Abstract
Objective(s):
Recent evidence have proposed that Tretinoin produced in the gut preferentially promote differentiation of FoxP3+Treg cells but inhibits Th17 lymphocytes, and this may be the main immunomdulatory mechanism of Tretinoin in vivo. This study was done to investigate the effects of Tretinoin in outbred white mice after challenge with sheep red blood cells (SRBC).
Materials and Methods:
Twenty male NMRI-mice randomly allocated in two equal groups. Mice were treated with 1×109 SRBCs emulsified in CFA intraperitoneally twice with one weak interval. Animals were bled 5 days after last injection. Moreover, 48 hr before bleeding time, 1×109 SRBCs were injected into the left hind foot pad of mice. Tretinoin (25 mg/kg-every other day) were intraperitoneally injected into the treatment group from the beginning of the study and continued throughout the study. The levels of anti-SRBC antibody and the specific cellular immune responses were measured by microhemagglutination test and footpad thickness, respectively. Moreover, splenocytes were checked for proliferation rate, respiratory burst, cytokine production and FoxP3+Treg cells frequency.
Results:
Tretinoin markedly alleviated cellular immunity and concurrently potentiated humoral immunity after mice challenge with SRBCs. Furthermore, aside from reducing NBT reduction and lymphocyte proliferation, Tretinoin markedly suppressed the secretion of interleukin-17 and conversely, increased the production of interleukin-10. However, the level of IFN-γ and the frequency of FoxP3+Treg cells did not alter significantly.
Conclusion:
The in vivo immunomudlatoty effects of Tretinoin may be partly due to immune deviation from pro-inflammatory cytokine interleukin-17 to anti-inflammatory cytokine interleukin-10, but not absolutely depend on the expansion of FoxP3+Treg cells.
Keywords: Immunomodulator, NMRI-mice, Tretinoin
Introduction
Retinoids are a class of natural and synthetic derivatives of vitamin A which play important functions in embryogenesis, vision, growth and reproduction (1, 2). Tretinoin (all-trans retinoic acid) is an active metabolite of retinoids and modulate a wide range of biologic processes such as cell proliferation, differentiation and survival via specific family of nuclear receptors, the retinoic acid receptors (RARs), which function as ligand-inducible transcription factors (2-5).
The concept that vitamin A derivatives are important for immunity goes back to the early 20th century when Edward Mellanby and his colleague Harry Green reported on vitamin A as anti-infective agents (6). In this regard, Tretinoin has been shown to exert anti-neoplastic and immunomodulatory properties (3, 4). It has been clinically administrated for the treatment of cancers such as acute promyelocytic leukemia and Kaposi sarcoma and inflammatory diseases including acne and psoriasis (3). Previous reports showed that Tretinoin alleviated inflammatory responses and tissue destruction and attenuated a variety of autoimmune diseases in animal models, such as rheumatoid arthritis, type I diabetes, inflammatory bowel disease and experimental autoimmune encephalomyelitis (7-11).
Recent evidence indicated that immune responses are finely regulated by the opposing effects of Th-17 and FoxP3+Treg lymphocytes (12, 13). On the other hand, some new documents indicated that Tretinoin produced by CD103+ dendritic cells in the gastrointestinal lumen preferentially promotes generation of FoxP3+ Treg cells but concurrently inhibits generation of Th17 cells in an antigen specific manner, and this may be the main immunomdulatory mechanism of Tretinoin in vivo (5, 14).
The present study was done to investigate the effect of Tretinoin on T-helper cells responses and its effects on humoral and cellular immunity in mice after challenge with sheep red blood cells (SRBC).
Materials and Methods
Reagents
Tretinoin, Complete Freund's adjuvant (CFA), Nitro blue tetrazolium, dioxin, dimethyl sulfoxide (DMSO) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St.Louis, MO). RPMI 1640 and fetal calf serum were bought from GIBCO/Life Technologies Inc. (Gaithersburg, MD). The cytokine assay by enzyme-linked immunosorbent assay (ELISA) kits for interferon gamma (IFN-γ), interleukin (IL)-10 and IL-17 were procured from Bender MedSystems (Vienna, Austria). Mouse Regulatory T Cell Staining Kit was purchased from eBioscience (San Diego, CA).
Animals
Six to eight-week-old male NMRI mice were purchased from Pasteur Institute of Iran. The animals were housed for one week before the experiment, maintained under constant condition of temperature (22-24°C) and 12 hr light/dark cycle and received food and water ad libitum. Animal welfare was observed in compliance with the regulations of the Ministry of Health, IR Iran approved by the Medical Ethics Committee of the University for Animal Studies.
