Abstract
Background:
Modern knee designs have popularized its use in younger patients due to its better performance. There remains uncertainty whether higher demands of these patients can affect implant survivorship.
Purpose:
To assess whether modern knee designs have provided similar results in patients younger than 70 years versus older patients.
Methods:
We included 203 consecutive patients (236 knees) who underwent knee replacement for osteoarthritis with a mean follow-up of 11.4 years (range: 8.8 to 12). The mean age was 70 years (range: 31 to 85). Knee replacements were stratified into two groups: 109 were younger than 70 years and 127 were older than 70 years (70 years of age is the mandatory retirement age).
Results:
There were no significant pre-operative differences between groups with regards to knee alignment, alpha or beta angles, knee score or function score. Fourteen implants were radiographically loose at last follow up visit. Groups were matched in terms of demographic data. We found that patients older than 70 years had significantly better mean survivorship at 12 years. (97% vs. 88%; P=0.010). Patients under 70 years presented with a higher rate of polyethylene wear which was further associated with radiolucent lines in the femur and tibia as well as the presence of osteolysis. There was also an association between migration and presence of osteolysis.
Conclusions:
Patients over 70 years old undergoing cemented total knee replacement for osteoarthritis showed better implant survivorship versus patients under 70 years old.
Key words: Elderly patients, Survival analysis, TKR
Introduction
Total knee arthroplasty has proved to have long term clinical success (1-3). Classically, total knee replacement has been advocated in elderly patients with severe degenerative changes while cemented total knee arthroplasty has showed to have satisfactory results in both young and old patients. Most of the results in young patients are after TKA in patients with rheumatoid arthritis (4-6). Patients with inflammatory diseases have a lower level of activity than patients with osteoarthritis. On the other hand, it is important to emphasize higher expectations of the middle-aged patients.
Recently, the favourable long term results of knee replacement and the improved performance of modern designs have popularized its use in younger patients. Higher congruency has shown less range of motion while reduction of edge loading in high flex designs resulted in less to intermediate congruency as a new modification in design. Moreover, new designs are moving toward better function using new materials (crosslink PE) for having less wear (7). This improvement in function allows more active patients to resume their previous level of activity. However, the increased demands of younger patients may generate greater stresses on their implants. A higher rate of polyethylene wear will compromise implant survivorship.
The main purpose of our study was to determine whether there was any difference in survivorship between patients under and over seventy years of age with osteoarthritis. We will then assess the rate of polyethylene wear and the presence of osteolysis in both groups to look for an explanation for different prosthetic survival.
Methods
We prospectively studied 236 consecutive cemented knee prosthesis implanted in 203 patients (37 men and 166 women). The study period was from January 1996 to December 2000. All patients were having grade 4 osteoarthritis based on Kellgren-Lawrence classification (8). The mean age at the time of surgery was 70.3 years (31-35). Patients were stratified by age into two groups: under 70 years old (109 patients) and over 70 years old (127 patients) based on the consideration that 70 years is the mandatory age of retirement in our country. We only included patients with idiopathic degenerative osteoarthritis and excluded the other diagnoses such as posttraumatic osteoarthritis and rheumatoid arthritis.
All knees were replaced using NexGen total knee arthroplasty system (Zimmer, Warsaw, IN). All were fixed bearing with normal designs. In patients < 70 years, 41 cruciate retained (CR) and 68 posterior cruciate ligament sacrificed (PS) were implanted while in patients >70 years, 43 CR and 84 PS were implanted. Indication for the prosthesis type was decided intraoperatively by the surgeon performing the procedure. The criteria for retaining PCL were the grade of deformity and the state of the ligament. A cemented femoral component and a cemented modular tibial implant were used in every case. All patients underwent patella resurfacing as well. There were 127 right and 109 left knees. Thirty three patients underwent bilateral knee replacement. Bilateral surgeries were not performed simultaneously. Both groups were comparable regarding preoperative varus and valgus alignment. The mean body mass index was 33 Kg/m2 (20 to 49).
