Abstract
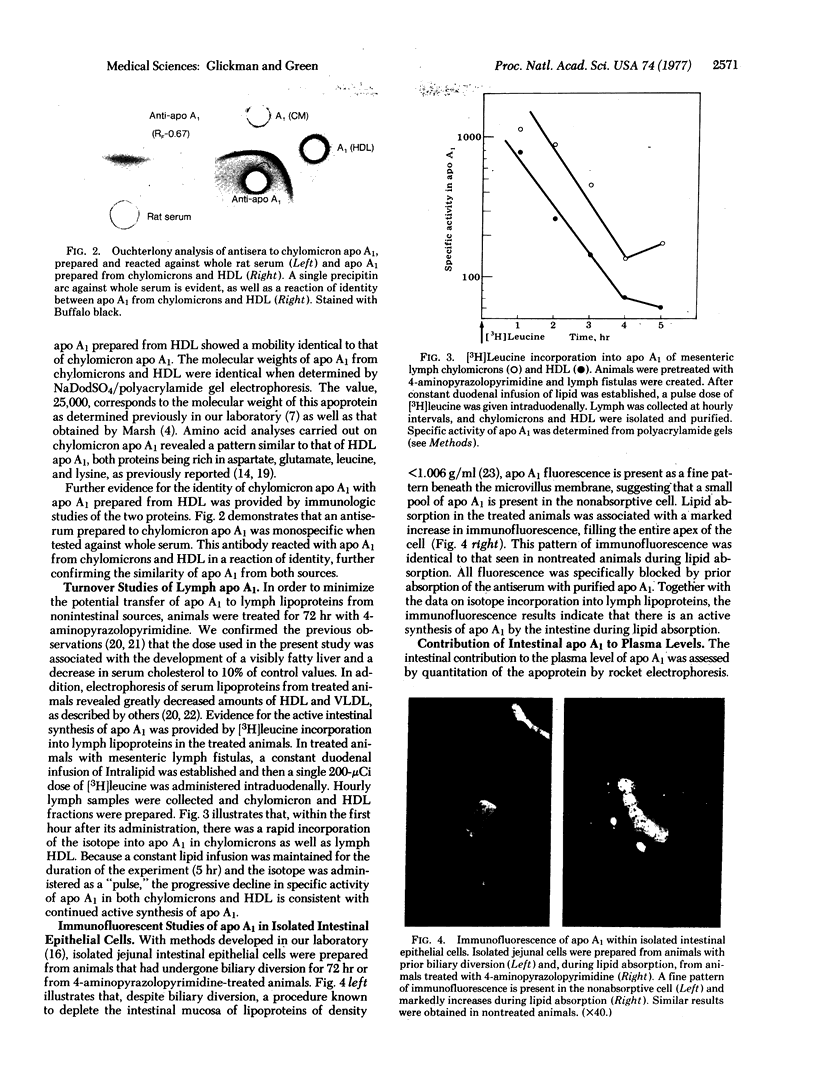
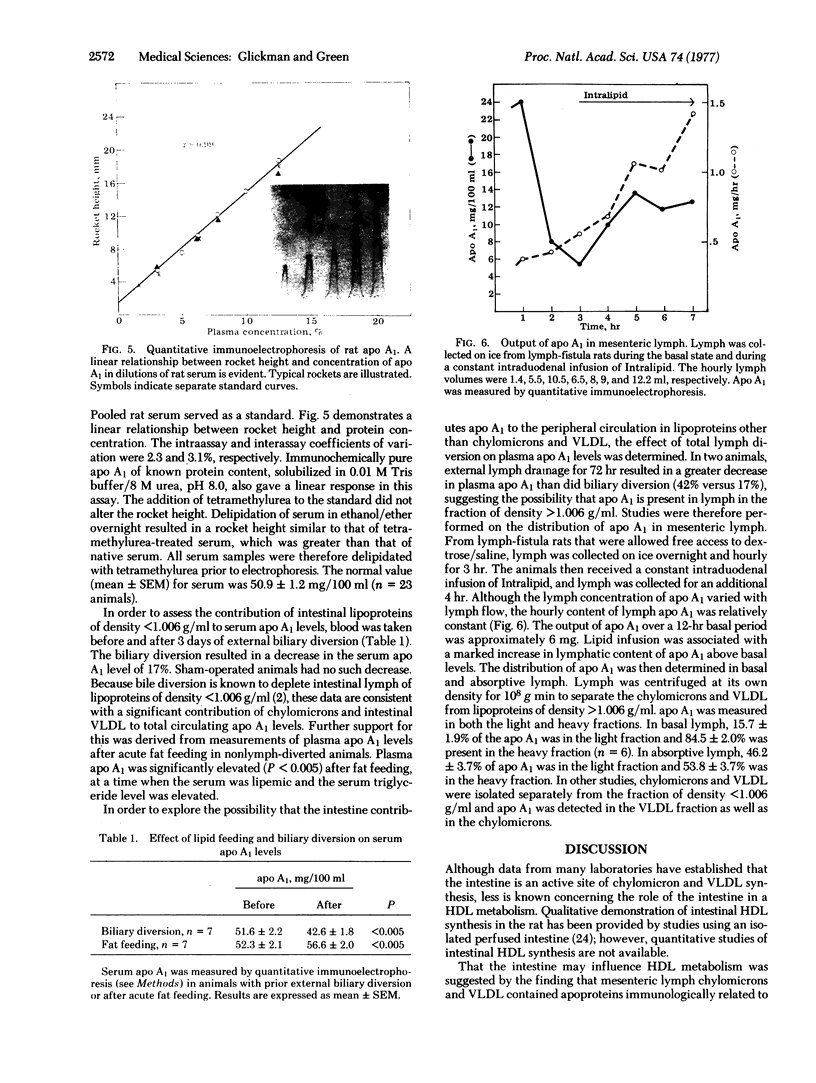
The major apoprotein of rat mesenteric lymph chylomicrons has been isolated and characterized and shown to be identical to apoprotein A1 (apo A1) isolated from serum high density lipoprotein (HDL). During intestinal lipid absorption, active synthesis of apo A1 was demonstrated by radioactive amino acid incorporation into lymph chylomicron A1 as well as lymph HDL. Immunofluorescence studies of intestinal epithelium demonstrated a marked increase in apo A1 fluorescence, confirming an active synthesis of this apoprotein during lipid absorption. Quantitative immunoelectrophoretic methods were used to measure apo A1 in lymph and peripheral blood during various conditions designed to estimate the quantitative importance of intestinal apo A1 to the levels of circulating lipoproteins. During lipid feeding there was an increase in lymph apo A1 that was associated with lymph lipoproteins (50%) of density less than 1.006 g/ml whereas in basal lymph most apo A1 (85%) was in the lipoproteins of density greater than 1.006 g/ml. Lipid feeding in animals without lymph fistulas resulted in a significant increase in serum apo A1 levels; biliary diversion, designed to eliminate intestinal lipoproteins of density less than 1.006 g/ml, resulted in a significant decrease in serum apo A1 levels. These studies demonstrate that the intestine actively synthesizes apo A1 and is a significant source of this apoprotein for circulating lipoproteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Wahl P. W., Cabana V. G., Hazzard W. R., Hoover J. J. Quantitation of apolipoprotein A-I of human plasma high density lipoprotein. Metabolism. 1976 Jun;25(6):633–644. doi: 10.1016/0026-0495(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2564–2568. doi: 10.1073/pnas.73.8.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Felker T. E. Radioimmunoassay of apolipoprotein A-I of rat serum. Biochim Biophys Acta. 1976 Sep 28;446(1):56–68. doi: 10.1016/0005-2795(76)90097-0. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Khorana J., Kilgore A. Localization of apolipoprotein B in intestinal epithelial cells. Science. 1976 Sep 24;193(4259):1254–1255. doi: 10.1126/science.183265. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K., Isselbacher K. J. Fat absorption during inhibition of protein synthesis: studies of lymph chylomicrons. J Clin Invest. 1972 Feb;51(2):356–363. doi: 10.1172/JCI106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K. Lymph chylomicron formation during the inhibition of protein synthesis. Studies of chylomicron apoproteins. J Clin Invest. 