Abstract
Objective(s):
Streptococcus pyogenes produces extracellular hyaluronidase enzyme. This enzyme is directly associated with the spread of the organism during infection. The objective of the present study was to clone and express the nucleotide sequence of the enzyme which is involved in hyaluronidase enzymatic activity.
Materials and Methods:
The enzymatic region of hyaluronidase gene was detected by bioinformatics method. The PCR method was used to amplify enzymatic region of hyaluronidase gene from chromosomal DNA of Streptococcus pyogenes. The eluted product was cloned into the prokaryotic expression vector pET32a which was digested by BamHI and HindIII restriction endonuclease enzymes. The target protein was expressed in the Escherichia coli. The bacteria including pET32a-hylA (hylA is abbreviation of Streptococcus pyogenes hyaluronidase gene and hylA is abbreviation of Streptococcus pyogenes hyaluronidase protein) plasmids were induced by IPTG and analyzed by SDS-PAGE. The enzymatic evaluation and antigenicity was finally studied.
Results:
Enzymes digestion analysis, sequencing results showed that the target gene (1296 base pair) was inserted correctly into the recombinant vector. The expressed protein (65 KDa) was purified successfully via affinity chromatography. Data also indicated that enzymatic region of hyaluronidase protein from Streptococcus pyogenes was recognized in all 5 patient's sera.
Conclusion:
In general, it is possible to produce the enzymatic regions of the Streptococcus pyogenes hyaluronidase in E. coli. The antigenic property of the produced protein is well retained. Considering the product's domestic demand and also low efficiency of production and pathogenicity of Streptococcus species, it is possible to produce it as recombinant product.
Keywords: Gene expression, Hemolytic streptococci, Hyaluronidase
Introduction
Hemolytic streptococci (group A) are spherical Gram-positive bacteria that form chains during growth, these chains are relatively short in pus and solid medium but they are longer in broth. Streptococci belong to Lancefield group A cause various purulent infections in humans (1, 2). The most important human infections caused by these bacteria include cellulitis, lymphangitis, pharyngitis, erysipelas and impetigo. In addition, Streptococcus pyogenes causes late septic complications in human (3). Two major complications in humans caused by group A streptococcus infection are rheumatic fever and glomerulonephritis.
Diagnosis of streptococcal infections is based on culture (isolation of the bacteria) and serologic tests. Streptococcus pyogenes produces a number of antigenic virulence factors. Some of them are known as spreading factors. Extracellular hyaluronate lyase is one of these factors (4).
Hyaluronic acid which is considered an important ground substance of connective tissue and known as interstitial cement breaks down by hyaluronidase enzyme of these bacteria. Therefore hyaluronidase plays an important role in invasion of Streptococcus pyogenes and is regarded as one of the effective factors in pathogenesis of this bacterium (5, 6).
This enzyme can be used in treatment of some diseases. The first therapeutic use of this enzyme as spreading factor was noted in 1928, so that subcutaneous injection of the enzyme increased subcutaneous penetration of vaccines and toxins. In terms of therapy, this enzyme is used together with topical anesthetics and pain relief medications (7, 8). Hyaluronidase also makes chemotherapy-resistant tumors more sensitive to treatment by enhancing the penetration of cancer medications (by spreading activity) (9). Another use of hyaluronidase is in the diagnosis of infection. This enzyme is inhibited by a specific antibody (anti-hyaluronidase) in human serum. These antibodiesspread several weeks after the beginning of the streptococcal infection and remain after the inflammation subsides, they appear in serum gamma globulin and specifically neutralize group A Streptococcus hyaluronidase (10). Research shows that the production of recombinant hyaluronidase does not have a suitable efficiency because of the large molecule size of the enzyme. However, production of this enzyme has always been considered due to its medicinal importance (11).
Therefore, this study tries to identify the enzymatic region of hyaluronidase gene which plays a role in its enzymatic activity by using bioinformatics methods and then to produce the desired region by induction of the gene in Escherichia coli. The purpose of this study is expression and production of recombinant protein part involved in the enzymatic activity of hyaluronidase in E. coli.