Experimental design, Immunological challenge and evaluation
Mice were randomly allocated into 2 groups: Control mice and Treatment group. Each group had 10 animals. Tretinoin (2.5 mg/ml) was suspended in 2% DMSO and stored at -80°C as aliquots before use. Tretinoin (25 mg/kg body weight, every other day) were intraperitoneally injected into the treatment group from the beginning of the study (onset of immunization) and continued throughout the study when the mice were bled. This dosage was chosen in accordance with pervious works on murine autoimmune models (8, 15-18). Normal control mice received an equal volume of PBS containing 2% DMSO with the same schedule of treatment group.
When the experiment began, animals were intraperitoneally immunized twice with one weak interval by 1×109 sheep red blood cells (SRBC) emulsified in CFA. Mice were bled from their hearts 5 days after last injection and the levels of anti-SRBC antibody were measured by microhemagglutination test as described previously (19).
Moreover, 48 hr before bleeding time, 1×109 SRBCs in 50 µl of PBS were administered subcutaneously into the left hind foot pad of each mouse and simultaneously the same volume of PBS was injected into the right foot pad as a negative control.
Footpad thickness was measured before bleeding time with a dial caliper and the mean percentage increase in footpad thickness was measured according to the following formula: [(Thickness of left footpad) _ (Thickness of right footpad] * 100/ (Thickness of right footpad).
Cytokines production
Spleen cells were aseptically isolated from mice at bleeding time. In brief, single-cell suspensions of splenocytes were prepared in RPMI 1640 medium supplemented with 10% fetal calf serum and red blood cells (RBCs) were removed by RBC lysis buffer. Next, cell suspensions (2×106 cells/ml) were incubated in 24-well plates and pulsed with 50 µl PHA solution (1 mg/ml). The culture supernatants were collected after 72 hr. IFN-γ, IL-17 and IL-10 production were assumed by ELISA according to the manufacturer's instructions.
Splenocytes proliferation
Proliferation potential of lymphocytes in splenocyte population was evaluated by MTT assay. The splenocytes were plated in 96-well flat-bottomed plates in RPMI 1640 medium supplemented with 10% fetal calf serum (1×105 cells/100 µl/well) and stimulated with 50 µl PHA solution (1 mg/ml) or medium alone. After 72 hr incubation, cultures were pulsed with 20 µl of the MTT solution (5 mg/ml) for 4 hr at 37°C. Then 150 ml DMSO was added and shaken vigorously to dissolve formazan crystal. The optical density (OD) at 550 nm was measured using microplate reader (Dynatech, Denkendorf, Germany). The experiments were done in triplicate sets. The results were expressed as the proliferation index according to the ratio of OD550 of stimulated cells with MOG35-55 to OD550 of non-stimulated cells.
Respiratory burst in splenocyte population
Respiratory burst of phagocytic cells in splenocyte population was checked using NBT dye reduction as described previously with some modification (20-22). In brief, 100 µl of suspension of splenocytes with 0.1 ml of Staphylococcus aureus suspension (108 cell/ml) and 0.1 ml of 0.1% NBT in PBS (pH=7.4) were mixed. The mixture was incubated at room temperature for 15 min and subsequently kept at 37°C for additional 15 min. The reduced dye was extracted in dioxan and quantitated at 520 nm.
Treg analysis by flow cytometry
Mouse Regulatory T Cell Staining Kit was used to check the FoxP3+CD4+CD25+ regulatory T (FoxP3+Treg) cells according to manufacturer's instructions. Briefly, 100 µl of freshly prepared splenocytes (1×106cells) were stained with anti-mouse CD4 and anti-mouse CD25 for 30 min at 4°C. After staining surface markers, cells were incubated with fixation/permeabilization working solution for 30 min at 4°C. Then, the cells were washed with permeablilization buffer and stained with anti-mouse Foxp3 antibody or isotype control at 40 for at least 30 min in dark. The cells were then washed with permeabilization buffer and suspended in flow cytometry staining buffer. All samples were analyzed on DAKO flow cytometer (Partec, Germany), using Cyflogic software (version: 1.2.1).
Statistical analysis
Data were analyzed by Student's_t-test and presented as means ± SD. P-values less than 0.05 were considered statistically significant.
