Abstract
Purpose
Adjuvant high-dose chemotherapy (HDC) with autologous hematopoietic stem-cell transplantation (AHST) for high-risk primary breast cancer has not been shown to prolong survival. Individual trials have had limited power to show overall benefit or benefits within subsets.
Methods
We assembled individual patient data from 15 randomized trials that compared HDC versus control therapy without stem-cell support. Prospectively defined primary end points were relapse-free survival (RFS) and overall survival (OS). We compared the effect of HDC versus control by using log-rank tests and proportional hazards regression, and we adjusted for clinically relevant covariates. Subset analyses were by age, number of positive lymph nodes, tumor size, histology, hormone receptor (HmR) status, and human epidermal growth factor receptor 2 (HER2) status.
Results
Of 6,210 total patients (n = 3,118, HDC; n = 3,092 control), the median age was 46 years; 69% were premenopausal, 29% were postmenopausal, and 2% were unknown menopausal status; 49.5% were HmR positive; 33.5% were HmR negative, and 17% were unknown HmR status. The median follow-up was 6 years. After analysis was adjusted for covariates, HDC was found to prolong relapse-free survival (RFS; hazard ratio [HR], 0.87; 95% CI, 0.81 to 0.93; P < .001) but not overall survival (OS; HR, 0.94; 95% CI, 0.87 to 1.02; P = .13). For OS, no covariates had statistically significant interactions with treatment effect, and no subsets evinced a significant effect of HDC. Younger patients had a significantly better RFS on HDC than did older patients.
Conclusion
Adjuvant HDC with AHST prolonged RFS in high-risk primary breast cancer compared with control, but this did not translate into a significant OS benefit. Whether HDC benefits patients in the context of targeted therapies is unknown.
INTRODUCTION
In the 1980s and 1990s, thousands of patients with breast cancer were treated with high doses of chemotherapy followed by bone marrow transplantation or autologous hematopoietic stem-cell transplantation.1 The major driver of the high-dose chemotherapy (HDC) movement was the preclinical rationale that predicted greater cytotoxicity for increasing dose-intensity. Chemotherapy is known to reduce tumor burden, so administering as high doses as possible would seem to be optimal.2 However, the National Surgical Adjuvant Breast and Bowel Project showed that outcomes were not improved by increasing the dose of cyclophosphamide from 600 to 1,200 mg/m2, nor from 1,200 to 1,800 and 2,400 mg/m2.3,4 The US Intergroup showed that increasing the dose of doxorubicin from 60 to 75 or 90 mg/m2 did not improve relapse-free survival (RFS) or overall survival (OS).5
Published reports of nonrandomized comparisons of HDC with adjuvant therapy not having stem-cell support (ie, control group) were encouraging,6 but the findings arose from potentially large patient selection biases, including different staging in the two groups. Women with breast cancer and advocates began demanding HDC. By 1995, autologous bone marrow transplantation was being used in the treatment of more occurrences of breast cancer than of any other type of cancer, mostly outside of clinical trials.
Initial reports of randomized, clinical trials of HDC appeared in 1999. Since then, there have been 15 known randomized trials for high-risk primary breast cancer that compared control groups with HDC plus autologous hematopoietic stem-cell transplantation as adjuvant therapy.7–21 The generally accepted conclusion from these trials has been negative (ie, that HDC has little or no benefit over control regarding OS).
A primary objective of this study was to address whether the conclusion that HDC is no better than control therapy is correct. In view of the individual trial reports, no overview could conclude that adjuvant HDC dramatically prolongs OS in primary breast cancer. However, the open question of whether HDC prolongs survival at all remains. Answering the question is complicated, because HDC is not a single regimen; the 15 trialists employed heterogeneous mixes of drugs, schedules, and doses. Moreover, the control regimens used in the trials also varied; some trialists used no therapy (ie, zero dose), and others used standard regimens containing agents not part of the trial HDC regimen.
Our second major objective was to address whether subsets of patients with primary breast cancer benefit from adjuvant HDC. The importance of evaluating treatment variability in subsets of patients with breast cancer was firmly established over a period of years and was supported by research in the 1970s through the 1990s. Knowledge accumulating during this time about the chemotherapy responsiveness of breast cancer suggested associations between such responsiveness and patient age,22,23 as well as tumor characteristics of hormone-receptor status,24–26 grade,26,27 and lymph node involvement.28 Evidence in support of HDC effect became available after the randomized trials were initiated.29–32 Some investigators proposed that younger women benefit from HDC,11 and others suggested that human epidermal growth factor receptor 2 (HER2) –negative tumors are sensitive to increasing dose.12,18 But individual trials have little power for distinguishing benefits within subsets of patients, because such analyses are subject to well-known subset biases.33
We addressed both major objectives by assembling a database that contained individual patient results of the 15 known randomized trials of adjuvant breast cancer. We specified the patient subsets in our institutional review board–approved protocol.
METHODS
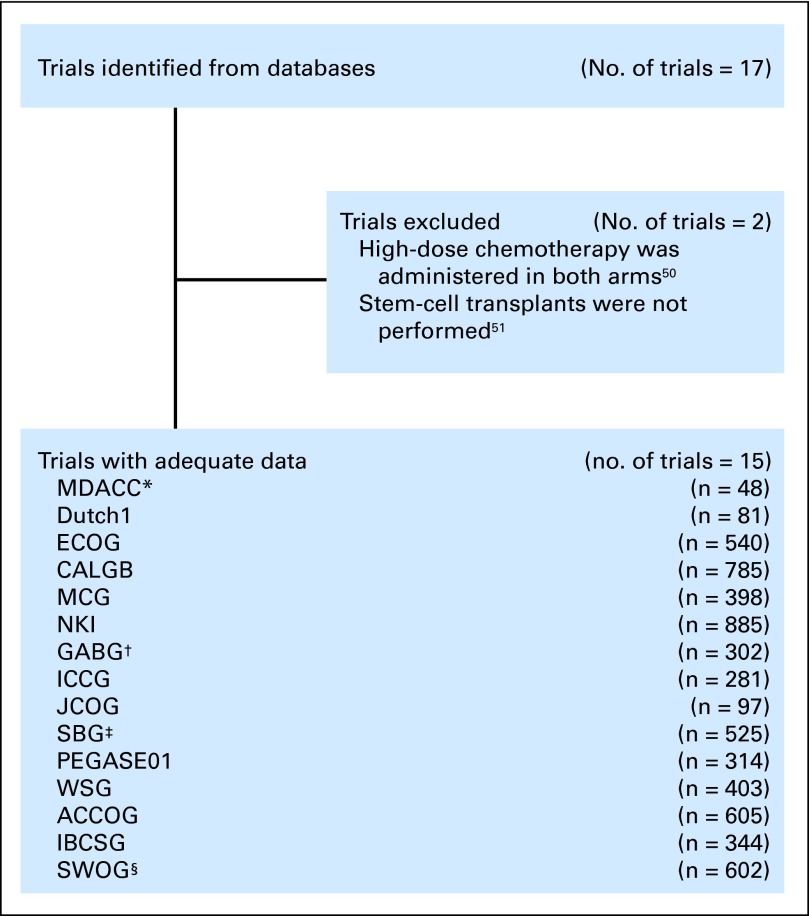
The trial selection process we used is illustrated in Figure 1and is described in the Appendix (online only). We assessed 15 randomized trials involving patients with primary high-risk breast cancer who were randomly assigned to HDC versus control therapy in the adjuvant setting between 1990 and 2002. We collected patient-level data from each study that included clinical characteristics, treatments, and outcomes, and we worked with the various trialists to merge the individual patient data into a single database. Details of the regimens used and of the demographic and clinical characteristics of patients in each of the studies we evaluated are listed in Tables 1 and 2.