Surgeries were performed under spinal anesthesia with a thigh tourniquet and an anteromedial parapatellar approach. Surgeries were performed by 6 surgeons (two are the authors of this article). Thrombo-prophylaxis was done using low weight heparin for 4 weeks. Every patient was given perioperative antibiotics with a second generation cephalosporin (or vancomycin if allergic) for 48 hours, until suction drains were removed.
Rehabilitation protocol was identical in both groups: On day 1, rehabilitation began almost immediately the day after surgery. Within the first 24 hours, patients began standing and walking using an assistive device with the help of a physical therapist (PT). Patients were shown to get in and out of the bed and move around with the aid of an assistive device such as a walker or crutches. Knee movement was encouraged with the goal of preventing the build-up of scar tissue and stiffness that can result from being immobile. On day 2, PT increased patients' activity level. On day 3, patients started longer distance walk in the ward, climbing up and down a flight of stairs, and move onto a chair or a toilet without assistance. At discharge, patients were able to get a minimum of 90-degree knee flexion.
Clinical evaluation was performed pre-operatively, and at 1, 3, and 6 months and annually thereafter. Clinical evaluation was performed with the Knee Society Knee Score and function was assessed using the Knee Society Function Score (9).
Radiological assessment included standing AP and lateral radiographs of the prostheses. All the radiographs were perpendicular to the cement-bone and cement-prosthesis interfaces. The exposures were aligned under fluoroscopic control. Serial radiographs at one year interval were analyzed by a single examiner who was blinded to the clinical outcome and had not been involved in the surgical procedure. Evaluation of the x-rays was performed according to the Knee Society rating system (10).
Alpha angle (α) is the medial angle between a line drawn parallel with the femoral component condyles and the anatomical axis of the femur. Beta angle (β) is the medial angle between a line drawn parallel to the tibial component on the anteroposterior (AP) X-ray and the anatomical axis of the tibia (12, 13).
HKA (hip-knee-ankle) angle: The mechanical axis of the femur (FM) is located as a line from the center of the femoral head running distally to the mid-condylar point between the cruciate ligaments (13). The mechanical axis (TM) of tibia is a line from the center of the tibial plateau (interspinous intercruciate midpoint) extending distally to the center of the tibial plafond (14). The angle between these 2 axes is the hip-knee-ankle (HKA) angle (15). In the neutrally aligned limb the HKA angle approaches 180°. At this point FM and TM are colinear, pass through the knee center, and are coincident with the load-bearing axis, which is the line of ground reaction force passing from the ankle to the hip (13). In varus knee, the center is lateral to the load-bearing axis, whereas in valgus knee, the center is displaced medially (16). As a convention, the HKA angle may be expressed as its angular deviation from 180° (i.e., HKA = 0° in neutral alignment). Varus deviations are negative and valgus deviations are positive. Our choice of varus as a negative value and valgus as positive was based on the general observations of a more serious problem of loading and damage within the varus knee.
Polyethylene wear was manually measured as a progression in the difference of the knee space seen in the anteroposterior view. Magnification was adjusted taking all radiographs at 1.1 meters distance. Radiolucent lines were assessed in all postoperative films. Progression of a lucent line was recorded when there was an increase in the width higher than 1 mm or when the line expanded to a neighbouring zone. Osteolysis was defined as expansive radiolucent lesions without trabecular markings and with scalloped margins (17,18). There were no differences between two groups in terms of Preoperative features [Tables 1; 2].
Table 1.
Preoperative details of both groups of patients
| Patients under 70 years | Patients over 70 years | P value | |
|---|---|---|---|
| Number of knees | 109 | 127 | |
| Age at time of surgery | 64.6 y | 75.2 y | |
| BMI | 33.4 Kg/m2 | 32.1 Kg/m2 | P=0.7 |
| PCL status | 41 CR 68PS | 43CR 84PS | P=0.58 |
| Preoperative beta angle | 88.10° | 88.29° | P=0.88 |
Table 2.
