Abstract
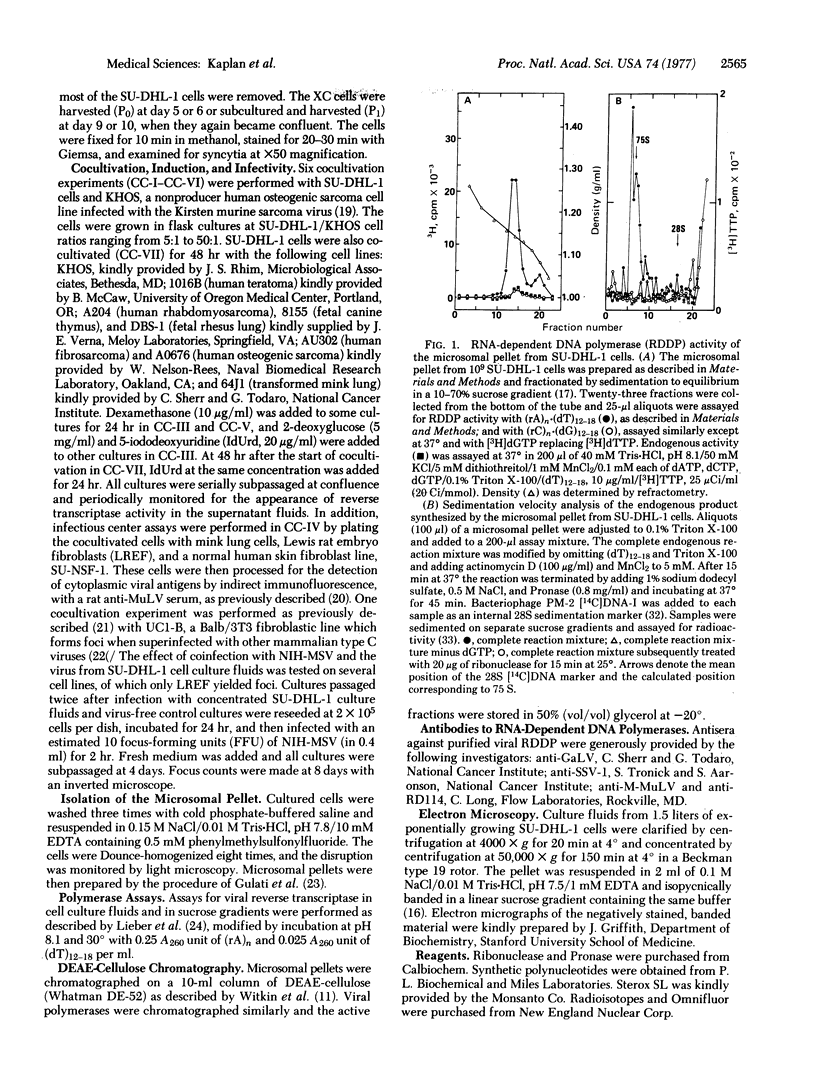
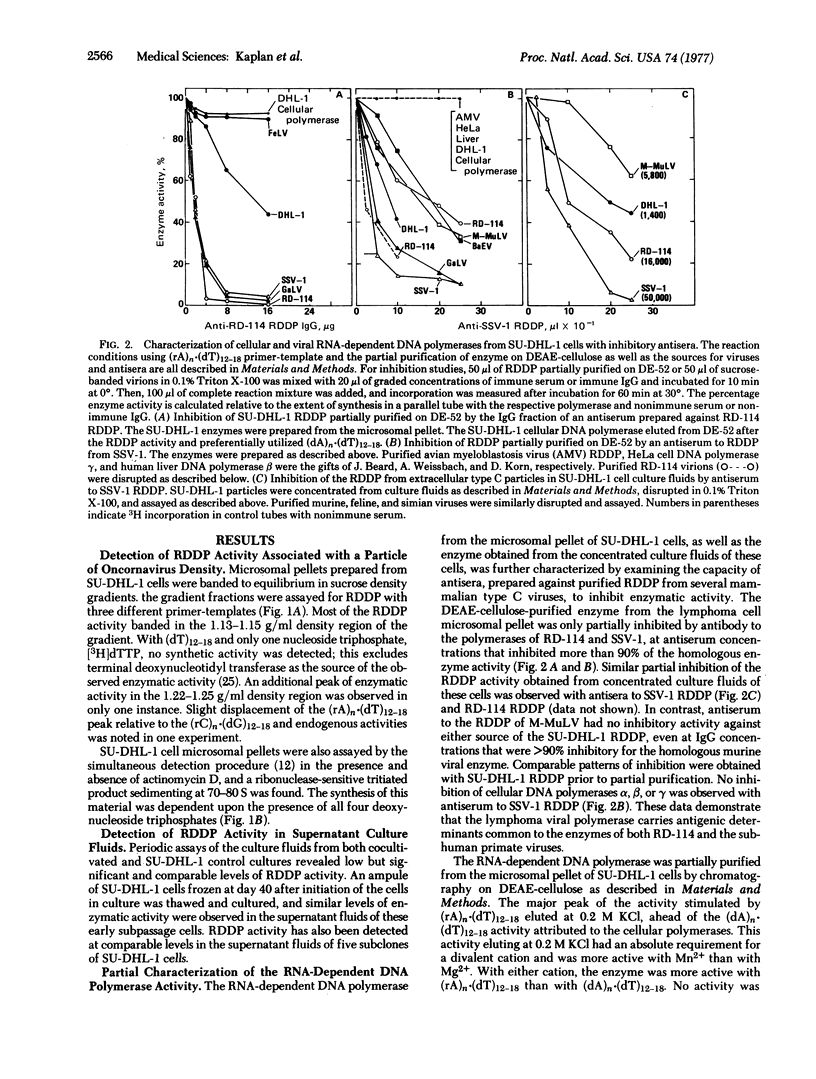
A type C RNA virus has been detected in the culture fluids of the SU-DHL-1 human histiocytic lymphoma cell line previously established in this laboratory. In electron micrographs, the virus closely resembled other typical mammalian type C RNA tumor viruses in size and morphology. Viral RNA-dependent DNA polymerase activity has been demonstrated in particles (densities of 1.15 and 1.22 g/ml) in the microsomal cytoplasmic fraction and in pellets of culture fluids. The enzyme is partially inhibited by antibodies to the RNA-dependent DNA polymerases of simian sarcoma virus and RD-114 virus but not by antibody to the polymerase of murine leukemia virus, suggesting some degree of relatedness to type C viruses of subhuman primate origin. Typical syncytial microplaques were induced when SU-DHL-1 cells were cocultivated with rat XC cells. Although no focus formation was noted in similarly cocultivated mouse UC1-B cell cultures, the numbers of foci induced in rat embryo fibroblasts by murine sarcoma virus were significantly increased by coinfection with the virus from SU-DHL-1 cell culture fluids. No other evidence of infectivity, inducibility, or capacity for helper rescue of defective murine sarcoma virus genomes has been detected to date in cocultivation studies with a spectrum of fibroblastic and other nonlymphoid indicator cell lines of human and other species of origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxt W., Hehlmann R., Spiegelman S. Human leukaemic cells contain reverse transcriptase associated with a high molecular weight virus-related RNA. Nat New Biol. 1972 Nov 15;240(98):72–75. doi: 10.1038/newbio240072a0. [DOI] [PubMed] [Google Scholar]
- Chan E., Peters W. P., Sweet R. W., Ohno T., Kufe D. W., Spiegelman S., Gallo R. C., Gallagher R. E. Characterisation of a virus (HL23V) isolated from cultured acute myelogenous leukaemic cells. Nature. 1976 Mar 18;260(5548):266–268. doi: 10.1038/260266a0. [DOI] [PubMed] [Google Scholar]
- Chezzi C., Dettori G., Manzari V., Aglianò A. M., Sanna A. Simultaneous detection of reverse transcriptase and high molecular weight RNA in tissue of patients with Hodgkin's disease and patients with leukemia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4649–4652. doi: 10.1073/pnas.73.12.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Kinetics of propagation of B-tropic murine leukemia virus on Fv-1b cell lines: requirement for multiple cycles of cell replication for transformation and viral antigen expression by RadLV. Virology. 