Fig 1.
Study selection process. (*) Thirty patients were excluded because they received neoadjuvant therapy rather than adjuvant therapy. (†) Five patients were excluded because of a lack of cooperation after random assignment. (‡) This trial was excluded from The Cochrane Collaboration review,52 because the study evaluated two experimental therapies and did not include a control group receiving conventional-dose chemotherapy; also noted, patients with bony micrometastases were not excluded from the study. (§) The 2007 Journal of Clinical Oncology publication21 for this trial included only 536 patients. This tiral was excluded from The Cochrane Collaboration review,52 because it was ongoing and the data were immature. ACCOG, Anglo-Celtic Cooperative Oncology Group; CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; GABG, German Adjuvant Breast Cancer Study Group; IBCSG, International Breast Cancer Study Group; ICCG, International Collaborative Cancer Group; JCOG, Japan Clinical Oncology Group; MCG, Michelangelo Cooperative Group; MDACC, MD Anderson Cancer Center; NKI, the Netherlands Cancer Institute; PEGASE01, Programme d'évaluation des greffes autologues dans le cancer du sein; SBG, Scandinavian Breast Group; SWOG, Southwest Oncology Group; WSG, West German Study Group.
Table 1.
Trial and Patient Characteristics
| Trial | Year of First Accrual | Year of Publication | Total No. of Patients | Follow-Up (years) | Median Age (years) | Induction Regimen | HDC Regimen | Control Regimen | SDI |
SDIP |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDC | Control | Difference | HDC | Control | Difference | |||||||||
| MDACC | 1990 | 2000,7 200634 | 48* | 7 | 46 | 1,000 F, 50 D, 500 C | 5,250 C, 1,200 Et, 165 P over 3 days 2 cycles | No additional chemotherapy | 2.32 | 2.07 | 0.25 | 70 | 50 | 20 |
| Dutch1 | 1991 | 1998,8 200235 | 81 | 6 | 47 | 500 F, 120 E, 500 C | 6,000 C, 480 T, 1,600 Cb over 4 days | No additional chemotherapy | 3.13 | 2.10 | 1.03 | 47 | 25 | 22 |
| ECOG | 1991 | 20039 | 540 | 7 | 44 | 1,400 C, 60 D, 1,000 F | 6,000 C, 800 T over 4 days | No additional chemotherapy | 2.25 | 2.10 | 0.15 | 68 | 50 | 18 |
| CALGB | 1991 | 200510 | 785 | 9 | 45 | 600 C, 60 D, 1,200 F | 5,625 C, 165 P, 600 BCNU over 3 days | 900 C, 90 P, 90 BCNU over 3 days | 2.16 | 1.90 | 0.26 | 52 | 36 | 16 |
| MCG | 1993 | 200111 | 398 | 7 | 44 | — | 7,000 C, 8,000 Mt, 240 E, 600 T, 160-180 A | 120 E 3 cycles then 600 C, 40 Mt, 600 F 6 cycles | 2.76 | 1.81 | 0.95 | 50 | 60 | −10 |
| NKI | 1993 | 200312 | 885 | 8 | 46 | 500 F, 90 E, 500 C | 6,000 C, 480 T, 1,600 Cb over 4 days | 500 F, 90 E, 500 C (1 additional cycle) | 2.72 | 1.70 | 1.02 | 49 | 25 | 24 |
| GABG | 1993 | 2004,13 200836 | 302 | 5 | 48 | 90 E, 600 C | 6,000 C, 600 T, 40 M over 4 days | 1,000 C, 80 Mt, 1,200 F 3 cycles (28 days) | 2.23 | 1.66 | 0.57 | 41 | 40 | 1 |
| ICCG | 1993 | 200514 | 281 | 4 | 47 | cycle 1: 600 F, 50 E, 600 C; cycles 2 and 3: 1,200 F, 50 E, 1200 C | 6,000 C, 500 T, 800 Cb | 1,200 F, 50 E, 1,200 C 2 additional cycles (28 days) | 2.21 | 1.39 | 0.82 | 38 | 33 | 5 |
| JCOG | 1993 | 200815 | 97 | 7 | 47 | 500 C, 40 D, 500 F | 6,000 C, 600 T | No additional chemotherapy | 1.80 | 1.56 | 0.24 | 43 | 28 | 15 |
| SBG | 1994 | 200016 200737 | 525 | 6 | 48 | HDC only: 600 C, 60 E, 600 F 3 cycles | 6,000 C, 500 T, 800 Cb over 4 days | Doses individually tailored, 6 plans. Start dose: 600 F, 75 E, 900 C, 9 cycles (21 days) | 2.33 | 1.86 | 0.47 | 35 | 50 | −15 |
| PEGASE 01 | 1994 | 200517 | 314 | 5 | 48 | 500 F, 100 E, 500 C | 120 mg/kg C, 45 M, 140 A | No additional chemotherapy | 2.98 | 1.82 | 1.16 | 54 | 22 | 32 |
| WSG | 1995 | 200518 | 403 | 5 | 49 | 90 E, 600 C | 3,000 C, 90 E 400 T every 28 days | 600 C, 40 Mt, 600 F 3 cycles (14 days) | 2.78 | 2.10 | 0.68 | 33 | 29 | 4 |
| ACCOG | 1995 | 200419 | 605 | 6 | 45 | 75 D | 4,000 C single dose then 6,000 C, 800 T over 4 days | 600 C, 50 Mt, 600 F 8 cycles (21 days) | 2.48 | 1.62 | 0.86 | 47 | 58 | −11 |
| IBCSG | 1995 | 2006,20 200938 | 344 | 5 | 47 | — | 4,000 C, 200 E 3 cycles (21 days) | 600 C, 90 E or 60 D 4 cycles (21 days), then 1,400 C, 1,200 F, 80 Mt over 14 days 3 cycles (28 days) | 4.73 | 1.84 | 2.89 | 42 | 44 | −2 |
| SWOG | 1996 | 200721 | 602 | 8 | 46 | HDC only: 80 D, 600 C | STAMP I: C, P, BCNU or STAMP V: C, Cb, T | 80 D: 3 cycles (14 days) then 200 Pac: cycles (14 days) then 3,000 C 3 cycles (14 days) | 3.00 | 2.50 | 0.50 | 36 | 45 | −9 |
| Total | 6,210 | 7 | 2.66 | 1.87 | 0.79 | 47 | 39.7 | 7.3 | ||||||
Abbreviations: A, melphalan; ACCOG, Anglo-Celtic Cooperative Oncology Group; BCNU, carmustine; C, cyclophosphamide; CALGB, Cancer and Leukemia Group B; Cb, carboplatin; D, doxorubicin; E, epirubicin; ECOG, Eastern Cooperative Oncology Group; Et, etoposide; F, fluorouracil; GABG, German Adjuvant Breast Cancer Study Group; HDC, high-dose chemotherapy; IBCSG, International Breast Cancer Study Group; ICCG, International Collaborative Cancer Group; JCOG, Japan Clinical Oncology Group; M, mitoxantrone; MCG, Michelangelo Cooperative Group; MDACC, MD Anderson Cancer Center; Mt, methotrexate; NKI, the Netherlands Cancer Institute; P, cisplatin; Pac, paclitaxel; PEGASE01, Programme d'évalution des greffes autologues dans le cancer du sein; SBG, Scandinavian Breast Group; SDI, summation dose intensity; SDIP, summation dose intensity product; STAMP I, Solid Tumor Autologous Marrow Transplant Program regimen I (C 1.85 g/m2/d and P 55 mg/m2/d, each for 3 days [days −6, −5, and −4], followed by BCNU 600 mg/m2 [day−3]); STAMP V, Solid Tumor Autologous Marrow Transplant Program regimen V (C 1.5 g/m2/d, Cb 200 mg/m2/d, and T 125 mg/m2/d for 4 days (days −7 through −4); SWOG, Southwest Oncology Group; T, thiotepa; WSG, West German Study Group.