Postoperative details of both groups of patients
| Patients under 70 years | Patients over 70 years | P value | |
|---|---|---|---|
| Number of deaths | 1 | 7 | |
| Number of lost f-u | 10 | 11 | |
| Postoperative alignment | 187.08° | 187.14 | P=0.88 |
| Postoperative alpha angle | 97.72 | 97.69 | P=0.93 |
| Absence of primary contact bone-prostheses | 20 knees | 37 knees | P=0.37 |
| Mean (SD) | Mean(SD) | P value | |
| Median KS pain score | 42.62 (13.7) | 45 (11.25) | 0.19 |
| Median KS knee score | 80.6 (14.1) | 82.9(11.0) | 0.21 |
| Median KS knee function | 80.5 (21.8) | 83.3(18.8) | 0.36 |
| Flexion Deformity after surgery | 1.67° (3.62°) | 1.94° (4.55°) | 0.65 (t- test) |
| Range of Movement after surgery | 95.36° (12.1) | 97.5° (12) | 0.23 (t-test) |
| Radiolucent lines (number of knees) | 71 | 78 | 0.59 (Chi-Square ) |
| Polyethylene wear (number of knees) | 24 | 15 | 0.04 (Fisher's) |
| Osteolysis (number of knees) | 12 | 7 | 0.15 (Fisher's) |
| Aseptic Loosening (number of knees) | 11 | 3 | 0.024 (Fisher's) |
| Revisions | 7 | 6 | 0.46 (Fisher's) |
| Survivorship | 88.13% | 97.49% | P=0.0103 |
Survival analysis
Survival analysis was stratified by age (<70 vs >70 years) and the log-rank test was used to assess whether there was a significant difference between survival estimates stratified by this variable.
Time to failure was analysed using Kaplan-Meier survival curves. Aseptic loosening or revision of any component were considered the end points and allowed calculation of asymmetrical 95% confidence intervals (CI). Cases were censored at their last clinical and radiographic evaluation or until the date of death if the implant was still in place at that time.
Results
The mean follow-up in the group <70 years was 10.5 (range 8.9 to 12); in the group >70 years, the mean follow-up was 10.3 (range 8.7 to 12). During the study period, 9 patients died for unrelated causes and 21 were lost to follow-up (7 in group < 70, 11 in group > 70, equally distributed between both groups). According to age differences, both groups of patients were comparable. In the preoperative clinical evaluation, there were not statistically significant differences in posterior cruciate ligament status (P=0.58) or body mass index (P=0.70). Pain score improved in a similar way in both groups (P=0.19). At final follow-up, there were no significant differences between both groups in terms of improvement in the knee score (P=0.21) or the knee function score (P=0.36). Postoperative flexion deformity (P=0.65) and range of motion (P=0.23) were also similar between both groups [Tables 1; 2].
Radiographically, we did not find significant differences in the preoperative tibiofemoral angle (P=0.37). After surgery, we did not find any significant differences in component alignment (P=0.88), in alpha angle (P=0.93), beta angle (P=0.49) and bone-prosthesis contact (P=0.37) between both groups.
