IR, and HbA1c also exhibited similar trends toward a positive relation, although their associations with serum 3,5-T2 concentrations did not reach statistical significance. In concordance, a study on obese women (41), who fasted for four days, reported a rise in serum 3,5-T2 concentrations after glucose ingestion. Challenging this trend in categorical analysis, continuous splines indicated an inverse association in mean glucose concentrations at serum 3,5-T2 concentrations >0.5 nM (Fig. 4). This observation is in line with a 3,5-T2-mediated increase in glucose sensitivity described for pharmacological interventions with high doses of 3,5-T2 in animal models (10,11). A possible indication to understand this discrepancy could be an effect of 3-iodothyronamine, which was suggested as a degradation product of 3,5-T2 and exhibited hyperglycemic effects (4,42). An antagonistic link between these two TH derivatives could be contributing to the observed nonlinear associations. Furthermore, one could argue that the observed associations might be mediated by the classical metabolic active T3 (or T4), since a recent study on euthyroid humans provided evidence for interference between T3 and fasting serum glucose (43). Depending on a previously published reference range for TSH concentrations that referred to our study region (0.25–2.12 mIU/L) (44), only a conspicuous subset of 64 subjects of the present study population was characterized by serum free T3 (fT3) and free T4 (fT4) concentrations. Based on these subjects, no significant correlation between circulating 3,5-T2 and fT3 or fT4 became obvious (Supplementary Table S2), further supporting an independent effect of 3,5-T2 on glucose metabolism. This finding is also in agreement with our previous study, where no correlation between 3,5-T2 and serum T4 and T3 concentrations was observed in healthy or T4-substituted individuals (20). However, since early experimental work suggested an extrathyroidal conversion of 3,5-T2 from T3 also in humans (45), the missing correlation observed within our tight subset does not necessarily exclude a physiological link between both, and further effort is needed for clarification.
Even leptin concentrations exhibited a nonlinear association with serum 3,5-T2 concentrations, whereby the highest mean leptin concentrations were observed for individuals with intermediate serum 3,5-T2 concentrations. Interdependency between serum leptin and TH concentrations is assumed to be mediated by a complex interplay with TSH, further involving thyrotropin-releasing hormone (46,47). In concordance, a recent study on young men provided evidence for a link between serum TSH and leptin concentrations, even in the euthyroid state (48). In recognition of these results, we tested for a possible effect of TSH on the association between 3,5-T2 and leptin. Indeed, further adjustment in ANOVA led to a loss of significance, thereby strengthening the dependency between serum TSH and leptin concentrations, as well as the relation between circulating TSH and 3,5-T2. In conclusion, further work is needed to elucidate the roles of circulating 3,5-T2 and leptin in TH homeostasis.
According to the promising effects of pharmacological interventions with 3,5-T2 on body weight or blood lipid profile (10,12,14,49), missing associations toward related measures require a clarifying note. To ensure an effect on resting metabolic rate, most of the studies used a daily intraperitoneal injection of 25 μg 3,5-T2 per 100 g body weight (10,12,14), and rather high doses were needed for oral administration in humans and rodents (49,50). In detail, a chronic treatment with 3,5-T2 at doses of up to 900 μg per day led to a significant weight loss in one human study (49), a 3,5-T2 dose ninefold higher than average daily thyroidal production of T4, the putative precursor of 3,5-T2, which is only a minor metabolite of T4 according to previous kinetic studies (17,18). Therefore, a dose-dependent effect could be reasonable, exceeding endogenous 3,5-T2 concentrations by far and pronouncing selected 3,5-T2 mediated effects. This might include for example a stimulatory effect on TH receptor β (39), which was attributed to enhance β-oxidation of long-chain fatty acids (46), a key mechanism for weight loss. Beside dose-dependent effects, when administered in comparable doses, 3,5-T2 was shown to act rapidly and complementary to T3 on resting metabolic rate (51), the major contributor to weight maintenance. Taking into account such moderate effects on anthropometry and/or lipid metabolism by 3,5-T2, the application of more sensitive clinical markers as provided by metabolomics could possibly validate reported associations (52).
