Abstract
Objective(s):
Spinal cord injury (SCI) is one of the most serious clinical diseases and its treatment has been a subject of interest to researchers. There are two important therapeutic strategies in the treatment of SCI: replacing lost tissue cells through cells implantation and scar elimination. Therefore, in this study we used human adipose-derived stem cells (hADSCs) implantation and injection of Chondroitinase ABC.
Aim of present study was to answer to this question: which one is more efficient for Improvement of locomotor recovery after SCI in rat? Transplantation of hADSCs or injection of ChABC.
Materials and Methods:
The spinal cord of rats was injured by contusion using a weight-drop at the level of T8-9, the hADSCs and Chondroitinase ABC were infused in to the spinal cord tissue after injury. BBB test was performed and recorded for each animal weekly for 8 weeks. After the 8th weeks, Serial cross-sections were stained with cresyl violet and examined under a light microscope and area of cavity in the spinal cord was measured.
Results:
At 8th weeks after injection, hADSCs and ChABC significantly promote locomotor function (P<0.01) and spinal cords of hADSCs and ChABC group had cavities much smaller than those of the control group (P<0.001).
Conclusion:
Results of the present study shows dealing with inappropriate neuro-inhibitory environment and glial scar by ChABC have equal role compare to cell therapy (with hADSCs) for improving motor function after SCI and this result in adoption of proper therapeutic strategies for SCI intervention is important.
Keywords: BBB, Chondroitinase ABC, Contusion, hADSCs, Spinal Cord Injury
Introduction
Spinal cord injury (SCI) is among the issues that affect individual lives and almost can be said all the individuals in society are at equal risk. In 2004, 11000 cases have been diagnosed in United States of America with this condition. SCI is one of the most serious clinical diseases and its prevalence is increasing year by year, although mortality rate from SCI has a decrement about 5%, but disability rate from SCI is almost remained at high level. Among these disabilities, "paraplegia" can be mentioned as one of the most important complications. Therefore expedition of locomotor recovery after spinal injury has been a subject of interest to researchers and professionals in neuroscience (1). The extension of spinal injury can be different in individuals depends on type and area of injury (2). The most common form of spinal cord injury is contusion that may damages spinal cord because of the physical impact and secondary cell damage by creating glial scarring around the injured spinal cord (3). Recovery after spinal cord injury will be difficult due to axonal damage (4), demyelination and scar (5). Spinal cord injury has two stages: Primary mechanical injuries and secondary injuries that exist through inflammatory responses. Neuropathology demonstrations of SCI contains: Edema, axonal damage, infiltration of inflammatory cells and increased astroglia (6). Improvement of locomotor recovery after spinal cord injury depends on intensity of tissue damage. Spontaneous recovery after spinal cord injuries is very low (7). In spinal Restoration process and Further Restoration, macrophages and astrocytes play significant roles through signals activation (8). The efforts which may be done to improve the performance of injured spinal cord include: reduction of secondary injury development, intervention in neuro-inhibitory environment in injured area, replacing lost tissue cells through cells implantation, renewal of axon myelin loss, increasing potential recovery of local progenitor cells (9). Different types of cells have been used by researchers to improve locomotor recovery in spinal cord injury (1, 10, 11). One of these cells, stem cells derived from human adipose tissue (hADSCs), due to the production of neurotrophic factors such as nerve growth factor, brain-derived neurotrophic factor and glial-derived neurotrophic factor, can be efficient for restoration of function because of supplying appropriate neurological environment at the site of spinal cord injury. In this regard, many studies have shown that stem cells derived from human adipose tissue have the ability to repair traumatic nerve damage (12). Cells that are extracted from the surface layer of abdominal fat have greater ability to create such an environment rather than cells that are extracted from deep layer (12). One of the reasons that inhibit axonal regeneration after CNS injury is existence of impermeable cement scar in the site of injury (13, 14). Several family of inhibitory molecules in the extracellular matrix along with reactive astrocytes create dense scar in site of injury that acts as a barrier to axonal regeneration (15). Chondroitin sulfate proteoglycan (CSPG) produced by astrocytes and oligodendrocytes in site of injury is increased drastically, which results in limiting axonal regeneration (16). Researchers have shown that CSPG is most abundant extracellular matrix molecules at the site of spinal cord injury and is associated with a Scar (17). Other therapeutic strategies in the treatment of spinal cord injury that can be used are scar elimination. Chondroitinase ABC has the ability to eliminate these scars and deal with Neuro-inhibitory environment that has been created at the site of spinal cord injury. ChondroitinaseABC (ChABC) is a bacterial enzyme that digests glycosaminoglycan chains (GAG) in CSPG, which is the main component of the extracellular matrix. One of the effects of ChABC which have focused recently by researchers is that the axonal regeneration and functional improvement after lesions of the central nervous system can be done through digestion of GAC and CSPG chains by ChABC (18-20).