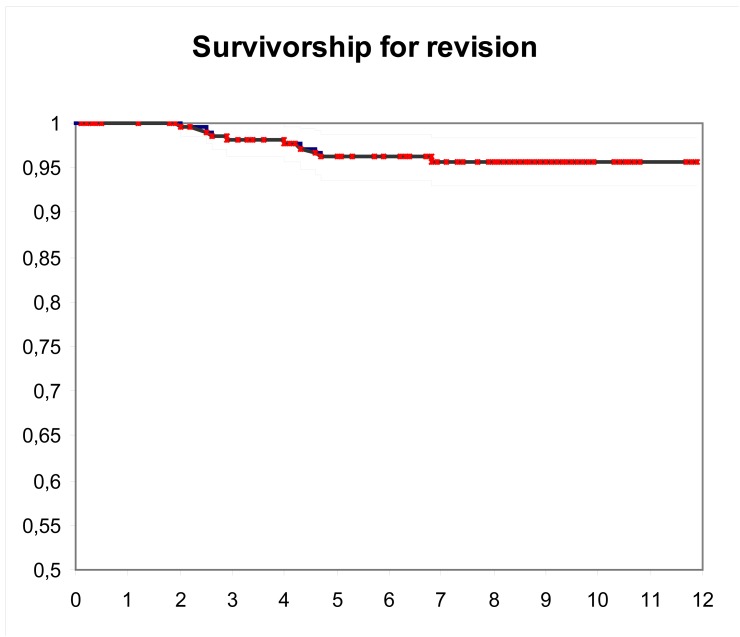
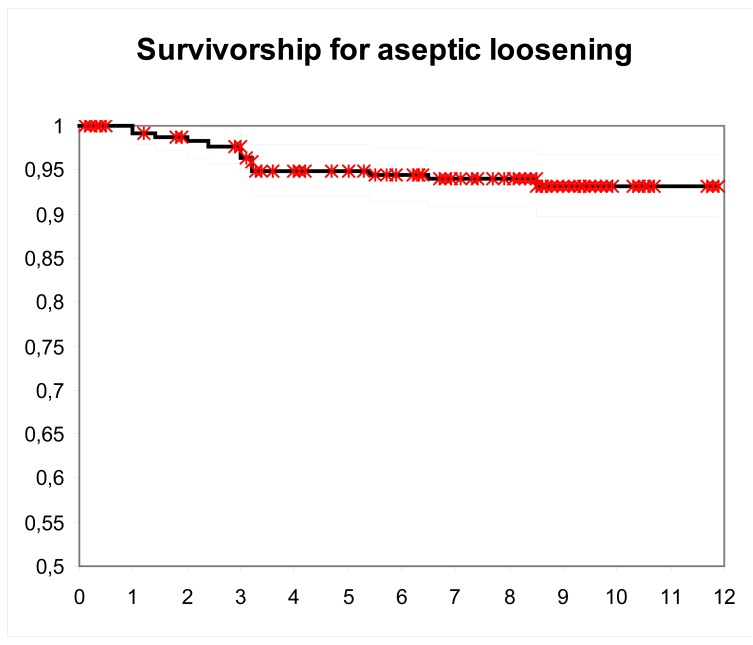
Survival of prostheses considering revision for any cause but infection as an end point was 95% (95% CI: 92 to 98) at 11.4 years [Figure 1]. Survival of prostheses at ten years with aseptic loosening as an endpoint was 93% (95% CI: 90 to 97) [Figure 2]. After stratifying by age, patients older than 70 years significantly had better mean survival at 12 years. (97% vs. 88%, P=0.010).
Figure 1.
Kaplan-Meier survival using revision for any cause but infection as the end point; blackline, the 11.4 year survival estimate (94.77%), grey lines, 95%CI (91.83 to 97.71).
Figure 2.
Kaplan-Meier survival using aseptic loosening as endpoint ; blackline, the 11.4 year survival estimate (93.24%), grey lines, 95% CI (89.8 to 96.68).
We found significant difference in the rate of polyethylene wear between both groups. Polyethylene wear was observed in 33 knees (14%). The rate of polyethylene wear was significantly higher in patients under 70 (22%) than in patients over 70 years (12%) (P=0.04). Polyethylene wear was more common in the medial joint space (26 knees). Only 6 knees showed polyethylene wear greater than 1 mm. We could not find any association between joint alignment and the presence of polyethylene wear (P=0.97). We could neither find any significant association between wear and the thickness of the polyethylene insert (P=0.85). The presence of polyethylene wear was associated with a higher incidence of radiolucent lines in the femoral and tibial components (P=0.02). Radiolucent lines were present in 102 femoral components and in 113 tibial components (affecting more than two zones in only 28 femoral components and 26 tibial components). The majority of the lucent lines appeared during the first year after surgery and were non progressive.