1975 Feb;63(2):367–383. doi: 10.1016/0042-6822(75)90310-4. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Hilgers J., Kaplan H. S. An improved murine leukemia virus immunofluorescence assay. Virology. 1974 Feb;57(2):491–502. doi: 10.1016/0042-6822(74)90188-3. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., BARR Y. M. CULTIVATION IN VITRO OF HUMAN LYMPHOBLASTS FROM BURKITT'S MALIGNANT LYMPHOMA. Lancet. 1964 Feb 1;1(7327):252–253. doi: 10.1016/s0140-6736(64)92354-2. [DOI] [PubMed] [Google Scholar]
- Epstein A. L., Kaplan H. S. Biology of the human malignant lymphomas. I. Establishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer. 1974 Dec;34(6):1851–1872. doi: 10.1002/1097-0142(197412)34:6<1851::aid-cncr2820340602>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Gabelman N., Waxman S., Smith W., Douglas S. D. Appearance of C-type virus-like particles after co-cultivation of a human tumor-cell line with rat (XC) cells. Int J Cancer. 1975 Sep 15;16(3):355–369. doi: 10.1002/ijc.2910160302. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Type C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science. 1975 Jan 31;187(4174):350–353. doi: 10.1126/science.46123. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Todaro G. J., Smith R. G., Livingston D. M., Gallo R. C. Relationship between RNA-directed DNA polymerase (reverse transcriptase) from human acute leukemic blood cells and primate type-C viruses. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1309–1313. doi: 10.1073/pnas.71.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Miller N. R., Saxinger W. C., Gillespie D. Primate RNA tumor virus-like DNA synthesized endogenously by RNA-dependent DNA polymerase in virus-like particles from fresh human acute leukemic blood cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3219–3224. doi: 10.1073/pnas.70.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S. C., Axel R., Spiegelman S. Detection of RNA-instructed DNA polymerase and high molecular weight RNA in malignant tissue. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2020–2024. doi: 10.1073/pnas.69.8.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. J., Sylvester S. S. Cell line derived from Balb-3T3 that is transformed by murine leukaemia virus: a focus assay for leukaemia virus. Nat New Biol. 1972 Oct 11;239(93):164–166. doi: 10.1038/newbio239164a0. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. Leukemia and lymphoma in experimental and domestic animals. Ser Haematol. 