Of the 78 total patients enrolled on this trial, 30 were randomly assigned to receive neoadjuvant therapy rather than adjuvant therapy; these patients were not included in the meta-analysis.
Table 2.
Patient Demographic and Clinical Characteristics
| Characteristic | HDC (n = 3,118) |
Control (n = 3,092) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 46.2 | 46 | ||
| Range | 21.7-66.1 | 20.6-67.0 | ||
| Menopausal status | ||||
| Pre | 2,146 | 68.8 | 2,134 | 69.0 |
| Post | 922 | 29.6 | 906 | 29.3 |
| Missing | 50 | 1.6 | 52 | 1.7 |
| ER status | ||||
| Negative | 1,024 | 32.8 | 1,043 | 33.7 |
| Positive | 1,415 | 45.4 | 1,382 | 44.7 |
| Missing | 679 | 21.8 | 667 | 21.6 |
| PR status | ||||
| Negative | 1,088 | 34.9 | 1,070 | 34.6 |
| Positive | 1,233 | 39.5 | 1,226 | 39.7 |
| Missing | 797 | 25.6 | 796 | 25.7 |
| HmR status | ||||
| Negative | 1,032 | 33.1 | 1,046 | 33.8 |
| Positive | 1,552 | 49.8 | 1,522 | 49.2 |
| Missing | 534 | 17.1 | 524 | 16.9 |
| HER2 status | ||||
| Negative | 648 | 20.8 | 612 | 19.8 |
| Positive | 220 | 7.1 | 215 | 7.0 |
| Missing | 2,250 | 72.2 | 2,265 | 73.3 |
| Histologic grade | ||||
| Low | 141 | 4.5 | 127 | 4.1 |
| Medium | 577 | 18.5 | 598 | 19.3 |
| High | 835 | 26.8 | 780 | 25.2 |
| Missing | 1,565 | 50.2 | 1,587 | 51.3 |
| Histologic type | ||||
| Invasive ductal | 1,227 | 39.4 | 1,215 | 39.3 |
| Invasive lobular | 242 | 7.8 | 257 | 8.3 |
| Mixed | 62 | 2.0 | 52 | 1.7 |
| Other | 108 | 3.5 | 104 | 3.4 |
| Missing | 1,479 | 47.4 | 1,464 | 47.3 |
| Positive lymph nodes | ||||
| < 10 | 1,157 | 37.1 | 1,119 | 36.2 |
| ≥ 10 | 1,943 | 62.3 | 1,947 | 63.0 |
| Missing | 18 | 0.6 | 26 | 0.8 |
| Tumor size, cm | ||||
| Median | 3.0 | 2.7 | ||
| Range | 0.01-17.5 | 0.03-20.0 | ||
| Assigned tamoxifen | ||||
| HmR negative | ||||
| No. | 170 | 16.5 | 170 | 16.3 |
| Total No. | 1,032 | 1,046 | ||
| HmR positive | ||||
| No. | 1,463 | 94.3 | 1,429 | 93.9 |
| Total No. | 1,551 | 1,522 | ||
| HmR missing | ||||
| No. | 484 | 90.6 | 470 | 89.7 |
| Total No. | 534 | 524 | ||
Abbreviations: HER2, human epidermal growth factor receptor 2; HDC, high-dose chemotherapy; HmR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor.
The primary end points were RFS and OS. RFS was defined as the time from surgery to disease recurrence or death as a result of any cause.39 OS was defined as the time from surgery to death as a result of any cause. We evaluated the results for both end points by using the Kaplan-Meier product limit method, and we compared the results across treatment groups by using the log-rank test. Additionally, we considered RFS and OS within patient subsets defined by age (< 50 v ≥ 50 years), number of positive lymph nodes (≥ 10 v < 10), tumor size (≥ 2 v < 2 cm), histology (invasive ductal v invasive lobular), hormone receptor status (estrogen- or progesterone-receptor positive v both negative), and HER2 status (positive v normal). In view of the multiplicities of subset analyses, we provided these analyses without P values or CIs. We used Cox proportional hazards regression models to assess the outcome of HDC versus control after the analysis was adjusted for trial, age, number of positive lymph nodes (square root transformation), and hormone receptor status (including a category for missing status). We provided the hazard ratio (HR) of HDC to control and its 95% CI (on the basis of the likelihood ratio) for RFS and OS for each of the 15 trials as well as overall.
Because the 15 trials used a variety of drugs and dose-intensities for the HDC and control regimens, we converted to dose-intensity by using the method of Hryniuk.40,41 This method determines the average weekly dose-intensity (the summation dose-intensity [SDI]) and the total dose-intensity over both the induction phase and the treatment phase (the summation dose intensity product [SDIP]). The SDI and SDIP for each trial are listed in Table 1 and are additionally defined in the Appendix. The analyses are based on intention to treat. All P values were based on two-sided tests, and significance was set at P ≤ .05. Missing data for the covariates were multiply imputed,42 but multiple imputation was not used for the subset analyses. P values were generated with the MIANALYZE procedure in SAS 9.1 (SAS Institute, Cary, NC). S-Plus, version 7.0 (Insightful Corporation, Seattle, WA), was also used to perform statistical analyses.
RESULTS
Of the 6,210 total patients included in these analyses, 3,118 were randomly assigned to HDC, and 3,092 were randomly assigned to control. The baseline characteristics of the two treatment groups were well balanced (Table 2). Relative to patients with primary breast cancer who receive adjuvant therapy, the women in these trials tended to be younger (median age, 46 years), to have larger tumors (median, 2.8 cm), and to have a greater number of positive axillary lymph nodes (median, 11). The rates of positivity of hormone receptor status and HER2 in this population were typical of high-risk breast cancer. Of patients with hormone receptor–positive tumors, 94% were treated with tamoxifen, with some variability across trials that ranged from 29% to 100%. The median follow-up was 6 years; 3,082 (50%) of the patients experienced disease recurrence, and 2,468 (40%) of the patients died.