Materials and Methods
Bacterial strains and plasmids
Streptococcus pyogenes (ATCC: 8668) used as the source of chromosomal DNA for the Polymerase Chain Reaction (PCR). E. coli DH5α (Stratagene) was used as the primary host for the construction and propagation of plasmid. For production of recombinant protein, a prokaryotic expression vector pET32a (Novagene) was used. The recombinant pET32a (pET32a-hylA) was transformed in E. coli BL21 (DE3) pLysS as host strain. LB agar and broth were used for bacterial culture. The antibiotics were added to media according to references recommendation (12). All chemicals were obtained from Merck (Germany) and all enzymes were purchased from Fermentas (Lithuania) or Cinagen (Iran) Companies.
Enzymatic region
To find out enzymatic region, the sequence of hylA gene (2607 bp) from a reference strain (NCBI GenBank, Accession number: EU078690.1) was submitted to ABCpred, Bcepred and Emboss enzymatic web servers (13).
Two specific PCR primers were designed with Oligo5 software. The forward primer (5'ATGGGATCCATGTATGAACACGCT3') starts from the beginning of the gene and contain BamHI site. Reverse primers (5'AACAAGCTTTATTTTGTTTCC-TAAGATA3') contain recognition site for HindIII. These two primers were designed to amplify a 1296 bp fragment of the hylA coding enzymatic region.
Chromosomal DNA isolation
Genomic DNA was extracted from culture according to the standard CTAB/NaCl protocol (14). 1.5 ml of full grown, overnight bacterial culture is centrifuged for 2 min at 13000 RPM. The pellet resuspended in TE buffer (Tris 10 mM, EDTA 1 mM, pH 8) by repeated pippeting. Further, the bacterial cell was lysed by sodium dodecyl sulphate (SDS 10%) and proteinase K (20 mg/ml) and the chromosomal DNA was extracted by CTAB/NaCl solution (10% CTAB and 0.7 M NaCl). Cell debris and proteins were removed by two times phenol/chloroform/isoamylalcohol (25:24:1) mixture. Genomic DNA was precipitated by isopropanol and washed in ethanol (70%), dried, and then resuspended in TE buffer.
Quality and quantity of the purified genomic DNA were assessed by 1% agarose gel electrophoresis in 1x TBE buffer containing 0.5 µg/ml ethidium bromide and spectrophotometrically (260/280 nm), respectively.
Gene amplification
PCR amplification was performed in a 50 µl total volume containing 500 ng of template DNA, 1 µM for each primers, 2 mM Mg2+, 200 µM each dNTP, 1x PCR buffer and 2.5 unit of pwo DNA polymerase (Roche, Germany). The following conditions were used for amplification: Hot start at 94°C (5 min), followed by 30 cycles of denaturation at 94°C (1 min), annealing at 50°C (1 min) and extension at 72°C (1 min) (14). The PCR product was analyzed by electrophoresis in 1% agarose gel in 1xTBE buffer and visualized by ethidium bromide staining on UV transilluminator. The PCR product was purified from agarose gel by high pure PCR product purification kit (Roche Diagnostic) according to the manufacturer guideline.
Cloning of hylA gene in bacterial expression vector
The PCR product and pET32a (containing N-terminal histidine tag) vector were digested with BamHI and HindIII restriction enzyme. The ligation performed at 16°C over night incubation using T4 DNA ligase (Cinagen) enzyme. E. coli DH5α and E. coli BL21 (DE3) pLYsS competent cells were prepared by calcium chloride method and were used for transformation of plasmid (15).
Gene expression and purification
E. coli BL21 (DE3) pLYsS was transformed with pET32a- hylA plasmids according to the standard method (12) and grown in 2 ml LB medium supplemented with Ampicillin (100 µg/ml) and chloramphenicol (34 µg/ml) at 37°C with agitation. A colony which contained recombinant plasmid was cultured on shaking incubator for overnight at 37°C in 2 ml LB medium containing 100 µg/ml Ampicillin and 34 µg/ml chloramphenicol. The next day, 0.5 ml of culture was removed and inoculated in 50 ml of LB broth (per liter contains: 10 g yeast extract (Difco), 20 g Bactotryptone broth (Difco), 0.2% (mass/vol) glucose, 10 g NaCl, 1 g KCl, 0.5 g MgCl2, 0.5 g CaCl2, 100 mg ampicillin), incubated at 37°C with at 200 RPM shaking until the optical density reached to an absorbance of 0.5 to 0.8 at 600 nm (16).