According to the above description, the aim of present study was to answer to the question: which one is more efficient for Improvement of locomotor recovery after a spinal cord contusion model? Transplantation of hADSCs or injection of ChABC.
Materials and Methods
Animals
In this study we used adult male wistar rats (N=24) weighting between 250-350g (Pasteur Institute, Tehran). The study approved by medical ethics committee of Iran University of Medical Sciences. Animals kept in animal house standard conditions (temperature 21±3°C, 12 hr light/dark cycle) with free access to food and water.
Isolation of hADSCs
After attainment of patients written consents, fatty tissue was prepared from superficial layer of abdomen during liposuction surgery from 25-46 years individuals in Rasul Akram hospital (Iran-Tehran). Isolation of human adipose-derived stem cells was performed according to Dubois et al protocol (21). Fatty tissue was warmed in 37°C water bath before the initiation of Isolation. Then all the Isolation stages were performed under hood sterilized condition. 200 mg of fatty tissue for washing purpose was transferred to the tube containing 1% Penicillin/Streptomycin (Invitrogen) dissolved with warm phosphate-buffered saline (PBS, Invitrogen) and washing was continued until elimination of blood vessels, and connective tissue (commonly 2 times washing). Fatty tissue sample was minced by sterilized scissors and was transferred to the tube containing collagenase type I (Gibco,17100-017, USA) 0.1% and BSA 1% (dissolved with warm PBS)(Invitrogen) for digestion, then kept in water bath for 30 min for total digestion and homogenization of sample. After tissue digestion, the tube containing the sample was centrifuged at room temperature for 5 min at 1200 rpm speed. After discharging supernatant, formed plate was resuspended with BSA 1% solution and was again centrifuged to remove red blood cells using RBC lysis buffer. Ultimately after centrifugation and discharging supernatant, formed plate was resuspended with medium containing DMEM/Ham's F-12, FBS 10% and Penicillin/Streptomycin 1% and transferred to the tissue culture flasks. Flasks were maintained in incubator (temperature 37°C, CO2 5%, humidity 98%).
Flowcytometry analysis
In order to characterization of hADSCs, isolated cells were fixed in 5th passages (after being harvested by trypsin) in paraformaldehyde 2% for 30 min. After two times washing with PBS, cells were incubated with antibodies against CD90, CD73, CD45, CD44, and CD31 for 30 min. CD44 and CD90 antibodies were directly conjugated with the allophycocyanin (APC). The Rat IgG2b was used for control isotope of CD90 and CD44 as substitute antibody. Goat anti-Rabbit IgG-FITC was used as a secondary antibody for CD31 and CD45 and Goat anti-mouse IgG-FITC was used as a secondary antibody for CD73. Rabbit polyclonal IgG was used as a substitute antibody for control isotope of CD31, CD45 and CD73. Flowcytometry was performed with a BD FACScalibur flow cytometer device (BD Biosciences, USA). Details of used antibodies are summarized in Table 1.
Table 1.