RFS and OS Estimates of HDC Versus Control
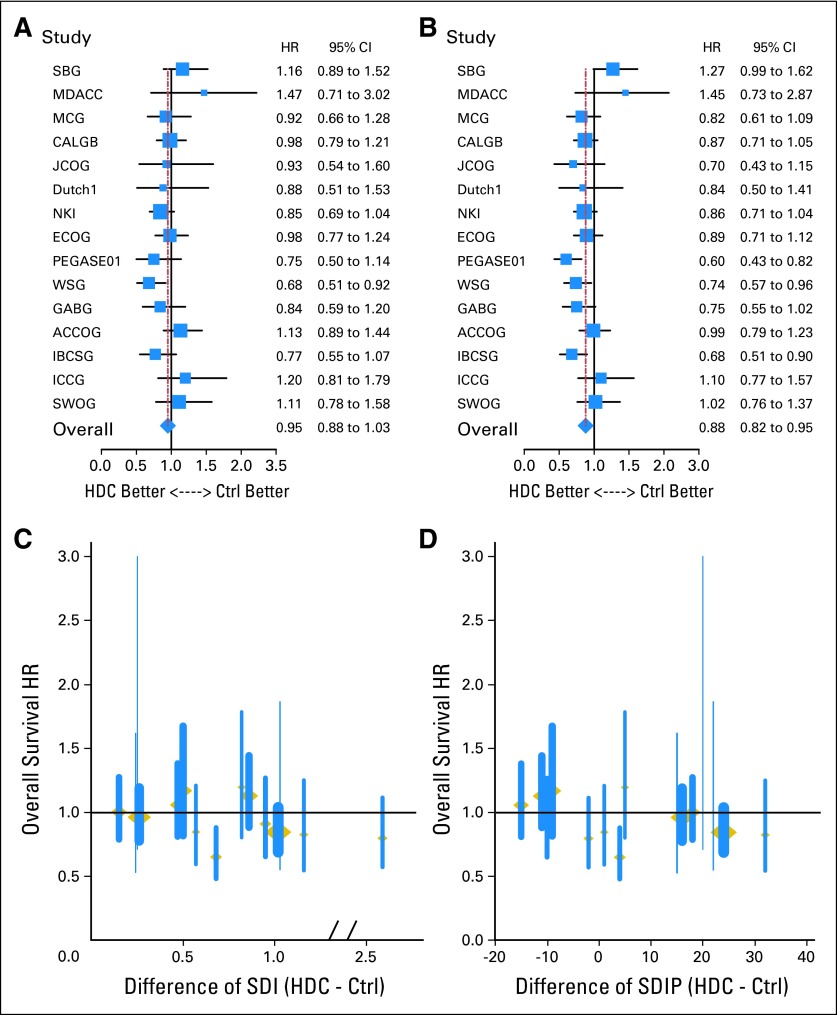
HRs for OS and RFS and the corresponding 95% CIs are shown in Figures 2A and 2B for the individual trials. Eleven of the 15 trials showed a numerical reduction in the risk of recurrence for the HDC group; three trials statistically significantly favored HDC. Ten of the 15 trials showed a numerical reduction in the risk of death for the HDC group; results of one trial statistically significantly favored HDC.
Fig 2.
Comparison of hazard ratios (HRs) of high-dose chemotherapy (HDC) versus control (Ctrl) therapy and HRs plotted against the dose-intensity for each individual trial. For (A) overall survival (OS) and (B) relapse-free survival (RFS), the HR (solid squares) and 95% CIs (shown by whiskers on both sides of the solid squares) were derived by univariable Cox regression models (on the basis of the likelihood ratio). Adjusted HRs of death among patients on HDC versus control therapy plotted against (C) the differences in summation dose-intensity product (SDIP) between HDC and control treatment arms and (D) the differences in summation dose-intensity product (SDIP) between HDC and control treatment arms. ACCOG, Anglo-Celtic Cooperative Oncology Group; CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; GABG, German Adjuvant Breast Cancer Study Group; IBCSG, International Breast Cancer Study Group; ICCG, International Collaborative Cancer Group; JCOG, Japan Clinical Oncology Group; MCG, Michelangelo Cooperative Group; MDACC, M.D. Anderson Cancer Center; NKI, the Netherlands Cancer Institute; PEGASE01, Programme d'évaluation des greffes autologues dans le cancer du sein; SBG, Scandinavian Breast Group; SWOG, Southwest Oncology Group; WSG, West German Study Group.
Kaplan-Meier curves of the OS (Fig 3A), RFS (Fig 3B), and OS minus RFS (Fig 3C) for all trials combined are shown in Figure 3. The corresponding proportional hazards model results are shown in Table 3. After analysis was adjusted for trial, age, number of positive lymph nodes, and hormone receptor status, HDC was associated with a nonsignificant 6% reduction in the risk of death (HR, 0.94; 95% CI, 0.87 to 1.02; P = .13) and a significant 13% reduction in the risk of recurrence (HR, 0.87; 95% CI, 0.81 to 0.93; P < .001).
Fig 3.
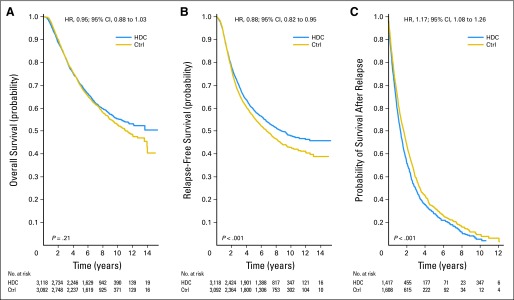
Kaplan-Meier estimates of survival outcomes. Hazard ratios (HRs) are presented with 95% CIs. P values are from the log-rank test. (A) Overall survival; (B) relapse-free survival; (C) probability of survival after relapse (overall survival − relapse-free survival). Ctrl, control; HDC, high-dose chemotherapy; OS, overall survival; RFS, relapse-free survival.
Table 3.
Cox Proportional Hazards Model Treating Missing HmR Status As a Separate Category and Using Multiple Imputation for Other Missing Covariates While Treating Age As Categoric
| Variable | OS |
RFS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| HDC v control | 0.94 | 0.87 to 1.02 | .13 | 0.87 | 0.81 to 0.93 | < .001 |
| Age ≥ 50 years v < 50 years | 0.97 | 0.89 to 1.06 | .55 | 0.91 | 0.84 to 0.98 | .019 |
| HmR status positive v negative | 0.59 | 0.54 to 0.64 | < .001 | 0.68 | 0.63 to 0.73 | < .001 |
| Square root of positive lymph nodes | 1.28 | 1.22 to 1.34 | < .001 | 1.26 | 1.20 to 1.31 | < .001 |
Abbreviations: HDC, high-dose chemotherapy; HmR, hormone receptor; HR, hazard ratio; OS, overall survival; RFS, relapse-free survival.
Kaplan-Meier curves of OS after disease recurrence are shown in Figure 3C. Patients in the HDC arm had a highly significant 16% increase in the risk of death after disease recurrence compared with patients in the control arm (HR, 1.16; 95% CI, 1.07 to 1.26; P < .001) after analysis was adjusted for trial, age, number of positive lymph nodes, and hormone receptor status.