Expression of the hylA protein was then induced by the addition of Isopropylthio β-D-galactosidase (IPTG) to a final concentration of 1 mM and incubated for four hours.
The expressed protein was purified by affinity chromatography with Ni-NTA agarose resin according to manufacture instruction (QIAGEN, USA). The purified protein was dialyzed twice against PBS (pH 7.2) at 4°C over night. The quality and quantity of purified recombinant hylA protein was analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE 12%) and Bradford methods, respectively (17).
Immunoblot analysis
The integrity of the product was confirmed by western-blot analysis. Five acute phase patients' sera from Immunology Department (the Arak University of Medical Sciences of Iran, Arak) were collected to be used in western blot experiment.
Western blotting was performed according to standard protocol (16) using normal human sera as negative control and hyla antibody. For western blot analyses, 0.5 µg of purified recombinant hylA protein was used per well. The SDS-PAGE gel were blotted on to polyvinylidine difluoride (PVDF) membrane (Roche, Germany) using transfer buffer containing 25 mM Tris (pH = 8.3), 192 mM glycine and 20% methanol at 90 v for 3 hr at 4°C. The blotted membrane was blocked with 2.5% (w/v) BSA in TBS buffer (0.5 M NaCl, 0.02 M Tris pH 8.5, 0.05% tween 20) for 1 hr at room temperature. Membrane was incubated for 2 hr at room temperature with diluted sera 1:100 from patient's sera, normal sera and hylA antibody. After reaction with the primary antibody, the blot was washed three times with TBS buffer and incubated with antihuman IgG (Bioscience) in 1:1000 dilution of TBS. The blots were then washed three times with TBS and reactions were developed by Diamino Banzidine (DAB) solution (Roche, Germany).
Hyaluronidase activity assay
Enzymatic assay of hyaluronidase enzymatic fragments was estimated as units/mg using a quantitative turbidimetric method based on Dorfman, A (18). In principle hyaluronic acid as substrate is hydrolyzed and depolymerized by hyaluronidase. Conditions of this method is T = 37°C, pH = 5.9, A600 nm, Light Path= 1 cm.
Briefly, recombinant protein was dissolved in 1 ml enzyme buffer (0.02 M phosphate buffer, 0.45% NaCI, 0.01% BSA (Sigma), pH 6.8-7.0) and mixed with 0.5 mg HA (Sigma), which was dissolved in 1 ml Substrate butter (0.3 M KH2PO4/Na2HPO, pH 5.35). Enzyme digestions were allowed to proceed for 45 min at 37°C. Turbidity was generated at the end of incubation by adding 10 ml acid albumin solution. Optical densities at 600 nm were determined exactly 5 min after addition of acid albumin.
National Formulary (NF) Standard hyaluronidase Solution (NF Std) (sigma) was used as a standard sample in different concentration and was used to establish a standard curve and units of activity were calculated by comparison to this curve. A standard curve was made using commercial hyaluronidase (sigma) with different known concentrations diluted from a stock of 400-1000 mg/ml as follows: (0.1, 0.2, 0.3, 0.4, 0.5 and 0.6).
Results
Enzymatic region
According to the results of three servers which were in accordance, amino acid sequence of 173 till 605 was selected as a region with enzymatic properties (Figure 1).
Figure 1.