Results
Footpad thickness after challenge with SRBC was performed as an indicator for evaluation of delayed type of hypersensitivity (DTH) reaction. As shown in Figure 1, Tretinoin-treated mice showed significantly lower DTH responses than the control mice. Conversely, mean antibody titer in treatment group (260±11.22) was significantly higher than the mean antibody titer in control mice (35.87±9.76) (P<0.001).
Figure 1.
Effects of administration of Tretinoin on cellular immunity. Mice were intraperitoneally immunized twice with one weak interval by 1×109 sheep red blood cells (SRBC) emulsified in CFA. 3 days after last intraperitoneal immunization, 1×109 SRBCs in 50 µl of PBS were administered subcutaneously into the left hind foot pad of each mouse and the same volume of PBS was injected into the right foot pad as a negative control.Footpad thickness was measured before bleeding time with a dial caliper and the mean percentage increase in footpad thickness was measured. The values were presented as mean ± S.D. (*P<0.001 versus control mice)
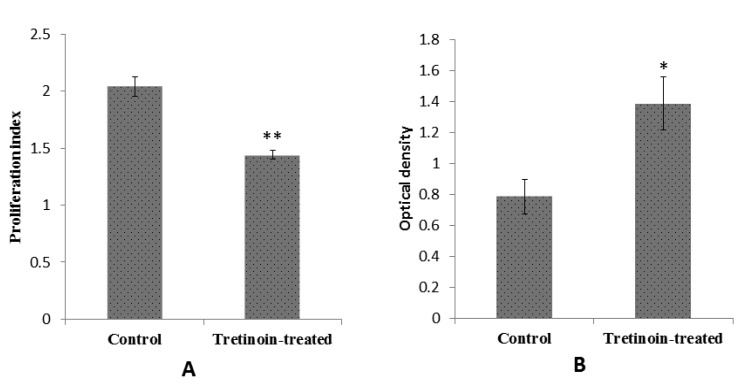
A significant decrease in IL-17 and a significant increase in IL-10 secretion in cells from Tretinoin-treated mice were found compared to cells from vehicle-treated group (Figure 2). However, the level of IFN-γ was diminished in treatment group but this reduction was not significant (Figure 2). Moreover, splenocyte proliferation and respiratory burst showed a significant reduction in Tretinoin-treated mice compared to the normal control animals (Figure 3).
Figure 2.
Cytokines production assay after treatment with Tretinoin. Spleen cells isolated from immunized mice with SRBC were cultured with 50 µl of PHA (1 mg/ml) for 72 hr. The levels of IFN-γ, IL-17 and IL-10 in culture supernatants were determined after 72 hr by ELISA. The results were shown as mean ± SD (*P<0.01, ** P<0.001 versus control mice)
Figure 3.
Effects of Tretinoin administration on lymphocytes proliferation and respiratory burst in phagocytic cells. Splenocytes were isolated from sensitized mice with SRBC. A) Splenocytes cultured with 50 µl PHA solution (1mg/ml) for 72 hr. Then, lymphocytes proliferation were evluated by MTT test. B) splenocytes with Staphylococcus aureus suspension and NBT were mixed and incubated for 30 min as detailed under materials and methods. The reduced dye was extracted in dioxan and quantitated at 520 nm. The values were presented as mean ± SD (*P<0.01, ** P<0.001 versus control mice)
Evaluation method of Foxp3+Treg cells is shown in Figure 4A, B, and C. No significant change in the expression of these cells was observed in the treatment group compared to the control mice (Figure 4D).
Figure 4.
Evaluation of Tretinoin administration on FoxP3+Treg cells frequency. At the bleeding time, spleens were removed from immunized mice with SRBC. A single-cell suspension was prepared, and the cells were stained as detailed under materials and methods. Representative dot plots illustrated the regions and gates for immune phenotypic analysis. Lymphocytes were gated on a forward vs. side scatter dot plot (A) and CD25+ T cells were gated by CD25/side scatter futures (B). Then, we analyzed CD4+ FoxP3+on CD25+ gated cells (C). (FL1, CD4+; FL2, CD25+; FL3, FoxP3+). Flow cytometry analysis demonstrated that. No significant change in the expression of CD4+CD25+FoxP3+ was observed in the treatment group compared to the control mice (D). Data were shown as mean±SD
Discussion
Recently, several lines of evidence showed that retinoic acid derivatives play an essential role in the differentiation of T cell subsets and the migration of T cells into tissues (6). T cell-mediated immunity has a substantial role in determining the extent of organ-specific autoimmune diseases (23-25). DTH is one of the typical response patterns of T cell-mediated immunity (25). The first requirement for DTH reaction is the priming of a special effector class of antigen specific T cells (26). DTH response has long been believed to be mediated by Th1 cells (27, 28). However, this concept was doubted because mice deficient in components of the IL-12/Th1 axis including IL-12α (IL-12p35), IFN-γ or IFN-γ receptor were more susceptible to autoimmunity. This deficiency in understanding of immunopathology was resolved by discovery of IL-17-producing CD4+ T cells (Th17) (23, 27, 29). IL-17 (also called IL-17A) has a potent pro-inflammatory property (30) and is a crucial factor for promotion of DTH induction (26). It seems that Th17 cells imitate the inflammatory response, while Th1 cells determine the tissue damage (28). Overall, it seems that Th17 cells are more pathogenic than Th1 cells in immunopathologic conditions (30).