Details of used antibodies in flowcytometry
| Dilution | Cat. number | Company | Antibody |
|---|---|---|---|
|
| |||
| 1/100 | 559869 | BD Biosciences | CD90 |
| 1/200 | 17-0441 | eBioscience | CD44 |
| 1/50 | ab81720 | Abcam | CD73 |
| 1/100 | ab10558 | Abcam | CD45 |
| 1/20 | ab28364 | Abcam | CD31 |
| 1/200 | 17-4031 | eBioscience | Rat IgG2b |
| 1/100 | Ab27478 | Abcam | Rabbit polyclonal IgG |
| 1/100 | AP156F | Millipore | Goat anti-Rabbit IgG |
| 1/40 | Sc-2010 | Santa cruz | Goat anti-mouse IgG-FITC |
Tagging Human Adipose- derived Stem cells (hADSCs) with GFP+ Recombinant lentiviral virus
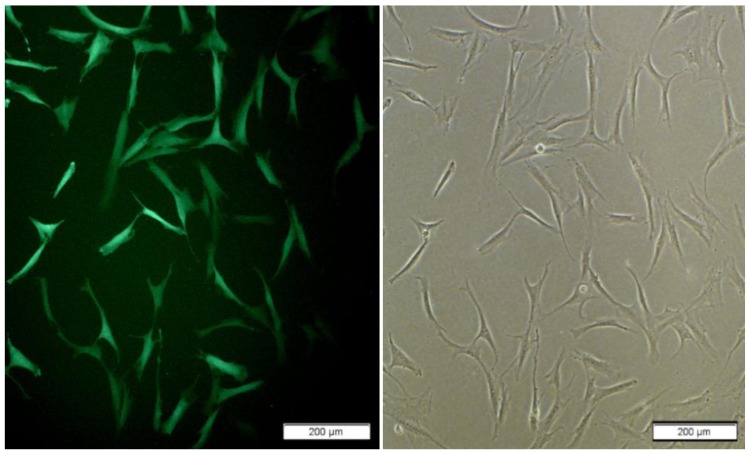
Lentivral vector carry Copa-GFP gens under EF1 promoter produce under calcium phosphate standard protocol. lentiviral vector pCDH-311B with EF1-CopGFP (System Bio Inc.) , pMD.2 and p.sPAx.2 (Kindly gift from Dr Trono) was used for transfection HEK293T in 10cm plate with CoPo4 reagents. After 18 h we change medium with fresh DMEM -10% FBS. Recombinant viral collected in 24, 48 and 72 hr after change the medium and any time add 12 ml fresh medium to plate. Collected recombinant viral titer measured with transduction HEK 293T in 6 well plate in different log. Titer was about 1×106-3×106vp/ml. hADSCs transduction achieved with application of polyberen and spinfection enhanced transduction protocol with MOI 4-6. For maximum transduction spinfection repeated with fresh recombinant viruses for 3 times. hADSCs transduction assay with florescent microscope after 72 hr (Figure 2).
Figure 2.
hADSCs express GFP in proliferation medium 10 days after transduction; left and right : cells grown in monolayer; right is GFP positive cells
Spinal cord injury model
Animals were anesthetized with IP injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Animals were placed in the prone position on the covered operating table with warm blankets. After shaving the skin in the thoracic spines area and prepping with Betadine, midline incision was created with a scalpel. After Pushing the subcutaneous fat and muscle to expose the vertebral lamina, laminectomy was performed at T8-T9 levels of spinal cord. Metal cylinder (weighing 10 g and 2 mm in diameter) was released on the exposed spinal cord from distance of 12.5 cm. Then the muscles and skin were sutured with 3/0 Suture. Postoperative care included: Ringer's solution administered to prevent dehydration (3 ml IP after surgery), administered gentamicin (0.8 mg/100 g, IP) for 4 days postoperatively and bladder massage twice a day, for all animals was performed.
Contusion model confirmation
To confirm contusion model, seven days after spinal cord injury one animal were selected to evaluation of cavity formation in lesion site. In order to this, mentioned animal were deeply anesthetized with ketamine and xylazine and transcardially perfused with 4% paraformaldehyde in 0.1 mol/l PBS (pH=7.4). A piece of spinal cord (length 1.5 cm), containing contusion location, was maintained in sucrose 30% overnight and was embedded in cryopreservation medium (OCT). Cross-sectional thickness of the 10 µm was made by cryostat from injured area. To observe formed cavity in the lesion site, slides were stained with Nissl staining.
Transplantation procedure
Animals were randomly divided into 4 groups include:
Sham operative group (n=6): laminectomy were performed in this group
Control group (n=6): SCI were achieved in this group
hADSCs group (n=6): 1×106 hADSCs in the volume of 10µl were injected through intraspinal injection
Chondroitinase ABC group (n=6):10 µl of 100 U/ml Chondroitinase ABC (Sigma, C3667)were injected [diluted with 0.01% bovine serum albumin (dissolved with PBS)].
Seven days after SCI, all animals were anesthetized and contusion site were exposed.1×106hADSCs were resuspended with 10µl PBS and aspirated by Hamilton syringe. Cell transplantation and chondroitinase ABC injection were performed by Hamilton syringe with a sterile 30 gauge needle. After connecting the syringe to the micro-injector device (model 780310), needle in the midline were inserted 1-1.5 cm deep in spinal cord and during 2 min 5 µl were injected 1mm rostrally and then 1 mm caudally from the site of injury. For prevention of cells and enzyme leakage from injection site, after 2 min needle was withdrawn from spinal cord at the end of injection.
After each transplantation session, 1 sample of hADSCs from the Hamilton syringe was mounted onto a slide and stained with trypan blue to assess cell viability. Approximately 90% of cells were alive.