1973 Nov;52(11):2910–2920. doi: 10.1172/JCI107487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L. Synthesis and secretion of plasma lipoproteins. Adv Exp Med Biol. 1972;26(0):7–24. doi: 10.1007/978-1-4684-7547-0_2. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Kashyap M. L. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973 Jan;52(1):32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Ockner R. K. An electron microscopic study of endogenous very low density lipoprotein production in the intestine of rat and man. J Lipid Res. 1971 Sep;12(5):580–589. [PubMed] [Google Scholar]
- Koga S., Bolis L., Scanu A. M. Isolation and characterization of subunit polypeptides from apoproteins of rat serum lipoprotein. Biochim Biophys Acta. 1971 May 25;236(2):416–430. doi: 10.1016/0005-2795(71)90222-4. [DOI] [PubMed] [Google Scholar]
- Kostner G., Holasek A. Characterization and quantitation of the apolipoproteins from human chyle chylomicrons. Biochemistry. 1972 Mar 28;11(7):1217–1223. doi: 10.1021/bi00757a016. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Marsh J. B. Apoproteins of the lipoproteins in a nonrecirculating perfusate of rat liver. J Lipid Res. 1976 Jan;17(1):85–89. [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Bloch K. J., Isselbacher K. J. Very-low-density lipoprotein in intestinal lymph: evidence for presence of the A protein. Science. 1968 Dec 13;162(3859):1285–1286. doi: 10.1126/science.162.3859.1285. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Jones A. L. An electron microscopic and functional study of very low density lipoproteins in intestinal lymph. J Lipid Res. 1970 Jul;11(4):284–292. [PubMed] [Google Scholar]
- Shiff T. S., Roheim P. S., Eder H. A. Effects of high sucrose diets and 4-aminopyrazolopyrimidine on serum lipids and lipoproteins in the rat. J Lipid Res. 1971 Sep;12(5):596–603. [PubMed] [Google Scholar]
- Stein Y., Glangeaud M. C., Fainaru M., Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975 Jan 24;380(1):106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Reese H., Eder H. A. Polypeptide composition of rat high density lipoprotein: characterization by SDS-gel electrophoresis. Biochem Biophys Res Commun. 1974 Jul 24;59(2):513–519. doi: 10.1016/s0006-291x(74)80010-0. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Windmueller H. G., Herbert P. N., Levy R. I. Biosynthesis of lymph and plasma lipoprotein apoproteins by isolated perfused rat liver and intestine. J Lipid Res. 1973 Mar;14(2):215–223. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]