Dose-Intensity and Dose-Intensity Product
The SDI and SDIP of the HDC and control arms of each trial are listed in Table 1. These values varied widely across trials, such that some control arms had larger SDIs or SDIPs than HDC arms in other trials. Figures 2C and 2D show the OS HRs and the corresponding 95% CIs for the individual trials plotted by the difference in SDI (Fig 2C) and the difference in SDIP (Fig 2D) between the two treatment arms. These plots show a positive trend with increasing dose-intensity. Multivariable analyses quantify this observation by considering SDI (and, separately, SDIP) as a substitute for HDC in the previously described multivariate analyses. After analysis was adjusted for trial, age, number of positive lymph nodes, and hormone receptor status status, an increasing SDI was associated with a statistically significant reduction in the risk of both disease recurrence (for one unit increase: HR, 0.85; 95% CI, 0.80 to 0.92; P < .001) and death (HR, 0.91; 95% CI, 0.84 to 0.99; P = .033). SDIP was associated with a statistically significant reduction in the risk of disease recurrence (for 0.05 unit increase: HR, 0.85, 95% CI, 0.78 to 0.93; P < .001) and death (HR, 0.91; 95% CI, 0.82 to 1.00; P = .045).
Subset Analyses
Figure 4 shows the OS comparison of HDC versus control in prespecified subset analyses, as follows: age (Fig 4A: < 50 v ≥ 50 years), number of positive axillary lymph nodes (Fig 4B: ≥ 10 v < 10), tumor size (Fig 4C: ≥ 2 v < 2 cm), histology (Fig 4D: invasive ductal v invasive lobular), hormone receptor status (Fig 4E: positive v negative), and HER2 status (Fig 4F: positive v normal). In view of the importance of tumor status for HER2 and hormone receptor in assessing chemotherapy effects in adjuvant breast cancer,31 we also considered subsets defined by the joint hormone receptor and HER2 status (Fig 5). Because a high rate of missing data for HER2 status may lead to biased comparisons, we also considered patients who had unknown HER2 status.
Fig 4.
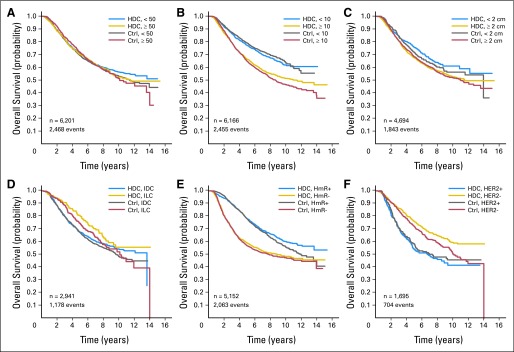
Kaplan-Meier estimates of overall survival (OS) comparison of high-dose chemotherapy (HDC) and control (Ctrl) therapy in prespecified subset analyses. Subsets of (A) patient age in years; (B) number of positive lymph nodes; (C) tumor size; (D) tumor histology; (E) tumor hormone receptor status (HmR); and (F) human epidermal growth factor receptor 2 status (HER2). IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Fig 5.
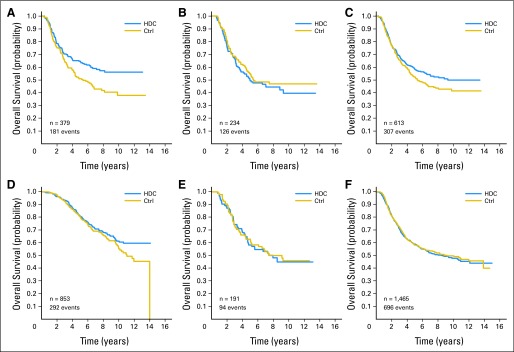
Kaplan-Meier estimates of overall survival comparison of high-dose chemotherapy (HDC) and control (Ctrl) therapy in subsets of patients defined by hormone receptor (HmR) status and human epidermal growth factor receptor 2 (HER2) status. (A) HmR negative, HER2 negative; (B) HmR negative, HER2 positive, (C) HmR negative, HER2 either positive or negative (known); (D) HmR positive, HER2 negative; (E) HmR positive, HER2 positive; (F) HmR negative, HER2 unknown.
We found that OS was not statistically different by treatment arm in any of the subgroups except for women with HER2-negative disease, for whom there was a 21% reduction in the risk of death (Fig 4F). The reduction was greatest (33%) among patients with both hormone receptor–negative and HER2-negative tumors—the so-called triple-negative breast cancer (Fig 5A). To address whether this observation is real, we compared patients who had hormone receptor–negative tumors and known HER2 status (Fig 5C) with those who had hormone receptor–negative tumors but unknown HER2 status (Fig 5F). The latter group showed little treatment effect, substantially less than those with hormone receptor–negative tumors for which HER2 status was available (Fig 5C).
Toxicity Deaths and Secondary Malignancies
In six of the 15 trials there were a total of 33 secondary malignancies categorized as myelodysplastic syndrome or acute myelogenous leukemia. Of the 33, 17 occurred in the HDC arms, and 16 occurred in the control arms.
Of 89 total deaths attributed to toxicity, 72 (6.0%) occurred among the 1,207 deaths in the HDC arms, and 17 (1.4%) occurred among the 1,261 deaths in the control arms. To evaluate survival separate from treatment-related mortality, we conducted an additional analysis that excluded patients whose deaths were attributed to toxicity. The HR was 0.90 (95% CI, 0.83 to 0.99; P = .011) for OS after analysis was adjusted for trial, age, hormone receptor status, and number of positive lymph nodes.
DISCUSSION
In a literature-based meta-analysis of 13 randomized trials, Farquhar et al43 found a statistically significant benefit for event-free survival but not for OS. Banna et al44 reviewed solid tumor trials and concluded that there was no overall benefit for the use of HDC. However, they suggested that additional trials could consider HDC in patients with triple-negative primary breast tumors, because there were no targeted therapies for these patients. Pedrazolli et al45 reviewed 14 randomized trials in solid tumors and supported the evaluation of regimens of HDC with low mortality rates in future breast cancer trials for subgroups most likely to benefit; a retrospective study by Rodenhuis et al46 suggested that such a subgroup include patients with HER2-normal tumors.
The largest individual studies had statistical power to detect a 30% improvement in survival outcomes.47 Our study had 80% power to detect a 10% improvement in RFS and a 12% improvement in OS. Our analyses showed that, compared with patients who were randomly assigned to receive control, those who were randomly assigned to receive HDC had a 13% improvement in RFS. Curiously, OS after disease recurrence (ie, OS minus RFS) was significantly worse in the HDC group (Fig 3C). As a consequence, the apparent RFS benefit translated to only a 6% improvement in OS.
In our experience, it is unusual to observe a significant benefit in RFS for one treatment group and then a significant benefit in OS after recurrence (ie, OS minus RFS) for the other group. There are several possible explanations. These studies were not blinded, so it is possible that some investigators assessed recurrence more diligently in the control group, perhaps fearing that they were most at risk of recurrence. Perhaps postrecurrence therapy was less feasible for the patients who were randomly assigned to HDC, whether as a result of residual toxicity, unwillingness of the patient to receive additional therapy, or exclusion from eligibility of clinical trials of targeted agents, such as trastuzumab or aromatase inhibitors. It is also possible that HDC reduces measurable disease burden but that it has a less dramatic effect on latent but insidious disease harbored in bone marrow, for example.