It has to be emphasized that all detected associations observed in our study, even when reaching statistical significance, were relatively weak. Hence, consequences for clinical practice cannot necessarily be expected. Since our study is cross-sectional, there are several limitations, especially in comparison to investigations on 3,5-T2 action using animal models. Most of these represent longitudinal studies on hypothyroid phenotypes including interventions by 3,5-T2 administration. In contrast, cross-sectional studies are limited in their prediction of time courses and intervention effects. In addition, the present study was performed in euthyroid humans. Therefore, the role of 3,5-T2 in thyroid diseases could not be delineated and requires separate analyses of appropriate patient cohorts. Moreover, nearly a third of study participants (n=274) exhibited serum 3,5-T2 concentrations below the functional sensitivity defined as limit of detection of the used assay, thereby limiting statistical analyses.
In conclusion, using a recently developed immunoassay, we were able to determine serum 3,5-T2 concentrations in a relatively large euthyroid study population and to test for associations with anthropometric and metabolic parameters. Our data point toward a physiological link between 3,5-T2 and glucose metabolism as well as TH homeostasis, while no significant associations with age, sex, and smoking or parameters of blood lipid profile and anthropometric measures were observed. Further analyses on even larger samples of euthyroid individuals, as well as pharmacological intervention studies, are needed to confirm the detected associations and to detect possible more moderate effects in order to obtain a more comprehensive picture of the role of 3,5-T2 in human metabolism.
Supplementary Material
Acknowledgments
This work was funded by grants from the German Federal Ministry of Education and Research (BMBF, Grants 01ZZ0403, 01ZZ0103, 01GI0883), the Ministry for Education, Research and Cultural Affairs, and the Ministry of Social Affairs of the Federal State of Mecklenburg-West Pomerania. This work is also part of the research project Greifswald Approach to Individualized Medicine (GANI_MED). The GANI_MED consortium is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg-West Pomerania (03IS2061A). The project was conducted within the framework of the DFG SPP 1692 “Thyroid Trans Act” (DFG WA 1328/5-1, KO 922/17-1 and VO 955/12-1) and the DFG GRK 1208-2 (TP 3 to J.K.).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Loeser A, Ruland H, Trikojus V.1938Darstellung, Eigenschaften und biologische Wirkungen von Derivaten (Äthern) des Thyroxins, Dijodthyronins und Dijodtyrosins. Naunyn Schmiedebergs Arch Pharmacol 189:664–678 [Google Scholar]
- 2.Gemmill CL.1951Effects of thyroxine, 3,5-diiodothyronine and thyronine on ascorbic acid oxidation. Am J Physiol 167:349–354 [DOI] [PubMed] [Google Scholar]
- 3.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F.2008Metabolic effects of thyroid hormone derivatives. Thyroid 18:239–253 [DOI] [PubMed] [Google Scholar]
- 4.Piehl S, Hoefig C, Scanlan T, Köhrle J.2011Thyronamines—past, present, and future. Endocr Rev 32:64–80 [DOI] [PubMed] [Google Scholar]
- 5.Lanni A, Moreno M, Cioffi M, Goglia F.1992Effect of 3,3′-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity. Mol Cell Endocrinol 86:143–148 [DOI] [PubMed] [Google Scholar]