We found areas of osteolysis in 19 knees (8%) at a mean of 3 years. There was a significant association between polyethylene wear and the presence of osteolysis (P= 0.013). In 9 knees, osteolysis affected only one zone and was not progressive. Osteolysis was more common on the femoral side (14 knees), and the most common site of osteolysis was the anterior aspect of the femur (areas 1 and 2). There was also a significant association between the presence of osteolysis and migration of the components (P< 0.001).
Fourteen implants (5.9%) were radiographically loose at a mean of 7.7 years after surgery (range 1 to 12 years): eight out of 14 required revision for symptomatic aseptic loosening, four patients with radiographic signs of loosening were asymptomatic and declined further surgery, one patient was deceased, and one patient is awaiting for the revision surgery. Among the revised implants, both components were revised in two knees, femur component was revised in five knees, and tibial component was revised in one knee. Eleven knees required revision: 2 patients underwent removal of well-fixed implants due to infection, and 8 knees required revision for aseptic loosening. One knee was revised for instability.
The overall infection rate in our series was 0.84% (2 out of 236 knees). The diagnosis of infection was confirmed by culture, laboratory, and clinical data. The infecting micro organism was a Staphylococcus aureus in both knees. Both infections were successfully treated with two-stage revision surgery.
Discussion
This study presents the clinical and radiographic results of a consecutive series of 236 knees with a contemporary knee design. We tried to determine whether modern knee designs provide similar results in all age groups. One of the limitations of this study was that all data were collected prospectively but were analysed retrospectively.
Our results are consistent with previous reports using modern knee designs (1-3, 19, 20). Similar clinical reports of the cemented NexGen implants have also shown excellent clinical results. Bertin reported a survivorship of 100% at seven years (21). In the Swedish registry, cemented cruciate retaining total knee replacements with this design showed 97% survival at eight years (22).
Patient's age may influence the survivorship of any arthroplasty. Some series of cemented knee replacements in young patients with rheumatoid arthritis have shown excellent survival rates with long follow-ups (4-6). However, these good results may be influenced by the reduced level of activity in patients with inflammatory arthritis. Generally, young patients have higher physical demands and a longer life expectancy generating more mechanical stresses over the implants. Modern designs with better clinical performance, more congruent articular geometry, larger contact areas, and less stress on the polyethylene might reduce the rate of polyethylene wear. More congruency has not shown less but different wear. On the other hand, the influence of better materials is questionable. We have found worse arthroplasty survivorship in patients under 70 years old than in older patients. This has been also found in other studies (2, 20, 23-28). In our study, patients over 70 years presented with significantly lower rate of polyethylene wear and a better survival of the prostheses.
Another study showed that the best results with 99% survival after ten years were achieved in non-obese women with osteoarthritis who were over 60 years of age. The worst results were in obese men with osteoarthritis who were less than 60 years of age in whom 36% had a ten-year survival (29). Roberts et al found that survival was significantly increased in women and older patients (30). The 5-year survival rates reported by Julin et al were 92% and 95% in patients aged ≤ 55 and 56-65 years, respectively, compared to 97% in patients who were > 65 years of age (31). Young age impaired the prognosis of TKR and was associated with increased revision rates for non-infectious reasons. Keenan et al reported the ten-year survival of a cemented total knee replacement (TKR) in patients aged < 55 years at the time of surgery, and compare the functional outcome with patients aged > 55 years (32). The ten-year survival using revision as the endpoint was 98% (95% confidence interval: 95 to 99). Based on the Oxford knee scores at five and ten years, the rate of dissatisfaction was 18% and 21%, respectively. This was no worse in patients aged < 55 years than in patients aged > 55 years. These results demonstrated that the cemented PFC Sigma knee has an excellent survival rate in patients aged < 55 after ten years post-operatively with clinical outcomes similar to those of an older group. Keenan et al concluded that TKR should not be withheld from patients on the basis of age.