The result of enzymatic fragments obtained by the applied bioinformatics softwares (ABCpred, Bcepred and Emboss enzymatic web servers)
DNA amplification
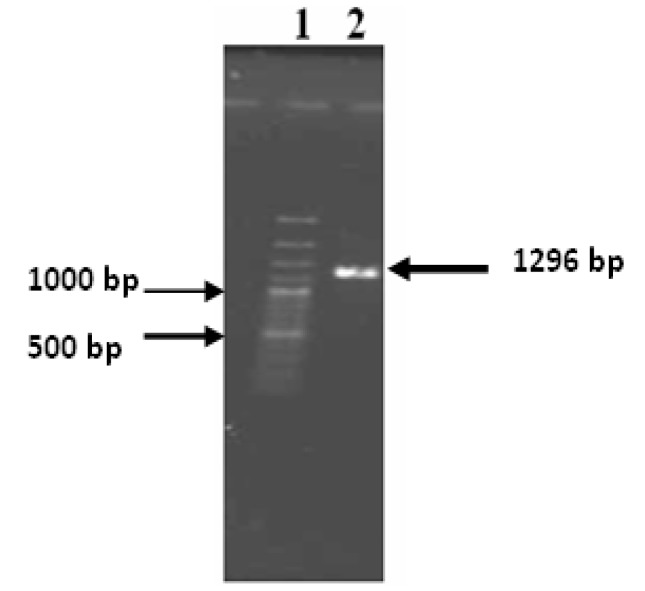
The chromosomal DNA of S. pyogenes was extracted for amplification of hylA gene. The amplified fragment had the expected size of 1296 bp comparing to 100 bp DNA ladder (Figure 2).
Figure 2.
hylA gene amplification by PCR. Lane 1, Molecular weight marker (100 bp ladder); Lane 2, amplified hylA
The recombinant plasmid (pET32a- hylA) was sequenced. The result was confirmed by comparing with databases and using basic local alignment search tool (BLAST) software (data not shown). To confirm the transformation of pET32a- hylA into E. coli BL21 (DE3) pLYsS, PCR reaction and enzymatic digestion were performed (BamHI, HindIII).
Expression and purification of recombinant hylA protein
pET32a- hylA in E. coli BL21 (DE3) pLYsS (from one colony) was induced and the expressed protein was purified by Ni-NTA column (Figure 3).
Figure 3.
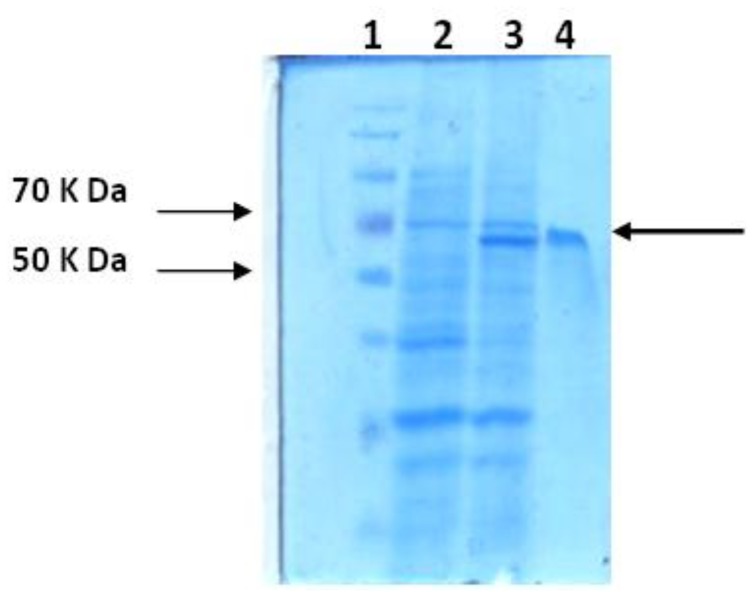
SDS-PAGE analysis of recombinant enzymatic fragment of hyaluronidase protein and its purification. Lane 1, Protein marker; Lane 2, pET32a-Hya before induction (2.5 µg /well); Lanes 3, pET32a-Hya after 2 hr (2.5 µg /well); Lane 4, elution of recombinant L7/L12 protein through Ni-NTA column
Purified recombinant hyaluronidase protein was assessed by SDS- polyacrylamide gel electrophoresis [SDS-PAGE (12%)]. The molecular weight of about 65 kDa for recombinant protein was measured by SDS-PAGE analyses and protein concentration was estimated using the Bradford method. The concentration of recombinant protein was calculated as 470 mg purified protein per liter of the initial culture (approximately %12 of total protein).