Behavioral assessment
Locomotor functions were assessed by the open-field walking test during 4 min. Each animal moved freely in a circular field (90 cm in diameter and height of 24 cm) during 4 min. The Animals movement was captured by digital camera during these 4 min. Basso, Beattie, and Bresnahan (BBB) test was performed and recorded for each animal weekly for 8 weeks. Two trained observers who were unaware of the treatment group were saw the films separately and were scored the animals movement on the basis of BBB scale. 21- point open field locomotion score was developed by BBB in order to study the sequence of locomotor recovery patterns and takes into consideration the early (BBB score from 0 to 7), intermediate (8-13) and late phases (14-21) of recovery (22). Mean scores of observers were recorded as a BBB score of each animal. BBB scores were recorded one week after SCI. Only the animals that had BBB scores under 2 were entered to study or else they were omitted.
Histology
After the 8th weeks animals were deeply anesthetized with ketamine and xylazine and were perfused transcardially with paraformaldehyde 4%. Segment of Spinal cords which contains lesion site (length 1.5 cm) removed and was maintained in sucrose 30% overnight and was embedded. Serial cross-sections (10 µm in thickness) were produced from samples with cryostat. These sections were stained with cresyl violet for the study of their general histology.
Confirmation of presence hADSCs in tissue
Mentioned sections were observed with flurocense microscope to confirm presence of hADSCs in tissue.
Measurement of the cavity size
For measurement of the cavity size, rats at postoperative 8 weeks were used. Serial cross-sections were stained with cresyl violet (in each animal 10 sections at an interval of 50 µm), and examined under a light microscope equipped with a camera. The area of cavity in the spinal cord was measured with an image processing and analysis program "Olysia Bio Report Soft Imaging System 3.2" on consecutive sections.
Statistical analysis
The statistical comparisons between groups were carried out using repeated measures analysis of variance (ANOVA) followed with the tukey test for Post hoc analysis. Statistical analysis was performed using SPSS version 15. A P<0.05 was accepted to denote statistically significance and all data were presented as mean ± SEM.
Results
Characterization of hADSCs Cell culture
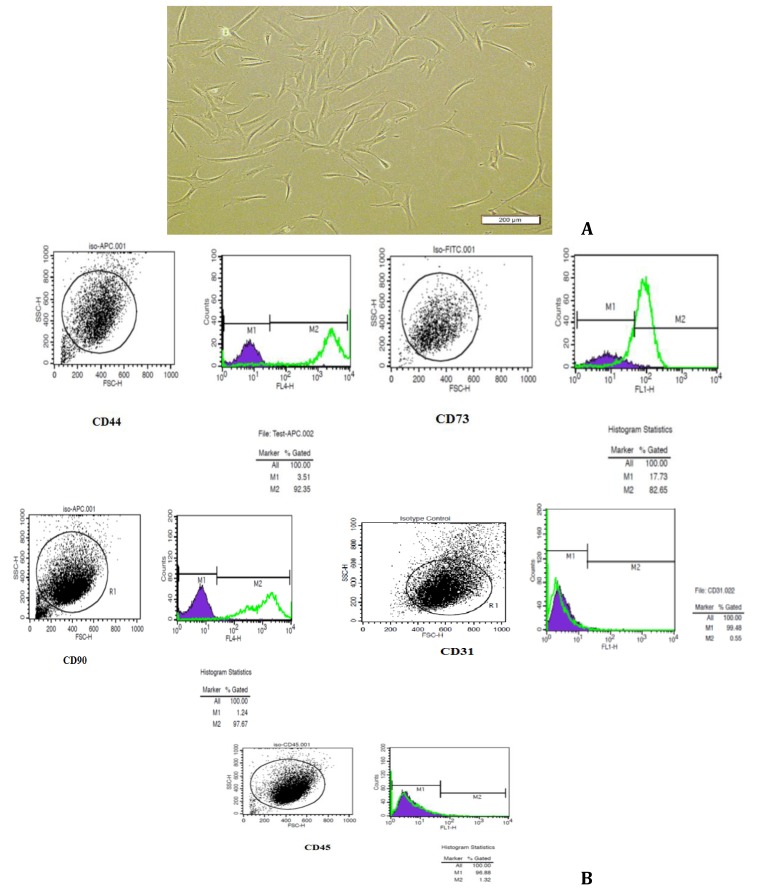
Human adipose derived stem cells were cultured in DMEM/F12 with 10% FBS, and the majority of the cells remained in suspension, including cell population. Erythrocytes had been removed during tissue digestion. In order to remove suspended cells, the culture was washed three times with PBS, after which a few attached single cells or cell clumps were observed. DMEM/F12 with 10% FBS added to flasks. Attached cells proliferated and reached approximately 90%confluence in 25-cm2 flasks, the primary culture was trypsinized using 0.25% trypsin-EDTA (Invitrogen) and passaged at a culture expansion ratio of 1:4 until passage 5. Spindle-shaped or fibroblast-like morphology of proliferated cells were observed using inverted microscope (Figure 1A).