Polyethylene failure is a major risk for the long-term survival of total knee replacement. To our knowledge, there is no standardized way of measuring polyethylene wear in total knee arthroplasty. In our study, wear was defined as a persistent asymmetry in joint space which was not present in previous radiographs. Wear was observed in 33 knees and it was more frequent in the medial compartment. We could not identify any additional radiographic sign which could be related to wear in this group of patients. Several studies have suggested that wear is influenced by the design and surface finish of the tibial base plate, patient's age, manufacturing technique of the insert, sterilization method of the polyethylene or by the orientation of the components (33-35). The only variable affecting wear in our study was the age of the patient. In a study comparing first and second generation total knee arthroplasties, patient age was the only variable correlated with wear-related failure in second generation prostheses (36). In our series, the rate of polyethylene wear was significantly higher in patients under 70.
We have found a high incidence of radiolucent lines in both the femoral and tibial components. Our radiographs were perpendicular to the prosthesis interface allowing detection of minor radiolucent lines less than 2mm (37). Other studies with the NexGen design have also shown a high incidence of radiolucent lines. Henricson et al found radiolucent lines in ten out of nineteen cemented NexGen modular tibial components (38). With this design, most of these lucent lines affecting the periphery of the tibial tray appear mostly during the first year after surgery and are non-progressive. These non-progressive peripheral radiolucent lines have no clinical relevance. When radiolucent lines extend around the stem or the central part of the implant, they are commonly associated with osteolysis, loosening, or migration of the component (39).
Previous reports with modular implants have shown an association between younger age and the presence of osteolysis (40). In our study, 19 knees had radiographic evidence of osteolysis, which were more frequent on the femoral side. Osteolysis is frequently underestimated, especially in the femoral side where it is not easily recognized. The anterior aspect of the femur was the area most frequently involved. When there is no primary contact between the femoral component and the anterior cortex of the femur, some cancellous bone is exposed and allows access of polyethylene particles which produce the osteolysis. In other designs with an extra posterior cut, osteolysis is more common in the posterior aspect of the femur for the same reason. This must be correlated with longer follow-up of the recently developed high-flexion designs.
In conclusion, we found that patients over 70 years old undergoing cemented total knee replacement for osteoarthritis showed better implant survivorship than patients under 70 years old. This is consistent with several published series (2, 20, 23-25). Patients under 70 years old showed higher rates of polyethylene wear which was associated with the presence of radiolucent lines, osteolysis, and loosening of the components. In second generation knee prostheses, patient's age seems to be the only variable correlated with wear-related failure (36).
Acknowledgments
The Authors wish to thank Dr. Elia Perez from the Department of Statistics, La Paz University Hospital for her help with the statistical analysis.
References
- 1. Pavone V, Boettner F, Fickert S, Sculco TP. Total condylar knee arthroplasty a long term follow-up. Clin Orthop Relat Res. 2001;388:18–25. doi: 10.1097/00003086-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 2. Rand JA, Ilstrup DM. Survivorship analysis of total knee arthroplasty: cumulative rates of survival of 9200 total knee arthroplasties. J Bone Joint Surg (Am) 1991;73A:397–409. [PubMed] [Google Scholar]