Immunoblotting analysis
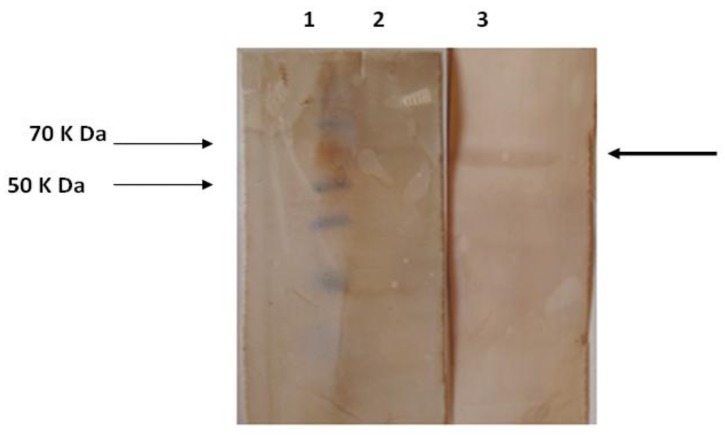
To determine the antigenicity of recombinant hylA protein in patients' sera, the recombinant hylA protein were assayed by western-blot analysis. Figure 4 shows the specific interaction between patients' serum and human normal sera. Human normal sera were used as a negative control.
Figure 4.
Western blot analyses of hyaluronidase recombinant enzymatic fragment using patient sera. Lane 1, protein marker; Lane 2, western blotting by human normal sera (negative control); Lane 3, western blotting by patients’ sera
Biological assay
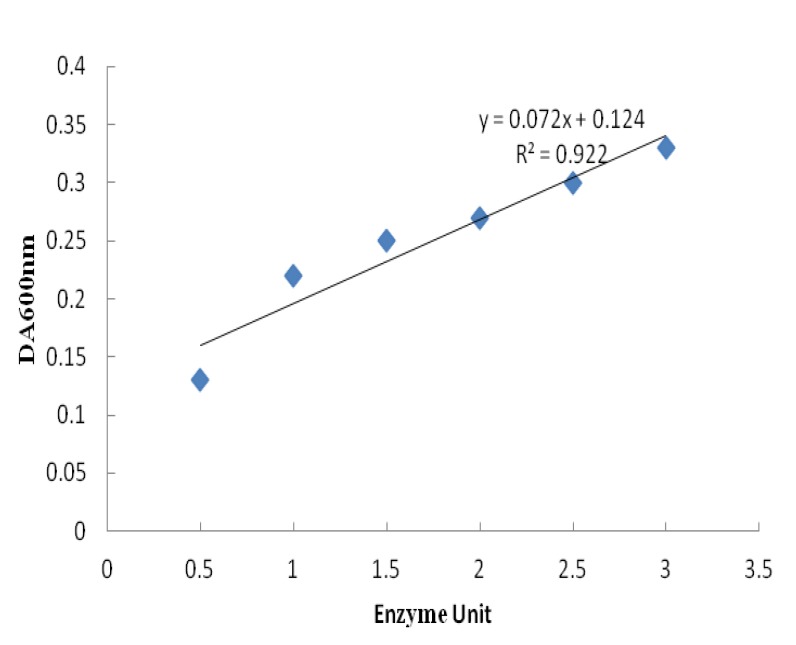
In order to determine the enzymatic activity of the recombinant protein, turbidimetric assay was performed. According to the standard curve from serial dilutions of a commercial hyaluronidase stock, results revealed that enzymatic activity of protein was about 30% of the activity of standard enzyme (Figure 5).
Figure 5.
Quantitative turbidimetric assay for determination of recombinant hyaluronidase activity. A standard curve was made using commercial hyaluronidase
Discussion
In this study, the enzymatic part of Streptococcus pyogenes hyaluronidase gene was identified using bioinformatics programs. The desired region of the gene was cloned in expression vectors after amplification and the desired protein was expressed. Then the produced protein was purified. Finally, the produced protein was verified by western blotting.
Our findings indicate that the enzymatic region of recombinant protein of streptococcal hyaluronidase can be produced in E. coli by pET32a plasmid.
In addition, hyaluronidase enzymatic fragments had enzymatic properties. Using bioinformatics software we could change hyaluronidase gene with genetic manipulation to express smaller protein with antigenic properties and enzymatic activity. Thus we can use in treatment of some diseases and production of diagnostic kits. Additional studies can be performed on this topic in future.