Our analyses by SDI and SDIP are revealing. They evince a dose response that extends into the high doses considered in these trials. However, the effect is not sufficiently clear to translate into clinical practice.
In clinical decision making, any benefit in recurrence or survival must be weighed against the greater toxicities of HDC. Individual studies have reported that the quality of life among patients receiving HDC is lower during treatment than that among the patients receiving control.43 There is less agreement regarding quality of life once treatment is complete. Farquhar et al43 reported that quality of life becomes comparable in the two groups over time, whereas Marino et al48 reported that physical functioning, role functioning, fatigue, and pain were negatively affected by HDC both during treatment and 1 year later.48
Individual studies have suggested that age, hormone receptor status, or HER2 expression may be predictive of the benefits of HDC. Our analyses showed that the only apparently significant OS benefit was among patients with HER2-negative tumors, and additionally, among patients with triple-negative tumors. However, only approximately 27% of the tumors had HER2 status available. (Anti-HER2 therapy was not available during this era and was specifically excluded for trials of HDC.) When broken out by HER2 status, we found that HDC is unlikely to show much of a benefit in triple-negative tumors. After analysis was adjusted for the missing data, we concluded that the triple-negative observation is likely to be spurious.
A limitation of our analysis is that we combined data that were highly heterogeneous, and variations exist among the patient populations, among the HDC regimens, and among the control regimens and also exist in dose-intensity differences between the HDC and control arms across the 15 trials. Indeed, the dose-intensity of the control arm was greater than that of the HDC arm in some of the trials and for some measures of intensity. For example, the SDIP for control was greater than that for HDC (difference < 0) in five trials (Table 1). These trials compared arms with different agents and not just differences in dose. Excluding these five trials effects a modest change in the HR of HDC versus control for OS of 0.90 (95% CI, 0.81 to 0.99; P = .031). Focusing on only the complementary set of trials, in the five with control arms that had more intensive doses, the HR was 1.03 (95% CI, 0.90 to 1.17; P = .69). Our adjustment for SDI and SDIP partially accounts for the differences in the treatment regimens, but no single number can perfectly measure the heterogeneity of the drug intensity of those regimens.
There are other differences in the trials as well, including that, although 94% of the patients with known hormone receptor–positive tumors were assigned tamoxifen, this proportion varied across the trials from 29% to 100%. We additionally adjusted for trial differences by incorporating patient-level covariates and by including an indicator of trial in the multivariate analyses.
An obvious caveat to our conclusions is that they apply for the settings of the 15 known randomized trials and the regimens considered. The relative benefits of HDC for other treatment regimens and in combination with targeted therapies for the treatment of breast cancer remain unknown.
Our conclusion in this article, that HDC does not have a statistically significant benefit in OS, is supported by the conclusion in our companion manuscript,49 that HDC does not have a statistically significant benefit in OS in metastatic breast cancer. Both studies leave open the possibility of a modest reduction in the hazards of OS in the range of 5% to 10%, but neither was able to identify subsets of patients who may benefit from HDC.
Acknowledgment
We thank William M. Hryniuk, MD, for calculating the SDI and SDIP for both treatment arms within the 15 trials as well as The University of Texas MD Anderson Cancer Center data managers V. Lopez, D. Smith, and L. Yancey for diligent work and L. Chastain for editorial assistance.
Appendix
Selection of Studies
We conducted a comprehensive review of the published literature by using MEDLINE and the following search terms: high-risk breast cancer, randomized trials, high-dose chemotherapy, standard-dose chemotherapy, stem-cell infusion, and autologous stem-cell transplant. We also reviewed presentations at all annual meetings of the American Society of Clinical Oncology by using the following search terms: breast cancer, adjuvant therapy, and transplant. From these reviews, our inclusion criteria required the studies to be randomized, controlled trials that compared high-dose chemotherapy with autologous stem-cell support and control (ie, standard-dose chemotherapy or another experimental control) in the treatment of primary breast cancer; to have occurred from 1988 to 2002; to have included outcome measurements of disease-free survival and overall survival; and to have included ongoing patient follow-up. Participants in the trials could be of any age, sex, race, ethnicity, or country of origin. Our searches resulted in the initial identification of 17 studies evaluating the treatment of primary breast cancer, two of which were excluded because of the use of high-dose chemotherapy in both treatment arms (no appropriate control)50 and because the adjuvant therapy did not include stem-cell transplantations.51 To confirm the comprehensiveness of our selection of 15 trials, we consulted with the European Blood and Marrow Transplant Group and the American Society for Blood and Marrow Transplantation, and we compared our search results with those in reviews published by The Cochrane Collaboration. The Cochrane Collaboration review included 13 of the 15 trials we selected.52 The review excluded the trial conducted by the Scandinavian Breast Group,16,37 because the study did not have a control group receiving conventional-dose chemotherapy: patients in the control arm received individually tailored fluororacil, epirubicin, cyclophosphamide–based therapy. The review also excluded the trial by the Southwest Oncology Group,21 because the trial was still ongoing and the data were immature.
Information Requested From the Trialists
We requested four categories of information for each patient enrolled on each of the 15 trials. First, we requested patient characteristics: sex (female or male), race/ethnicity, date of birth, date of diagnosis of invasive breast cancer, date of random assignment, and menopausal status at the time of random assignment (premenopausal, postmenopausal, unknown). Second, we requested disease characteristics: Eastern Cooperative Oncology Group performance status at the time of random assignment, tumor histologic subtype, nuclear grade (high or low), estrogen receptor status and progesterone receptor status (including method, enzyme-linked immunoassay or immunohistochemistry), and human epidermal growth factor receptor 2 status of the primary tumor (including method, fluorescent in situ hybridization or immunohistochemistry). Third, we requested treatment variables: date of surgery, type of surgery, size of tumor (pathology assessment), number of lymph nodes involved at the time of primary surgical therapy, number of lymph nodes sampled, extranodal extension, date of first systemic treatment, whether adjuvant hormonal therapy was received and duration, and whether radiotherapy was received, and date of initiation. If the patient received a control treatment, we requested information about what drugs, doses, and schedules the patient received. If the patient received high-dose chemotherapy, we requested information about what conditioning regimen the patient received (drugs, doses, and schedule), the date of transplantation, the number of high-dose chemotherapy cycles received, the type of graft (peripheral-blood stem-cell, marrow, or both), the number of cells infused (total nucleated cells or CD34-positive cells), and the date of engraftment. Fourth, we requested outcome variables: date of last follow-up visit; treatment-related grade 3 and 4 toxicities (according to the Common Terminology Criteria of Adverse Events, version 3.0), including date of occurrence; if the patient received autologous stem-cell transplantation, response status at transplantation (ie, complete response, partial response, stable disease, progressive disease); occurrence of secondary malignancy (including contralateral breast) during follow-up, and site (local, regional, distant, or relapse) and date; survival status at last follow-up; date of death; and cause of death.
Hormone Receptor Status
We did not receive hormone receptor data from all trialists, and some hormone receptor data were missing from other trials. We did not receive details regarding assessment methods for determining hormone receptor status in the individual trials.