Figure 1.
Characterization of isolated human adipose-derived stem cells. (A) Inverted microscopy of the spindle-shape and fibroblast- like morphology of hADSCs in the fourth passage. (B) Flowcytometry analysis of hADSCs: the results showed that hADSCs expressed, CD44, CD73 and CD90 but did not express CD31 and CD45. The experiments were repeated three times
Flow cytometry analysis
hADSCs displayed positive staining for the specific mesenchymal surface markers CD44, CD73 and CD90 (Figuer 2B). hADSCs at passage 4 exhibited high levels of CD44, CD73 and CD90 which expressed from 92.35%, 82.65% and 97.67% of the total cell population, respectively. In contrast, only a small proportion of hADSCs expressed hematopoietic stem cell surface markers, including CD31 and CD45 which were expressed at 0.55% and 1.32% of cells, respectively (Figure 1B).
Contusion model confirmation
Seven days after the contusion injury, evaluation of sections stained with cresyl violet revealed the formation of several differently sized vacuoles and cystic cavities at the site of injury.
Locomotor function
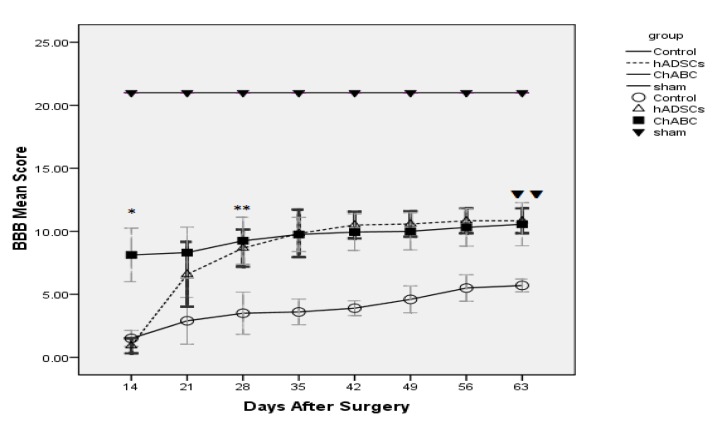
At the 7th days after SCI, the contusion site was injected with hADSCs and ChABC. For assessment of locomotor function after injection of hADSCs and ChABC, we used the BBB scale. At 8th weeks after injection (63 days after surgery), injection of hADSCs and ChABC promote locomotor function (P<0.01) such that hADSCs (10.83) and ChABC (10.56) groups show significant increase in BBB Score compared with the control group (5.70) (P<0.01) and this trend indicate that hADSCs and ChABC is necessary for locomotor function recovery. At1st week after injection (14 days after surgery) the ChABC group (8.12) show significant increase in BBB Score compared with the control group (1.50) (P<0.001) and this increment was maintained until 8th weeks. In hADSCs group until 3th weeks after injection (28 days after surgery) BBB Score was not shown significant difference compared with control group but at 3th weeks after injection, hADSCs group (8.67) shown significant increase in BBB Score compared with the control group (3.50) (P<0.01) and BBB Score was increased gradually until 8th weeks after injection (63 days after surgery) (Figure 3).
Figure 3.