After immunizations with CFA, killed mycro-bacteria produce the microenvironment for the Th17 polarization of the T cells that are specific for the antigen mixed into the adjuvant (20). Interestingly, immunizations with complete Freund's adjuvant (CFA) initiate mixed Th1 and Th17 immunity in the absence of Th2 immunity, while injection with alum or incomplete Freund's adjuvant (IFA) promote highly polarized Th2 responses (31).
Our results showed that treatment with Tretinoin after challenge with SRBC significantly suppressed IL-17 production and consequently DTH reaction. However, the level of IFN-γ did not show any significant differences between groups. These findings offer new insight into the potential mechanisms underlying the immumodulatory effects of Tretinoin.
FoxP3+Treg cells have an crucial role in maintaining of self-tolerance through their inhibitory properties on effector T lymphocytes especially during Th17 immune responses (32). In vitro studies have been demonstrated that Tretinoin can diminish Th17 differentiation and reciprocally promote differentiation of FoxP3+ Treg cells (3, 33, 34). Despite the in vitro studies, Tretinoin in our study did not have any significant effect on FoxP3+ Treg cell population. A previous study showed a significant reduction in Th17 cells generation without any significant change in Treg cells development in mice orally infected with Listeria monocytogenes and treated with Retinoic acid. Authors reported that TGF-β might be a limiting factor in the decline of Treg cell expression induced by exogenous Retinoic acid in vivo (35). Also, it has been showed that the high level of IL-6 could suppress de novo generation of antigen specific Treg cells (36). Of note, TGF-β is an essential factor for development of FoxP3+Treg, while TGF-β plus IL-6 promote generation of Th17 cells (12). Therefore, we propose that minor expansion of Treg cells following Tretinoin administration may be due to the profound production of inflammatory cytokines (such as IL-6) or conversely low level production of TGF-β in cytokinic milieu. However, these remain to be clarified.
IL-10 is a cytokine with anti-inflammatory properties and plays an important role in suppressing and terminating macrophage activity in DTH reactions and subsequent preventing of tissue destruction (37, 38). This cytokine was profoundly increased in Tretinoin treatment group.
Significant reduction in IL-17 production and conversely increase in the level of IL-10 could explain the decrease of the respiratory burst in mononuclear phagocytic cells in mice treated with Tretinoin.
In general, cellular and humoral arms of immunity are reciprocally regulated (25). Therefore, increasing the humoral immune response following reducing DTH reaction in the Tretinoin treatment group is not far-fetched. In this regard, pervious evidence showed that retinoic acid promotes antibody responses to T cell dependent antigens (6).
Altogether, immune deviation from cellular immunity to humoral responses concurrent with a significant decrease in lymphocyte proliferation may be explaining some beneficial effect that reported following Tretinoin therapy in some organ-specific autoimmune models.
Conclusion
The in vivo immunomudlatoty effects of Tretinoin may be partly due to immune deviation from pro-inflammatory cytokine IL-17 to anti-inflammatory cytokine IL-10, but not absolutely depend on the expansion of FoxP3+Treg lymphocytes. However, other mechanisms may also be involved, and these remain to be clarified.
Acknowledgment
This research has been supported by Urmia University, Urmia, Iran. The authors thank staffs of the Urmia University for their kindly help.