- 3. Ritter MA. The Anatomical Graduated Component total knee replacement. A long-term evaluation with 20-year survival analysis. J Bone Joint Surg (Br) 2009;91:745–9. doi: 10.1302/0301-620X.91B6.21854. [DOI] [PubMed] [Google Scholar]
- 4. Duffy GP, Trousdale RT, Stuart MJ. Total knee arthroplasty in patients 55 years old or younger. Clin Orthop Relat Res. 1998;356:22–7. doi: 10.1097/00003086-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 5. Ranawat CS, Padgett DE, Ohashi Y. Total knee arthroplasty for patients younger than 55 years. Clin Orthop Relat Res. 1989;248:27–33. [PubMed] [Google Scholar]
- 6. Stuart MJ, Rand JA. Total knee arthroplasty in young adults who have rheumatoid arthritis. J Bone Joint Surg (Am) 1988;70:84–7. [PubMed] [Google Scholar]
- 7. Bertin KC, Komistek RD, Dennis DA, Hoff WA, Anderson DT, Langer T. In vivo determination of posterior femoral roll back for subjects having a NexGen posterior cruciate-retaining total knee arthroplasty. J Arthroplasty. 2002;17:1040–8. doi: 10.1054/arth.2002.35793. [DOI] [PubMed] [Google Scholar]
- 8. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494– 502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Insall JN, Dorr LD, Scott RD, Scott WN. Rationales of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–4. [PubMed] [Google Scholar]
- 10. Ewald FC. The knee society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 11. Kilincoglu V, Unay K, Akan K, Esenkaya I, Poyanli O. Component alignment in simultaneous bilateral or unilateral total knee arthroplasty. Int Orthop. 2011;35:43–6. doi: 10.1007/s00264-010-0993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCaskie AW, Deehan DJ, Green TP, Lock KR, Thompson JR, Harper WM, et al. Randomised, prospective study comparing cemented and cementless total knee replacement. Results of pressfit condylar knee replacement at five years. J Bone Joint Surg Br. 1998;80:971–5. doi: 10.1302/0301-620x.80b6.8558. [DOI] [PubMed] [Google Scholar]
- 13. Yoshioka Y, Siu D, Cooke TD. The anatomy and functional axes of the femur. J Bone Joint Surg Am. 1987;69:873–80. [PubMed] [Google Scholar]
- 14. Yoshioka Y, Siu DW, Scudamore RA, Cooke TD. Tibial anatomy and functional axes. J Orthop Res. 1989;7:132–7. doi: 10.1002/jor.1100070118. [DOI] [PubMed] [Google Scholar]
- 15. Cooke TDV, Scudamore A. Healthy knee alignment and mechanics. In: Callaghan JJ, Rosenberg AG, Rubash HE, et al., editors. The adult knee. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 175–86. [Google Scholar]
- 16.Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–77. [PubMed] [Google Scholar]
- 17. Claus AM, Engh CA , Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvis osteolysis following total hip arthroplasty. J Bone Joint Surg (Am) 2003;85:1519–26. doi: 10.2106/00004623-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 18. Freeman MA. Radiolucent lines: A question of nomenclature. J Arthroplasty. 1999;14:1–2. doi: 10.1016/s0883-5403(99)90195-x. [DOI] [PubMed] [Google Scholar]
- 19. Dalury DF, Barrett WP, Mason JB, Goldstein WM, Murphy JA, Roche MW. Midterm survival of a contemporary modular total knee replacement. A multicentre study of 1970 knees. J Bone Joint Surg (Br) 2008;90:1594–6. doi: 10.1302/0301-620X.90B12.21064. [DOI] [PubMed] [Google Scholar]
- 20. Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone and Joint Surg (Am) 2003;85:259–65. doi: 10.2106/00004623-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 21. Bertin KC. Tibial component fixation in total knee arthroplasty: a comparison of pegged and stemmed designs. J Arthroplasty. 2001;16:670–8. doi: 10.1016/j.arth.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22. Sundberg M, Lidgren L, Dahl AW, Robertsson O. Swedish Knee Arthroplasty Register. Annual Report. 2013. Available at: www.myKnee.se/pdf/SKAR2013_Eng.pdf .
