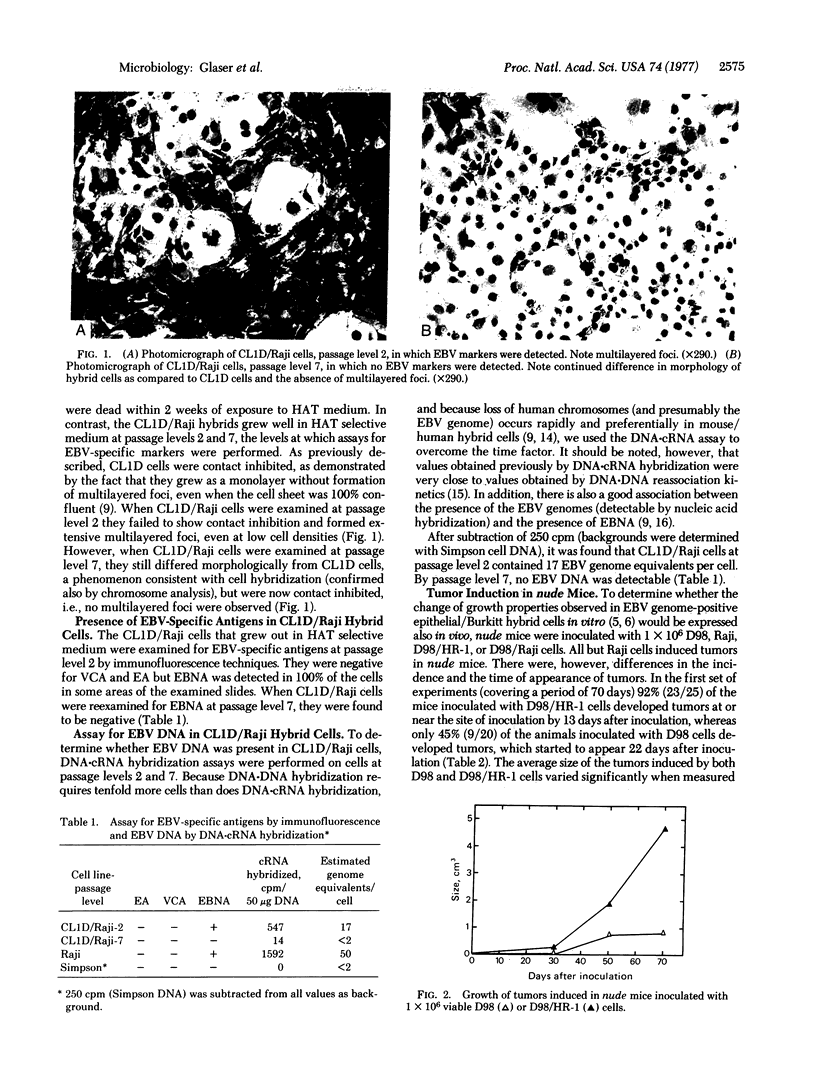
Abstract
Studies were made of the expression of the Epstein-Barr virus (EBV) in somatic hybrids of Burkitt tumor cells and human or mouse cells to determine whether EBV genetic information associated with the capacity to transform leukocytes of human and non-human primates could be maintained and expressed in nonlymphoblastoid cells. Data obtained thus far suggest that at least one characteristic associated with cellular transformation (loss of contact inhibition) is expressed only in nonlymphoblastoid cells in which the EBV genome is maintained. In addition, we have demonstrated that human epithelial/Burkitt hybrid cells (D98/HR-1 and D98/Raji) are more oncogenic in nude (athymic) mice than are cells of the human epithelial parental line, D98, or one of the Burkitt lymphoblastoid parent cell lines (Raji); the HR-1 Burkitt parent cell line was as oncogenic as the hybrid cell lines but the time required to induce tumors was much longer. Thus, human epithelial cells show alteration of growth properties in vitro and in vivo after cellular hybridization with Burkitt tumor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desgranges C., Wolf H., De-Thé G., Shanmugaratnam K., Cammoun N., Ellouz R., Klein G., Lennert K., Muñoz N., Zur Hausen H. Nasopharyngeal carcinoma. X. Presence of epstein-barr genomes in separated epithelial cells of tumours in patients from Singapore, Tunisia and Kenya. Int J Cancer. 1975 Jul 15;16(1):7–15. doi: 10.1002/ijc.2910160103. [DOI] [PubMed] [Google Scholar]
- Glaser R., Decker B., Farrugia R., Shows T., Rapp F. Growth characteristics of Burkitt somatic cell hybrids in vitro. Cancer Res. 1973 Sep;33(9):2026–2029. [PubMed] [Google Scholar]
- Glaser R., Farrugia R., Brown N. Effect of the host cell on the maintenance and replication of Epstein-Barr virus. Virology. 1976 Jan;69(1):132–142. doi: 10.1016/0042-6822(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Glaser R., Nonoyama M., Decker B., Rapp F. Synthesis of Epstein-Barr virus antigens and DNA in activated Burkitt somatic cell hybrids. Virology. 1973 Sep;55(1):62–69. doi: 10.1016/s0042-6822(73)81008-6. [DOI] [PubMed] [Google Scholar]
- Glaser R., Nonoyama M. Epstein-Barr virus: detection of genome in somatic cell hybrids of Burkitt lymphoblastoid cells. Science. 1973 Feb 2;179(4072):492–493. doi: 10.1126/science.179.4072.492. [DOI] [PubMed] [Google Scholar]