References
- 1.Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci. 2008;1143:170–187. doi: 10.1196/annals.1443.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Drew PD. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol . 2006; 171:135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemann C, Raveney BJ, Oki S, Yamamura T. Retinoid signals and Th17-mediated pathology. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:20–28. doi: 10.2177/jsci.32.20. [DOI] [PubMed] [Google Scholar]
- 4.Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, et al. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol . 2009; 174:2234–2245. doi: 10.2353/ajpath.2009.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol . 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr . 2012; 96:1166S–1172S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massacesi L, Abbamondi AL, Giorgi C, Sarlo F, Lolli F, Amaducci L. Suppression of experimental allergic encephalomyelitis by retinoic acid. J Neurol Sci . 1987;80:55–64. doi: 10.1016/0022-510x(87)90220-6. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clin Immunol. 2006; 119:272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular retinoic acid bioavailability determines epithelial integrity Role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol . 2007; 71:250–258. doi: 10.1124/mol.106.029579. [DOI] [PubMed] [Google Scholar]
- 10.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, et al. Retinoid treatment of experimental allergic encephalomyelitis IL-4 production correlates with improved disease course. J Immunol . 1995; 154:450–458. [PubMed] [Google Scholar]
- 11.Zunino SJ, Storms DH, Stephensen CB. Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in nonobese diabetic mice. J Nutr . 2007; 137:1216–1221. doi: 10.1093/jn/137.5.1216. [DOI] [PubMed] [Google Scholar]
- 12.Dong C. Mouse Th17 cells current understanding of their generation and regulation. Eur J Immunol. 2009; 39:640–644. doi: 10.1002/eji.200839076. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells CD4 T helper cells functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010; 159:137–147. doi: 10.1111/j.1365-2249.2009.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med . 2007; 204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eller P, Eller K, Wolf AM, Reinstadler SJ, Tagwerker A, Patsch JR, et al. Atorvastatin attenuates murine anti-glomerular basement membrane glomerulonephritis. Kidney Int . 2010; 77:428–435. doi: 10.1038/ki.2009.478. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita K, Yoo BS, Nozaki Y, Sugiyama M, Ikoma S, Ohno M, et al. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1 mice. J Immunol. 2003; 170:5793–5798. doi: 10.4049/jimmunol.170.11.5793. [DOI] [PubMed] [Google Scholar]
- 17.Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor atorvastatin promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature . 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 19.Hay FC, Westwood OMR. Practical Immunology. 4th ed. New Jersey: Wiley-Blackwell; 2002. [Google Scholar]
- 20.M ller J, Alf ldy P, Lemmel E-M. Nitroblue-tetrazolium test for the functional evaluation of phagocytic cells A critical analysis of the methodology. Agents Actions. 1981;11:384–390. doi: 10.1007/BF01982475. [DOI] [PubMed] [Google Scholar]
- 21.Hamaliaka A, Novikova I. Nitric oxide production disorders in leukocytes of patients with recurrent furunculosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub . 2010; 154:163–167. doi: 10.5507/bp.2010.025. [DOI] [PubMed] [Google Scholar]
- 22.Nabi AH, Islam LN, Rahman MM, Biswas KB. Polymorphonuclear neutrophil dysfunctions in streptozotocin-induced type 1 diabetic rats. J Biochem Mol Biol . 2005; 38:661–667. doi: 10.5483/bmbrep.2005.38.6.661. [DOI] [PubMed] [Google Scholar]
- 23.Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS) . Allergol Int . 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 24.Hofstetter H, Gold R, Hartung HP. Th17 Cells in MS and Experimental Autoimmune Encephalomyelitis. Int MS J . 2009;16:12–18. [PubMed] [Google Scholar]
- 25.Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech . 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 26.Kuerten S, Lehmann PV. The immune pathogenesis of experimental autoimmune encephalomyelitis lessons learned for multiple sclerosis? J Interferon Cytokine Res. 2011;31:907–916. doi: 10.1089/jir.2011.0072. [DOI] [PubMed] [Google Scholar]
- 27.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011;6:487–495. doi: 10.1007/s11739-011-0517-7. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010; 162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tigno-Aranjuez JT, Lehmann PV, Tary-Lehmann M. Dissociated induction of cytotoxicity and DTH by CFA and CpG. J Immunother. 2009; 32:389–398. doi: 10.1097/CJI.0b013e31819d79a7. [DOI] [PubMed] [Google Scholar]
- 32.Oukka M. Interplay between pathogenic Th17 and regulatory T cells. Ann Rheum Dis. 2007; 66:87–90. doi: 10.1136/ard.2007.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood . 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagami S, Owada T, Kanari H, Saito Y, Suto A, Ikeda K, et al. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol. 2009; 21:679–689. doi: 10.1093/intimm/dxp037. [DOI] [PubMed] [Google Scholar]
- 35.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science . 2007; 317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007; 13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 38.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol . 2010; 10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]