- 6.Moreno M, Lanni A, Lombardi A, Goglia F.1997How the thyroid controls metabolism in the rat: different roles for triiodothyronine and diiodothyronines. J Physiol 505:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goglia F, Lanni A, Horst C, Moreno M, Thoma R.1994In vitro binding of 3,5-di-iodo-L-thyronine to rat liver mitochondria. J Mol Endocrinol 13:275–282 [DOI] [PubMed] [Google Scholar]
- 8.Lanni A, Moreno M, Lombardi A, Goglia F.1994Rapid stimulation in vitro of rat liver cytochrome oxidase activity by 3,5-diiodo-L-thyronine and by 3,3′-diiodo-L-thyronine. Mol Cell Endocrinol 99:89–94 [DOI] [PubMed] [Google Scholar]
- 9.Lombardi A, Beneduce L, Moreno M, Diano S, Colantuoni V, Ursini MV, Lanni A, Goglia F.20003,5-Diiodo-L-thyronine regulates glucose-6-phosphate dehydrogenase activity in the rat. Endocrinology 141:1729–1734 [DOI] [PubMed] [Google Scholar]
- 10.de Lange P, Cioffi F, Senese R, Moreno M, Lombardi A, Silvestri E, De Matteis R, Lionetti L, Mollica MP, Goglia F, Lanni A.2011Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 60:2730–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang G, Gao P, Zhao Z, Chen Q, Jiang T, Zhang N, Li H.20133,5-Diiodo-L-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Biochim Biophys Acta 1832:674–684 [DOI] [PubMed] [Google Scholar]
- 12.Lanni A, Moreno M, Lombardi A, de Lange P, Silvestri E, Ragni M, Farina P, Baccari GC, Fallahi P, Antonelli A, Goglia F.20053,5-Diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J 19:1552–1554 [DOI] [PubMed] [Google Scholar]
- 13.Lombardi A, de Lange P, Silvestri E, Busiello RA, Lanni A, Goglia F, Moreno M.20093,5-Diiodo-L-thyronine rapidly enhances mitochondrial fatty acid oxidation rate and thermogenesis in rat skeletal muscle: AMP-activated protein kinase involvement. Am J Physiol Endocrinol Metabol 296:E497–E502 [DOI] [PubMed] [Google Scholar]
- 14.Moreno M, Silvestri E, De Matteis R, de Lange P, Lombardi A, Glinni D, Senese R, Cioffi F, Salzano AM, Scaloni A, Lanni A, Goglia F.20113,5-Diiodo-L-thyronine prevents high-fat-diet-induced insulin resistance in rat skeletal muscle through metabolic and structural adaptations. FASEB J 25:3312–3324 [DOI] [PubMed] [Google Scholar]
- 15.Kirkegaard C, Faber J, Siersbæk-Nielsen K, Friis T.1981. A radioimmunoassay of serum 3,5-diiodothyronine. Acta Endocrinol (Copenh) 97:196–201 [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa M, Inada M, Naito K, Ishii H, Tanaka K, Mashio Y, Imura H.1981Age-related changes of serum 3,3′-diiodothyronine, 3′,5′-diiodothyronine, and 3,5-diiodothyronine concentrations in man. J Clin Endocrinol Metabol 52:517–522 [DOI] [PubMed] [Google Scholar]
- 17.Faber J, Heaf J, Kirkegaard C, Lumholtz I, Siersbaek-Nielsen K, Kollendorf K, Friis T.1983Simultaneous turnover studies of thyroxine, 3,5,3′- and 3,3′,5′-triiodothyronine, 3,5-,3,3′-, and 3′,5′-diiodothyronine, and 3′-monoiodothyronine in chronic renal failure. J Clin Endocrinol Metab 56:211–217 [DOI] [PubMed] [Google Scholar]
- 18.Faber J, Kirkegaard C, Thomson H, Lumholtz I, Siersbaek-Nielsen K, Friis T.1983The extrathyroidal conversion of 3,5,3′-triiodothyronine to 3,5-diiodothyronine in patients with liver cirrhosis. J Clin Endocrinol Metab 57:428–431 [DOI] [PubMed] [Google Scholar]
- 19.Pinna G, Meinhold H, Hiedra L, Thoma R, Hoell T, Graf KJ, Stoltenburg-Didinger G, Eravci M, Prengel H, Brodel O, Finke R, Baumgartner A.1997Elevated 3,5-diiodothyronine concentrations in the sera of patients with nonthyroidal illnesses and brain tumors. J Clin Endocrinol Metab 82:1535–1542 [DOI] [PubMed] [Google Scholar]