Hyaluronidase is an effective extracellular enzyme which breaks down hyaluronic acid, the important ground substance of connective tissue. Therefore, hyaluronidase plays an effective role in the invasion of microorganism and is considered as one of the important factors in the pathogenesis of this bacterium. This enzyme is produced by various Gram-positive microorganisms including Streptococcus spp, Staphylococcus, Propionibacterium, Peptostreptococcus and Streptomyses (19).
This enzyme is not usually produced by its natural and main sources because of the low amount of product and also the risk of streptococcal contamination. Therefore, research related to the production of this enzymemostly focus on its recombinant form.
Streptococcus pyogenes hyaluronidase protein has a weight of 99,636 Daltons. This enzyme has been previously produced. But unfortunately the protein was broken down due to heaviness of the protein and therefore low amount of product has been purified (11, 20).
The importance of the therapeutic role of this protein has made its production economically important especially in recombinant form (9). Therefore in this research, only the part of the hyaluronidase protein with enzymatic properties was determined using bioinformatics methods, to increase the protein product, and production of additional parts (which lack enzymatic properties) were abandoned. Thus, by producing the enzymatic part a smaller protein was produced and it's breaking down during the various stages of production and purification was prevented.
The target gene with 1296 nucleotides encodes a 43,200 daltons protein. However, the produced protein has a weight of 65 kDa (Figure 3). The expressed protein from pET system contains several extra amino acids (6xHis tag) linked to the C or N terminal extension of the recombinant protein. Additional amino acids increase the size of expressed protein by approximately 20 kDa, as shown in Figure 3 (12).
E. coli is widely used both in research and in industry as a host for the expression of recombinant proteins. In this study, BL21-pLysS strain of E. coli was used as host for production of recombinant protein, which lacks cytoplasmic proteases such as Lon, OmpT, DegP and HtpR. Thus, high expression of hyaluronidase in BL21 strain of E. coli is due to the absence of protease in this bacterium (21).
One of the most powerful systems for gene expression and production of recombinant proteins in E. coli is pET system. In this system, the target gene is under the control of T7 powerful promoter and in the host genome (E. coli BL21-pLysS) transcription of the gene under the control of this promoter is performed by phage T7 RNA polymerase which is cloned in the bacterial cell (15). Thus, mRNA production and ultimately protein production in this system are not affected by the cellular factors involved in protein synthesis due to the independence of the gene transcription system from the host cell. Therefore, production in this system is higher than the systems that transcription depends on the host cell polymerases (15). As the protein concentration was estimated to about 470 micrograms per liter in this research.
In general, in this research we show that, it is possible to produce the enzymatic regions of the Streptococcus pyogenes hyaluronidase in E. coli. The enzymatic activity and antigenic property of the produced protein is well retained.
Western blotting results indicate a similar structure between the produced recombinant protein and its natural form.
Conclusion
Our present data confirm that it is possible to clone the part of Streptococcus pyogenes hyaluronidase gene which is involved in the enzymatic activity into E. coli and express and produce its recombinant protein. This protein has antigenic properties similar to the commercial form. Results showed that the activity of enzymatic region of recombinant protein was about 30% of the activity of standard enzyme.
Acknowledgment
This study was conducted with financial assistance from Arak University of Medical Sciences, Iran, and we are grateful for their invaluable contribution to this study. This study is a part of the research proposal (562, ethical code: 88-5-12) by Dr.Hamid Abtahi and the thesis (102) by Ms Nafise Al-Sadat Mirjamali, master student of microbiology, Islamic Azad University of Arak, Iran.