The graph shows mean BBB after SCI until 9 weeks. Significant difference between Ch ABC and Control groups started At once week after injection (14 days after surgery) (*P<0.001). Significant difference between hADSCs and Control groups started At third week after injection (28days after surgery) (**P<0.01).At eighth week after injection (63 days after surgery) there was significant difference between Ch ABC, hADSCs and Control groups(▼P<0.01)
Histology and Confirmation presence of hADSCs in tissue
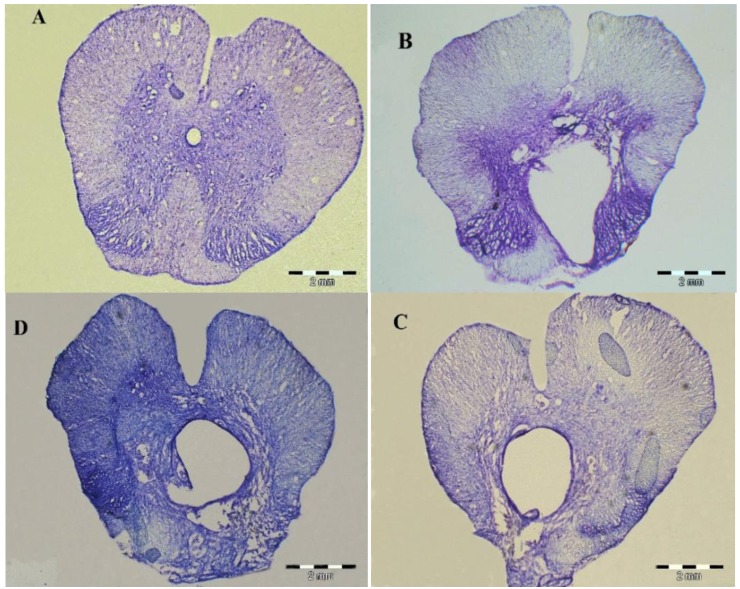
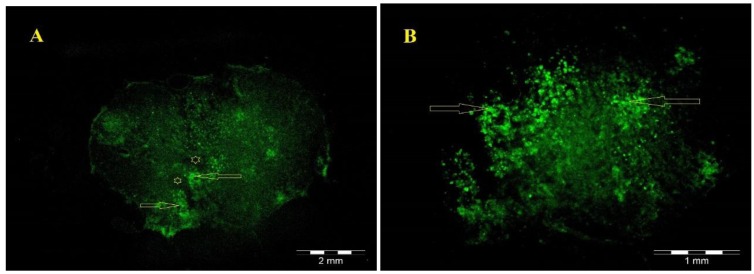
For assessment of changes in cavity volume, the host spinal cord tissue was stained with cresyl violet. Non-treated animals with SCI showed the formation of large cavities (Figure 4B). The spinal cords of treated animals had cavities much smaller than those of non- treated animals (Figure 4C, D). These results showed that hADSCs transplant and Chondroitinase ABC injection reduced the formation of cavities after contusion model of spinal cord injury. Fluorescence microscopy (Olympus AX 70) reveals that GFP-positive hADSCs, transplanted at the site of injury, survived and reorganized around the cavity center (Figure 5).
Figure 4.
10 µm thick cross sections of spinal cord segments T8-9 of sham operative group (A), control group (B), hADSCs group (C) and ChABC group (D) at 8 weeks after surgery. Spinal cord tissue was stained with Cresyl violet. A large cavity was shown from non treated animals. The cavity formation was reduced in treated animals
Figure 5.
(A) Low magnification of the GFP-positive hADSCs (green) located within the lesion 8 weeks after transplantation. (B) hADSCs in A are shown at higher magnification. Stars indicates the cystic cavity and arrow indicates GFP-positive hADSCs that migrating into the host tissue around the cystic cavity
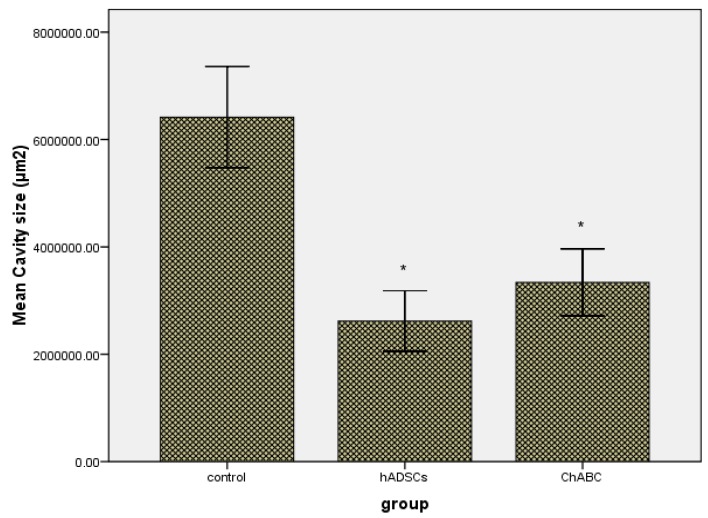
Cavity size
The spinal cords of hADSCs and ChABC-injected group had cavities much smaller than those of the control group (Figures 4C, D). In the hADSCs-injected rats showed a cavity size of 2618461.44 µm2 and ChABC-injected rats showed a cavity size of 3338041.09 µm2 on average, whereas the control rats showed a value of 6417159.48 µm2 on average. These values of cavity volume were significantly different between the hADSCs, ChABC-injected groups and control rats (P<0.001)(Figure 6).
Figure 6.