Method of Assessing Dose-Intensity
The method of Hryniuk assesses summation dose -intensity (SDI) and summation dose-intensity product (SDIP) of the treatment regimen.40,41 The goal of this approach that uses SDI and SDIP is to assign a numerical dose-intensity to regimens even though they may contain different drugs, different schedules, and different doses. These measures allow for comparisons of dose-intensity within trials as well as across trials. The SDI is the average weekly dose-intensity, and the SDIP is the total dose-intensity over both the induction phase and the treatment phase. Our basis for assessing the dose-outcome is the difference in SDI and the difference in SDIP for the HDC and control regimens within each of the trials. We provided the hazard ratio and its 95% CI for the overall survival for unit increases in the difference in SDI and difference in the SDIP. Analyses were based on intention to treat, with the same intensity scores assigned for all patients within the same treatment group and trial; we had no information about the dose an individual patient actually received.
Footnotes
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Russell L. Basser, CSL Limited (C) Consultant or Advisory Role: Donald A. Berry, Berry Consultants LLC (co-owner) (C); Gabriel N. Hortobagyi, Novartis (C), SanofiAventis (C), Genentech (C) Stock Ownership: None Honoraria: Ulrike A. Nitz, Amgen Research Funding: Jonas Bergh, Roche, Amgen, Pharmacia; Ulrike A. Nitz, Amgen; R. Charles Coombes, Pfizer; Gabriel N. Hortobagyi, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Donald A. Berry, Naoto T. Ueno, Sjoerd Rodenhuis, William P. Peters, Robert C. Leonard, William E. Barlow, Martin S. Tallman, Jonas Bergh, Ulrike A. Nitz, Alessandro M. Gianni, Russell L. Basser, Axel R. Zander, R. Charles Coombes, Henri Roché, Yutaka Tokuda, Elisabeth G.E. de Vries, Gabriel N. Hortobagyi, John P. Crown, Paolo Pedrazzoli, Marco Bregni, Taner Demirer
Administrative support: Donald A. Berry, Marcella M. Johnson, Taner Demirer
Provision of study materials or patients: Sjoerd Rodenhuis, William P. Peters, Robert C. Leonard, William E. Barlow, Martin S. Tallman, Jonas Bergh, Ulrike A. Nitz, Alessandro M. Gianni, Russell L. Basser, Axel R. Zander, R. Charles Coombes, Henri Roché, Yutaka Tokuda, Elisabeth G.E. de Vries, Gabriel N. Hortobagyi, John P. Crown
Collection and assembly of data: Donald A. Berry, Marcella M. Johnson, Jean Caputo, Gabriel N. Hortobagyi, Taner Demirer
Data analysis and interpretation: Donald A. Berry, Marcella M. Johnson, Xiudong Lei, John P. Crown
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.National Cancer Institute. Washington, DC: National Cancer Institute; 2001. High-Dose Chemotherapy for Breast Cancer: History. http://www.cancer.gov/clinicaltrials/developments/high-dose-chemo-history0501. [Google Scholar]
- 2.Antman KH. New developments in clinical oncology: The interdependence of bench and bedside. Cancer Res. 1991;51(suppl 18):5060s–5064s. [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 5.Henderson IC, Berry DA, Demetri GD. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Peters WP, Ross M, Vredenburgh JJ. High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high-risk primary breast cancer. J Clin Oncol. 1993;11:1132–1143. doi: 10.1200/JCO.1993.11.6.1132. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Buzdar AU, Theriault RL, et al. Randomized trial of high-dose chemotherapy and blood cell autografts for high-risk primary breast carcinoma. J Natl Cancer Inst. 2000;92:225–233. doi: 10.1093/jnci/92.3.225. [DOI] [PubMed] [Google Scholar]
- 8.Rodenhuis S, Richel DJ, van der Wall E, et al. Randomised trial of high-dose chemotherapy and haemopoietic progenitor-cell support in operable breast cancer with extensive axillary lymph-node involvement. Lancet. 1998;352:515–521. doi: 10.1016/S0140-6736(98)01350-6. [DOI] [PubMed] [Google Scholar]
- 9.Tallman MS, Gray R, Robert NJ, et al. Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med. 2003;349:17–26. doi: 10.1056/NEJMoa030684. [DOI] [PubMed] [Google Scholar]
- 10.Peters WP, Rosner GL, Vredenburgh JJ, et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: A report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005;23:2191–2200. doi: 10.1200/JCO.2005.10.202. [DOI] [PubMed] [Google Scholar]
- 11.Gianni A, Bonadonna G, Michelangelo Cooperative Group Five-year results of the randomized clinical trial comparing standard versus high-dose myeloablative chemotherapy in the adjuvant treatment of breast cancer with > 3 positive nodes (LN+) Proc Am Soc Clin Oncol. 2001;20:21a. abstr 80. [Google Scholar]
- 12.Rodenhuis S, Bontenbal M, Beex LV, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med. 2003;349:7–16. doi: 10.1056/NEJMoa022794. [DOI] [PubMed] [Google Scholar]
- 13.Zander AR, Kröger N, Schmoor C, et al. High-dose chemotherapy with autologous hematopoietic stem-cell support compared with standard-dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: First results of a randomized trial. J Clin Oncol. 2004;22:2273–2283. doi: 10.1200/JCO.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Coombes RC, Howell A, Emson M, et al. High dose chemotherapy and autologous stem cell transplantation as adjuvant therapy for primary breast cancer patients with four or more lymph nodes involved: Long-term results of an international randomised trial. Ann Oncol. 2005;16:726–734. doi: 10.1093/annonc/mdi166. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda Y, Tajima T, Narabayashi M, et al. Phase III study to evaluate the use of high-dose chemotherapy as consolidation of treatment for high-risk postoperative breast cancer: Japan Clinical Oncology Group study, JCOG 9208. Cancer Sci. 2008;99:145–151. doi: 10.1111/j.1349-7006.2007.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergh J, Wiklund T, Erikstein B, et al. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial—Scandinavian Breast Group 9401 study. Lancet. 2000;356:1384–1391. doi: 10.1016/s0140-6736(00)02841-5. Erratum in: Lancet 356:2196, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Roche H, Viens P, Biron P, et al. High-dose chemotherapy for breast cancer: The French PEGASE experience. Cancer Control. 2003;10:42–47. doi: 10.1177/107327480301000105. [DOI] [PubMed] [Google Scholar]
- 18.Nitz UA, Mohrmann S, Fischer J, et al. Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: Results of a multicentre phase III trial. Lancet. 2005;366:1935–1944. doi: 10.1016/S0140-6736(05)67784-7. [DOI] [PubMed] [Google Scholar]
- 19.Leonard RC, Lind M, Twelves C. Conventional adjuvant chemotherapy versus single-cycle, autograft-supported, high-dose, late-intensification chemotherapy in high-risk breast cancer patients: A randomized trial. J Natl Cancer Inst. 2004;96:1076–1083. doi: 10.1093/jnci/djh188. [DOI] [PubMed] [Google Scholar]
- 20.International Breast Cancer Study Group. Basser RL, O'Neill A, et al. Multicycle dose-intensive chemotherapy for women with high-risk primary breast cancer: Results of International Breast Cancer Study Group Trial 15-95. J Clin Oncol. 2006;24:370–378. doi: 10.1200/JCO.2005.03.5196. [DOI] [PubMed] [Google Scholar]