References
- 1.Ikebe T, Endo M, Ueda Y, Okada K, Suzuki R, Minami T, et al. The genetic properties of Streptococcus pyogenes emm49 genotype strains recently emerged among severe invasive infections in Japan. Jpn J Infect Dis. 2004; 57:187–188. [PubMed] [Google Scholar]
- 2.Morosini M, Canton R, Loza E, Del Campo R, Almaraz F, Baquero F. Streptococcus pyogenes isolates with characterized macrolide resistance mechanisms in Spain: in vitro activities of telithromycin and cethromycin. J Antimicrob Chemother. 2003; 52:50. doi: 10.1093/jac/dkg303. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham MW. Pathogenesis of group a strepto-coccal infections. Clin Microbiol Rev. 2000; 139:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhtar M, Bhakuni V. Streptococcus pneumoniae hyaluronate lyase: An overview. Curr Sci . 2004; 86:285–295. [Google Scholar]
- 5.Butler L, Rainger G, Nash G. A role for the endothelial glycosaminoglycan hyaluronan in neutrophil recruitment by endothelial cells cultured for prolonged periods. Exp Cell Res. 2009; 315:3433–3441. doi: 10.1016/j.yexcr.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starr C, Engleberg N. Role of hyaluronidase in sub-cutaneous spread and growth of group A streptococcus. Infect Immun. 2006; 74:40–48. doi: 10.1128/IAI.74.1.40-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze C, Bittorf T, Walzel H, Kundt G, Bader R, Mittelmeier W. Experimental evaluation of hyaluronidase activity in combination with specific drugs applied in clinical techniques of interventional pain management and local anaesthesia. Pain Phys. 2008; 11:877–883. [PubMed] [Google Scholar]
- 8.Stern R, Jedrzejas M. Hyaluronidases: their genomics structures and mechanisms of action. Chem Rev . 2006; 106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol Biol (Paris) 2005; 53:372–382. doi: 10.1016/j.patbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Steer A, Vidmar S, Ritika R, Kado J, Batzloff M, Jenney A, et al. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin Vaccine Immunol . 2009; 16:172–175. doi: 10.1128/CVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abtahi H, Moradkhani A , Pakzad I. Cloning and expression of the Streptococcus pyogenes hyaluronidase gene in Escherichia coli . Aust J of Basic App Sci. 2012; 6:95–99. [Google Scholar]
- 12.Mahmoudi S, Abtahi H, Bahador H, Mosayebi G, Salmanian AH. Production of Recombinant Streptokinase in E. coli and Reactivity with Immunized Mice. Pak J Biol Sci. 2010; 13:380–384. doi: 10.3923/pjbs.2010.380.384. [DOI] [PubMed] [Google Scholar]
- 13.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett . 1990; 276:172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 14.Molaee N, Abtahi H, Mosayebi G. Expression of recombinant streptokinase from streptococcus pyogenes and its reaction with infected human and murine sera. Iran J Basic Med Sci. 2013; 16:985–989. [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 16.Farjadi V, Abtahi H, Zolfaghari, Safieh Soufian MR, Hasanzadeh L. Expression purification and evaluation of antigenicity of cagA antigenic fragment of helicobacter pylori. Jundishapur J Microbiol . 2013; 6:e7367. [Google Scholar]
- 17.Hasanzadeh L, Ghaznavi-Rad E, Soufian S, Farjadi V, Abtahi H. Expression and antigenic evaluation of vacA antigenic fragment of helicobacter pylori. Iran J Basic Med Sci . 2013; 16:836–839. [PMC free article] [PubMed] [Google Scholar]
- 18.Dorfman A. Mucopolysaccharidases. In: Holowick SP, Kaplan NO, editors. Methods in Enzymology. N.Y: Academic Press; 1995. pp. 166–173. [Google Scholar]
- 19.Zhu H, Liu M, Sumby P, Lei B. The secreted esterase of group a streptococcus is important for invasive skin infection and dissemination in mice. Infect Immun . 2009; 77:5225–5232. doi: 10.1128/IAI.00636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes W, Dixon A, Walton S, Aridgides L. The extracellular hyaluronidase gene (hylA) of Streptococcus pyogenes. FEMS Microbiol Lett. 2000; 184:109–112. doi: 10.1111/j.1574-6968.2000.tb08999.x. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoudi S, Abtahi H, Bahador A, Mosayebi G, Salmanian AH , Teymuri M . Optimizing of nutrients for high level expression of recombinant streptokinase using pET32a expression system. J Clin Med . 2012; 7:109–114. [PMC free article] [PubMed] [Google Scholar]