- 23. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Early failures among 7174 primary total knee replacements a follow-up study from the Norwegian Arthroplasty Register 1994-2000. Acta Orthop Scan. 2002;73:117–29. doi: 10.1080/000164702753671678. [DOI] [PubMed] [Google Scholar]
- 24. Harryson LO, Robertsson O, Nayfeh JF. Higher cumulative revision rate of the knee arthroplasties in younger patients with osteoarthritis. Clin Orthop Relat Res. 2004;421:162–8. doi: 10.1097/01.blo.0000127115.05754.ce. [DOI] [PubMed] [Google Scholar]
- 25. Robertson O, Knutson K, Lewold S, Lidgren L. The Swedish Knee Arthroplasty Register 1975- 1997: an update with special emphasis on 41223 knees operated in 1988-1997. Acta Orthop Scand. 2001;72:503–13. doi: 10.1080/000164701753532853. [DOI] [PubMed] [Google Scholar]
- 26. Vazquez-Vela Johnson G, Worland RL, Keenan J, Norambuena N. Patient demographics as a predictor of the ten-year survival rate in primary total knee replacement. J Bone Joint Surg (Br) 2003;85:52–6. doi: 10.1302/0301-620x.85b1.12992. [DOI] [PubMed] [Google Scholar]
- 27. Roberts VI, Esler CN, Harper WM. A 15-year followup study of 4606 primary total knee replacements. J Bone Joint Surg (Br) 2007;89:1452–6. doi: 10.1302/0301-620X.89B11.19783. [DOI] [PubMed] [Google Scholar]
- 28. Julin J, J msen E, Puolakka T, Konttinen YT, Moilanen T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis. A follow-up study of 32,019 total knee replacements in the Finnish Arthroplasty Register. Acta Orthop. 2010;81:413–9. doi: 10.3109/17453674.2010.501747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keenan AC, Wood AM, Arthur CA, Jenkins PJ, Brenkel IJ, Walmsley PJ. Ten-year survival of cemented total knee replacement in patients aged less than 55 years. J Bone Joint Surg Br. 2012;94(7):928–31. doi: 10.1302/0301-620X.94B7.27031. [DOI] [PubMed] [Google Scholar]
- 30. Collier MB, Engh CA, McAuley JP, Ginn SD, Engh GA. Osteolysis after total knee arthroplasty: Influence of tibial baseplate surface finish and sterilization of polyethylene insert. J Bone Joint Surg (Am) 2005;87:2702–8. doi: 10.2106/JBJS.E.00074. [DOI] [PubMed] [Google Scholar]
- 31. Lombardi AV, Ellison BS, Berend KR. Polyethlylene wear is influenced by manufacturing technique in modular TKA. Clin Orthop Relat Res. 2008;466:2798– 805. doi: 10.1007/s11999-008-0470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reay E, Wu J, Holland J, Deehan D. Premature failure of the Kinemax plus total knee replacements. J Bone Joint Surg (Br) 2009;91:604–11. doi: 10.1302/0301-620X.91B5.21525. [DOI] [PubMed] [Google Scholar]
- 33. Griffin WL, Fehring TK, Pomeroy DL, Grune TA, Murphy JA. Sterilization and wear related failure in first and second generation press-fit condylar total knee arthroplasty. Clin Orthop Relat Res. 2007;464:16–20. [PubMed] [Google Scholar]
- 34. Vyskocil P, Gerber C, Bamert P. Radiolucent lines and component stability in knee arthroplasty standard versus fluoroscopically-assisted radiographs. J Bone Joint Surg (Br) 1999;81:24–6. doi: 10.1302/0301-620x.81b1.9213. [DOI] [PubMed] [Google Scholar]
- 35. Henricson A, Linder L, Nilsson KG. A trabecular metal tibial component in total knee replacement in patients younger than 60 years. J Bone Joint Surg (Br) 2008;90:1585–93. doi: 10.1302/0301-620X.90B12.20797. [DOI] [PubMed] [Google Scholar]
- 36. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system: Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg (Br) 1998;80:850–8. doi: 10.1302/0301-620x.80b5.8368. [DOI] [PubMed] [Google Scholar]
- 37. Lachiewicz PF, Soileau ES. Fifteen-year survival and osteolysis associated with a modular posterior stabilized knee replacement. A concise follow-up of a previous report. J Bone Joint Surg (Am) 2009;91:1419–23. doi: 10.2106/JBJS.H.01351. [DOI] [PubMed] [Google Scholar]
- 38. Huang CH, Ma HM, Liau JJ, Ho FY, Cheng CK. Osteolysis in failed total knee arthroplasty: A comparison of mobile-bearing and fixed-bearing knees. J Bone Joint Surg (Am) 2002;84:2224–9. [PubMed] [Google Scholar]
- 39. Kim YH, Oh JH, Oh SH. Osteolysis around cementless porous coated anatomic knee prostheses. J Bone Joint Surg (Br) 1995;77:236–41. [PubMed] [Google Scholar]
- 40. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Why are total knee arthoplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]