- 20.Lehmphul I, Brabant G, Wallaschofski H, Ruchala M, Strasburger CJ, Kohrle J, Wu Z.2014Detection of 3,5-diiodothyronine in sera of patients with altered thyroid status using a new monoclonal antibody-based chemiluminescence immunoassay. Thyroid 24:1350–1360 [DOI] [PubMed] [Google Scholar]
- 21.Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Jünger M, Mayerle J, Kraft M, Lerch MM, Dörr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Gläser S, Ewert R, Fietze I, Penzel T, Dören M, Rathmann W, Haerting J, Hannemann M, Röpcke J, Schminke U, Jürgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kühn J-P, Kühn J, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Völker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W.2011Cohort profile: the study of health in Pomerania. Int J Epidemiol 40:294–307 [DOI] [PubMed] [Google Scholar]
- 22.Müller H-P, Raudies F, Unrath A, Neumann H, Ludolph AC, Kassubek J.2011Quantification of human body fat tissue percentage by MRI. NMR Biomed 24:17–24 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC.1985Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 24.Stone CJ, Koo C-Y.1985Additive splines in statistics. Proc Stat Comp Sect Am Statist Assoc 27:45–48 [Google Scholar]
- 25.R Core Team 2013R: A Language and Environment for Statistical Computing 3.0.1. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 26.Piehl S, Heberer T, Balizs G, Scanlan TS, Köhrle J.2008Development of a validated liquid chromatography/tandem mass spectrometry method for the distinction of thyronine and thyronamine constitutional isomers and for the identification of new deiodinase substrates. Rapid Commun Mass Spectrom 22:3286–3296 [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Stapleton H.2010Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 397:1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Conrad AH, Thoma R, Conrad GW.2006Differentiation of diiodothyronines using electrospray ionization tandem mass spectrometry. J Mass Spectrom 41:162–168 [DOI] [PubMed] [Google Scholar]
- 29.Meinhold H, Schurnbrand P.1978. A radioimmunoassay for 3,5-diiodothyronine. Clin Endocrinol (Oxf) 8:493–497 [DOI] [PubMed] [Google Scholar]
- 30.Maciel RM, Chopra IJ, Ozawa Y, Geola F, Solomon DH.1979. A radioimmunoassay for measurement of 3,5-diiodothyronine. J Clin Endocrinol Metab 49:399–405 [DOI] [PubMed] [Google Scholar]
- 31.Pangaro L, Burman KD, Wartofsky L, Cahnmann HJ, Smallridge RC, O'Brian JT, Wright FD, Latham K.1980Radioimmunoassay for 3,5-diiodothyronine and evidence for dependence on conversion from 3,5,3′-triiodothyronine. J Clin Endocrinol Metab 50:1075–1081 [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa M, Inada M, Naito K, Ishii H, Tanaka K, Mashio Y, Imura H.1983Serum concentrations of 3,3′-diiodothyronine, 3′,5′-diiodothyronine, and 3,5-diiodothyronine in altered thyroid states. Endocrinol Jpn 30:167. [DOI] [PubMed] [Google Scholar]
- 33.Engler D, Burger A.1984The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev 5:151–184 [DOI] [PubMed] [Google Scholar]
- 34.Ball S, Sokolov J, Chin W.19973,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J Mol Endocrinol 19:137–147 [DOI] [PubMed] [Google Scholar]
- 35.Baur A, Bauer K, Jarry H, Köhrle J.19973,5-Diiodo-L-thyronine stimulates type 1 5′ deiodinase activity in rat anterior pituitaries in vivo and in reaggregate cultures and GH3 cells in vitro. Endocrinology 138:3242–3248 [DOI] [PubMed] [Google Scholar]
- 36.Horst C, Harneit A, Seitz HJ, Rokos H.19953,5-Di-iodo-L-thyronine suppresses TSH in rats in vivo and in rat pituitary fragments in vitro. J Endocrinol 145:291–297 [DOI] [PubMed] [Google Scholar]