1974;7(2):94–163. [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Liberman M., Kaplan H. S. Adaptation of plaque assay methods to the in vitro quantitation of the radiation leukemia virus. J Virol. 1973 Jul;12(1):68–73. doi: 10.1128/jvi.12.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooter K., Aarssen A. M., Bentvelzen P., De Groot F. G., Van Pelt F. G. Isolation of infectious C-type oncornavirus from human leukaemic bone marrow cells. Nature. 1975 Aug 14;256(5518):595–597. doi: 10.1038/256595a0. [DOI] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M., Stephenson J. R., Gallagher R. E., Aaronson S. A., Gallo R. C., Tronick S. R. Immunological and biochemical characterisation of type C viruses isolated from cultured human AML cells. Nature. 1976 Mar 18;260(5548):264–266. doi: 10.1038/260264a0. [DOI] [PubMed] [Google Scholar]
- Panem S., Prochownik E. V., Reale F. R., Kirsten W. H. Isolation of type C virions from a normal human fibroblast strain. Science. 1975 Jul 25;189(4199):297–299. doi: 10.1126/science.49927. [DOI] [PubMed] [Google Scholar]
- Rangan S. R. C-type oncogenic viruses of nonhuman primates. Lab Anim Sci. 1974 Feb;24(1):193–203. [PubMed] [Google Scholar]
- Rangan S. R., Moyer P. P., Cheong M. P., Jensen E. M. Detection and assay of feline leukemia virus (FeLV) by a mixed-culture cytopathogenicity method. Virology. 1972 Jan;47(1):247–250. doi: 10.1016/0042-6822(72)90258-9. [DOI] [PubMed] [Google Scholar]
- Rangan S. R., Wong M. C., Ueberhorst P. J., Ablashi D. V. Mixed culture cytopathogenicity induced by virus preparations derived from cultures infected by simian sarcoma virus. J Natl Cancer Inst. 1972 Aug;49(2):571–577. [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Vernon M. L., Arnstein P., Huebner R. J., Gilden R. V. Characterization of non-producer human cells induced by Kirsten sarcoma virus. Int J Cancer. 1975 Nov 15;16(5):840–849. doi: 10.1002/ijc.2910160516. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin P. S., Gallo R. C. Terminal deoxynucleotidyltransferase in chronic myelogenous leukemia. J Biol Chem. 1974 Dec 25;249(24):8051–8053. [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Salahuddin S. Z., Gallagher R. E., Gillespie D. H., Gallo R. C. Infective transmission and characterisation of a C-type virus released by cultured human myeloid leukaemia cells. Nature. 1975 Aug 14;256(5518):551–555. doi: 10.1038/256551a0. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Ohno T., Spiegelman S. Purification of RNA-instructed DNA polymerase from human leukemic spleens. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4133–4136. doi: 10.1073/pnas.72.10.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]