These graph show the difference in size of cavities between the hADSCs, ChABC-injected and control groups at 8 weeks after injection. The values of the cavity size were significantly different between the hADSCs, ChABC-injected and control groups (*P<0.001)
Discussion
The most of the human spinal cord injury during the accident, falling down and Sports Injuries occurs due to contusion. Damage to the vertebrae column and eventually inserting bone or inter vertebrae discs into the spinal canal space, is the main cause of the SCI (23). Therefore, in this study due to the common natural models of the SCI, contusion model is used. In most cases progressive necrosis of spinal cord tissue is caused formation of cavity (24-26). Also, in this study cavity was formed at SCI site (Figure 3). Flux of inflammatory cells causes a fluid-filled cavity or cyst that this process is known as secondary injury. Secondary injury caused the activation and proliferation of astrocytes that is able to cover holes or cysts with glial scar. The glial scar as an inhibitory factor, degeneration of myelin, prevents proper nerve conduction to correct path (23). Axonal regeneration with reduction of cavity size as a treatment strategy can improve motor function in SCI (27-29).
In this study, the groups treated with hADSCs and ChABC, the Cavity volume was significantly decreased compared to controls. Also in treatment groups, BBB score shows the significant increment rather to control group that Cavity volume in treatment groups is consistent with increased BBB score. This study shows that implantation of hADSCs leads to improvement of locomotor function by reduction in volume of cavity.
Presence of hADSCs in the injury site at least in 8 weeks, causes in volume cavity reduction (30, 31). Fluorescence microscopy confirms the presence of cells in the end of 8th weeks. Other studies are also reported cavity volume loss after SCI with transplantation of cells such as bone marrow stromal cells (BMSCs) (24, 32, 33) and neural progenitor cells (NPCs) (34, 35). Like to BMSCs (24, 32, 33) and NPCs (34, 35), hADSCs (36) cannot differentiate totally into neurons after transplanting into the injured spinal cord and remain undifferentiated. According to these explanations, BBB score increase and improve motor function in hADSCs maybe not due to neuronal differentiation of hADSCs but may be due to the production of useful growth factors for neural tissue. Cytokine secretion by hADSCs, such as interleukins, stem cell factor (36, 37), NGF and BDNF (38) have been reported. Glial cell proliferation and in addition axonal growth at the site of SCI can reduce Cavity volume. Cavity volume reduction maybe not related to a specific factor, but the result is the accumulation of several factors (39).
One of the therapeutic strategies for SCI is dealing with chondroitin sulfate proteoglycans in order to prevent the formation of glial scar because this inhibitory environment is one of the barriers for axonal growth and recovery of motor function. Attenuation the role of chondroitin sulfate proteoglycans leads to wide range of axonal regeneration cascade and consequently restoring nerve regeneration in injured site. ChABC degrades glial scar (40), particularly chondroitin sulfate proteoglycans, reduces expression of glial fibrillary acidic protein (41) in injured site and in this way reduces inappropriate microenvironment in injured site. Field studies showed that this enzyme, ChABC, significantly increases the expression of growth-associated protein-43 and significantly reduces the extent of necrotic area containing Cavity (41) and improves motor function in SCI (18). Therefore in this study ChABC was used to counters the Neuro-inhibitory environment. The results of present study also confirm findings of previous studies in such a way that significant cavity volume reduction and motor function improvement was observed in ChABC group. One week after SCI, astrocytes and glial scarring at the site of a lesion creates a Neuro-inhibitory environment where become one of the major challenges for therapeutic interventions in SCI. Currently, the most common intervention in the treatment of SCI is cell therapy in such a way that inappropriate microenvironment does not take into consideration adequately. The aim of our studyis comparing of motor function improvement in adopting of two treatment strategies for SCI, containing counter with inappropriate microenvironment and cell therapy in lesion site.
Conclusion
Our results shows dealing with Neuro-inhibitory environment by ChABC causes reduction in cavity volume and motor function improvement is equal to cell therapy with hADSCs. Innovation of this study regarding its results is that dealing with inappropriate Neuro-inhibitory environment and glial scar by ChABC have equal role compare to cell therapy for improving motor function after SCI and this result in adoption of proper therapeutic strategies for SCI intervention is important.
It is suggested that, Differentiation of transplanted cells into mature neurons also synapse of them with endogenous neurons to be investigated, Locomotor Function tests to be performed at 16 weeks and Scar changes at the cellular and molecular level tobe investigated.
Acknowledgment
The results described in this paper were part of student thesis. The present study was supported by a grant from Iran University of Medical Sciences and was performed in department of anatomy. The cell culture stage was performed at Cellular and Molecular Research Center of Iran University of Medical Sciences. The Sectioning and staining of tissue sections part of this research was performed at Department of Medical basic Sciences at Iran University of Medical Sciences so we express our deep thanks to Dr Behnam Jameie for her help.