- 37.Garcia GC, Lopez-Bojorquez L, Nunez J, Valverde RC, Orozco A.20073,5-Diiodothyronine in vivo maintains euthyroidal expression of type 2 iodothyronine deiodinase, growth hormone, and thyroid hormone receptor beta1 in the killifish. Am J Physiol Regul Integr Comp Physiol 293:R877–883 [DOI] [PubMed] [Google Scholar]
- 38.Kvetny J.19923,5-T2 stimulates oxygen consumption, but not glucose uptake in human mononuclear blood cells. Horm Metab Res 24:322–325 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza A, Navarrete-Ramirez P, Hernandez-Puga G, Villalobos P, Holzer G, Renaud JP, Laudet V, Orozco A.20133,5-T2 is an alternative ligand for the thyroid hormone receptor beta1. Endocrinology 154:2948–2958 [DOI] [PubMed] [Google Scholar]
- 40.Mollica MP, Lionetti L, Moreno M, Lombardi A, De Lange P, Antonelli A, Lanni A, Cavaliere G, Barletta A, Goglia F.20093,5-diiodo-L-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J Hepatol 51:363–370 [DOI] [PubMed] [Google Scholar]
- 41.Jaedig S, Faber J.1982The effect of starvation and refeeding with oral versus intravenous glucose on serum 3,5-,3,3′-and 3′-5′-diiodothyronine and 3′-monoiodothyronine. Acta Endocrinol (Copenh) 100:388–392 [DOI] [PubMed] [Google Scholar]
- 42.Galli E, Marchini M, Saba A, Berti S, Tonacchera M, Vitti P, Scanlan TS, Iervasi G, Zucchi R.2012Detection of 3-iodothyronamine in human patients: a preliminary study. J Clin Endocrinol Metab 97:E69–74 [DOI] [PubMed] [Google Scholar]
- 43.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, Kaufman JM.2014Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 24:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Völzke H, Alte D, Kohlmann T, Lüdemann J, Nauck M, John U, Meng W.2005Reference intervals of serum thyroid function tests in a previously iodine-deficient area. Thyroid 15:279–285 [DOI] [PubMed] [Google Scholar]
- 45.Faber J, Kirkegaard C, Lumholtz IB, Siersbaek-Nielsen K, Friis T.1982Simultaneous measurement of 3,5-diiodothyronine and 3,5,3′-triiodothyronine turnover kinetics in euthyroid hyperthyroid, and hypothyroid subjects. J Clin Endocrinol Metab 55:8–12 [DOI] [PubMed] [Google Scholar]
- 46.Mullur R, Liu YY, Brent GA.2014Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce EN.2012Thyroid hormone and obesity. Curr Opin Endocrinol Diabetes Obes 19:408–413 [DOI] [PubMed] [Google Scholar]
- 48.Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, Taes Y.2012Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol 167:719–726 [DOI] [PubMed] [Google Scholar]
- 49.Antonelli A, Fallahi P, Ferrari S, Di Domenicantonio A, Moreno M, Lanni A, Goglia F.20103, 5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J Biol Regul Homeost Agents 25:655–660 [PubMed] [Google Scholar]
- 50.Goldberg IJ, Huang LS, Huggins LA, Yu S, Nagareddy PR, Scanlan TS, Ehrenkranz JR.2012Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology 153:5143–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno M, Lombardi A, Beneduce L, Silvestri E, Pinna G, Goglia F, Lanni A.2002Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology 143:504–510 [DOI] [PubMed] [Google Scholar]
- 52.Bictash M, Ebbels TM, Chan Q, Loo RL, Yap IKS, Brown IJ, de Iorio M, Daviglus ML, Holmes E, Stamler J, Nicholson JK, Elliott P.2010Opening up the “Black Box”: metabolic phenotyping and metabolome-wide association studies in epidemiology. J Clin Epidemiol 63:970–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.