- 21.Moore HC, Green SJ, Gralow JR, et al. Intensive dose-dense compared with high-dose adjuvant chemotherapy for high-risk operable breast cancer: Southwest Oncology Group/Intergroup study 9623. J Clin Oncol. 2007;25:1677–1682. doi: 10.1200/JCO.2006.08.9383. [DOI] [PubMed] [Google Scholar]
- 22.Albain K, Green S, Rivkin S, et al. Age < 35 years is an adverse prognostic feature in premenopausal node-positive breast cancer patients in Southwest Oncology Group studies. J Clin Oncol. 1990;9(suppl):35. abstr 32. [Google Scholar]
- 23.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Redmond CK, Wickerham DL, et al. Relation of estrogen and/or progesterone receptor content of breast cancer to patient outcome following adjuvant chemotherapy. Breast Cancer Res Treat. 1983;3:355–364. doi: 10.1007/BF01807588. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SG, 4th, Knuiman MW, Sleeper LA, et al. Six-year results of the Eastern Cooperative Oncology Group trial of observation versus CMFP versus CMFPT in postmenopausal patients with node-positive breast cancer. J Clin Oncol. 1989;7:879–889. doi: 10.1200/JCO.1989.7.7.879. [DOI] [PubMed] [Google Scholar]
- 26.Neville AM, Bettelheim R, Gelber RD, et al. Factors predicting treatment responsiveness and prognosis in node-negative breast cancer: The International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:696–705. doi: 10.1200/JCO.1992.10.5.696. [DOI] [PubMed] [Google Scholar]
- 27.Davis BW, Gelber RD, Goldhirsch A, et al. Prognostic significance of tumor grade in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Cancer. 1986;58:2662–2670. doi: 10.1002/1097-0142(19861215)58:12<2662::aid-cncr2820581219>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Bonadonna G, Valagussa P, Rossi A, et al. Ten-year experience with CMF-based adjuvant chemotherapy in resectable breast cancer. Breast Cancer Res Treat. 1985;5:95–115. doi: 10.1007/BF01805984. [DOI] [PubMed] [Google Scholar]
- 29.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 31.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 32.Albain KS, Paik S, van't Veer L. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast. 2009;18(suppl 3):141–145. doi: 10.1016/S0960-9776(09)70290-5. [DOI] [PubMed] [Google Scholar]
- 33.Berry DA. The difficult and ubiquitous problems of multiplicities. Pharm Stat. 2007;6:155–160. doi: 10.1002/pst.303. [DOI] [PubMed] [Google Scholar]
- 34.Hanrahan EO, Broglio K, Frye D, et al. Randomized trial of high-dose chemotherapy and autologous hematopoietic stem cell support for high-risk primary breast carcinoma: Follow-up at 12 years. Cancer. 2006;106:2327–2336. doi: 10.1002/cncr.21906. [DOI] [PubMed] [Google Scholar]
- 35.Schrama JG, Faneyte IF, Schornagel JH, et al. Randomized trial of high-dose chemotherapy and hematopoietic progenitor-cell support in operable breast cancer with extensive lymph node involvement: Final analysis with 7 years of follow-up. Ann Oncol. 2002;13:689–698. doi: 10.1093/annonc/mdf203. [DOI] [PubMed] [Google Scholar]
- 36.Zander AR, Schmoor C, Kröger N, et al. Randomized trial of high-dose adjuvant chemotherapy with autologous hematopoietic stem-cell support versus standard-dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: Overall survival after 6 years of follow-up. Ann Oncol. 2008;19:1082–1089. doi: 10.1093/annonc/mdn023. [DOI] [PubMed] [Google Scholar]
- 37.Wilking N, Lidbrink E, Wiklund T, et al. Long-term follow-up of the SBG 9401 study comparing tailored FEC-based therapy versus marrow-supported high-dose therapy. Ann Oncol. 2007;18:694–700. doi: 10.1093/annonc/mdl488. [DOI] [PubMed] [Google Scholar]
- 38.Colleoni M, Sun Z, Martinelli G, et al. The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: Results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up. Ann Oncol. 2009;20:1344–1351. doi: 10.1093/annonc/mdp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 40.Hryniuk W, Frei E, 3rd, Wright FA. A single scale for comparing dose-intensity of all chemotherapy regimens in breast cancer: Summation dose-intensity. J Clin Oncol. 1998;16:3137–3147. doi: 10.1200/JCO.1998.16.9.3137. [DOI] [PubMed] [Google Scholar]
- 41.Hryniuk WM, Peters WP, Ragaz J. Determinants of outcome in adjuvant chemotherapy of breast cancer: Dose intensity versus total dose versus dose size. Breast Cancer Res Treat. 2000;64:66. abstr 66. [Google Scholar]
- 42.Rubin DB. New York, NY: John Wiley and Sons; 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 43.Farquhar CM, Marjoribanks J, Lethaby A, et al. High dose chemotherapy for poor prognosis breast cancer: Systematic review and meta-analysis. Cancer Treat Rev. 2007;33:325–337. doi: 10.1016/j.ctrv.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Banna GL, Simonelli M, Santoro A. High-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation for the treatment of solid tumors in adults: A critical review. Curr Stem Cell Res Ther. 2007;2:65–82. doi: 10.2174/157488807779316964. [DOI] [PubMed] [Google Scholar]
- 45.Pedrazzoli P, Rosti G, Secondino S, et al. High-dose chemotherapy with autologous hematopoietic stem cell support for solid tumors in adults. Semin Hematol. 2007;44:286–295. doi: 10.1053/j.seminhematol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Rodenhuis S, Bontenbal M, van Hoesel QG, et al. Efficacy of high-dose alkylating chemotherapy in HER2/neu-negative breast cancer. Ann Oncol. 2006;17:588–596. doi: 10.1093/annonc/mdl001. [DOI] [PubMed] [Google Scholar]
- 47.Elias AD. High dose chemotherapy with stem cell rescue for breast cancer: Current status and future directions. Breast Dis. 2001;14:51–68. doi: 10.3233/bd-2001-14107. [DOI] [PubMed] [Google Scholar]
- 48.Marino P, Roché H, Biron P, et al. Deterioration of quality of life of high-risk breast cancer patients treated with high-dose chemotherapy: The PEGASE 01 Quality of Life Study. Value Health. 2008;11:709–718. doi: 10.1111/j.1524-4733.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 49.Berry DA, Ueno NT, Johnson MM, et al. High-dose chemotherapy with autologous hematopoietic stem-cell transplantation in metastatic breast cancer: Overview of six randomized trials. J Clin Oncol. 2011;29:3224–3231. doi: 10.1200/JCO.2010.32.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bliss JM, Vigushin D, Kanfer E, et al. qj> Randomised trial of high dose therapy using cyclophosphamide, thiotepa and carboplatin in primary breast cancer patients with 4 or more histologically involved positive nodes (ISRCTN: 52623943) Proc Am Soc Clin Oncol. 2003;22:15. abstr 58. [Google Scholar]
- 51.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in local advanced breast cancer: An EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 52.Farquhar C, Marjoribanks J, Basser R, et al. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database Sys Rev. 2005;3:CD003139. doi: 10.1002/14651858.CD003139.pub2. [DOI] [PubMed] [Google Scholar]