References
- 1.Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival migration axon association nd functional recovery. Glia . 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 2.Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002; 26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 3.Dietz V, Curt A. Neurological aspects of spinal-cord repair: promises and challenges. Lancet neurol . 2006;5:688–694. doi: 10.1016/S1474-4422(06)70522-1. [DOI] [PubMed] [Google Scholar]
- 4.Kurnellas MP, Nicot A, Shull GE, Elkabes S. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. FASEB J. 2005; 19:298–300. doi: 10.1096/fj.04-2549fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006; 23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- 6.Von Euler M, Seiger A, Sundstrom E. Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp Neurol. 1997; 145:502–510. doi: 10.1006/exnr.1997.6481. [DOI] [PubMed] [Google Scholar]
- 7.Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res. 2002; 68:315–322. doi: 10.1002/jnr.10215. [DOI] [PubMed] [Google Scholar]
- 8.Jung K, Min DS, Sim KB, Ahn M, Kim H, Cheong J, et al. Upregulation of phospholipase D1 in the spinal cords of rats with clip compression injury. Neurosci Lett. 2003; 336:126–130. doi: 10.1016/s0304-3940(02)01155-2. [DOI] [PubMed] [Google Scholar]
- 9.Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol. 2004; 12:343–358. doi: 10.1016/j.trim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006; 20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 11.Cummings BJ, Uchida N, Tamaki SJ, Anderson AJ. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol Res. 2006; 28:474–481. doi: 10.1179/016164106X115116. [DOI] [PubMed] [Google Scholar]
- 12.Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011; 344:251–260. doi: 10.1007/s00441-011-1142-5. [DOI] [PubMed] [Google Scholar]
- 13.Dyer JK, Bourque JA, Steeves JD. The role of complement in immunological demyelination of the mammalian spinal cord. Spinal cord. 2005;43:417–425. doi: 10.1038/sj.sc.3101737. [DOI] [PubMed] [Google Scholar]
- 14.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? . Nat Rev Neurosci. 2006; 7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004; 5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 16.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin- reactive glia- and scar-derived CNS axon growth inhibitors: expression receptor signaling and correlation with axon regeneration. Glia . 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 17.Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003; 23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature . 2002;4164:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 19.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006; 26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramer LM, Ramer MS, Steeves JD. Setting the stage for functional repair of spinal cord injuries: a cast of thousands. Spinal cord . 2005;43:134–161. doi: 10.1038/sj.sc.3101715. [DOI] [PubMed] [Google Scholar]
- 21.Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008; 449:69–79. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- 22.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 23.Kundi S, Bicknell R, Ahmed Z. Spinal Cord Injury: current Mammalian Models. Am J Neurosci. 2013;4:1–12. doi: 10.1016/j.neures.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002; 99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999; 19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Krebs CJ, Guth L. Experimental analysis of progressive necrosis after spinal cord trauma in the rat: etiological role of the inflammatory response. Exp Neurol. 1997; 143:141–152. doi: 10.1006/exnr.1996.6355. [DOI] [PubMed] [Google Scholar]
- 27.Michele Basso D, Murray M, Goldberger ME. Differential recovery of bipedal and overground locomotion following complete spinal cord hemisection in cats. Restor Neurol Neurosci. 1994; 7:95–110. doi: 10.3233/RNN-1994-7205. [DOI] [PubMed] [Google Scholar]
- 28.Edgerton VR, Roy RR, Hodgson JA, Prober RJ, de Guzman CP, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J Neurotrauma. 1992; 9:S119–128. [PubMed] [Google Scholar]
- 29.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987; 412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 30.Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004; 190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 31.Lee TH. Transplantation of human adipose-derived stromal cells promotes functional recovery of rat spinal cord injury. Korean J Anat . 2005;38:461–468. [Google Scholar]
- 32.Wu S, Suzuki Y, Ejiri Y, Noda T, Bai H, Kitada M, et al. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res. 2003; 72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- 33.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000; 11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 34.Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, et al. Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryonic rat spinal cord. Brain Res. 2000; 874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998; 176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Eaves CJ , Cashman JD , Kay RJ , Dougherty GJ , Otsuka T , Gaboury LA , et al. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in longterm human marrow cultures. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood . 1991;78:110–117. [PubMed] [Google Scholar]
- 38.Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002; 69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 39.Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004; 187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008; 25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, He X, Li H, Wang G. Chondroitinase ABC plus bone marrow mesenchymal stem cells for repair of spinal cord injury. Neural Regen Res . 2013;8:965–974. doi: 10.3969/j.issn.1673-5374.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]