- Glaser R., Nonoyama M. Host cell regulation of induction of Epstein-Barr virus. J Virol. 1974 Jul;14(1):174–176. doi: 10.1128/jvi.14.1.174-176.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., O'Neill F. J. Hybridization of Burkitt lymphoblastoid cells. Science. 1972 Jun 16;176(4040):1245–1247. doi: 10.1126/science.176.4040.1245. [DOI] [PubMed] [Google Scholar]
- Glaser R., Rapp F. Rescue of Epstein-Barr virus from somatic cell hybrids of Burkitt lymphoblastoid cells. J Virol. 1972 Aug;10(2):288–296. doi: 10.1128/jvi.10.2.288-296.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., de Thé G., Lenoir G., Ho J. H. Superinfection epithelial nasopharyngeal carcinoma cells with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):960–963. doi: 10.1073/pnas.73.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Huang D. P., Ho J. H., Henle W., Henle G. Demonstration of Epstein-Barr virus-associated nuclear antigen in nasopharyngeal carcinoma cells from fresh biopsies. Int J Cancer. 1974 Nov 15;14(5):580–588. doi: 10.1002/ijc.2910140504. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Nonoyama M., Pagano J. S. Reassociation kinetics for Epstein-Barr virus DNA: nonhomology to mammalian DNA and homology of viral DNA in various diseases. J Virol. 1973 Nov;12(5):1006–1012. doi: 10.1128/jvi.12.5.1006-1012.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Giovanella B. C., Lindahl T., Fialkow P. J., Singh S., Stehlin J. S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Wiener F., Zech L., zur Hausen H., Reedman B. Segregation of the EBV-determined nuclear antigen (EBNA) in somatic cell hybrids derived from the fusion of a mouse fibroblast and a human Burkitt lymphoma line. Int J Cancer. 1974 Jul 15;14(1):54–64. doi: 10.1002/ijc.2910140108. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Trumper P. A., Epstein M. A., Giovanella B. C. Activation in vitro by BUdR of a productive EB virus infection in the epithelial cells of nasopharyngeal carcinoma. Int J Cancer. 1976 May 15;17(5):578–587. doi: 10.1002/ijc.2910170505. [DOI] [PubMed] [Google Scholar]
- Weiss M. C. Further studies on loss of T-antigen from somatic hybrids between mouse cells and SV40-transformed human cells. Proc Natl Acad Sci U S A. 1970 May;66(1):79–86. doi: 10.1073/pnas.66.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., zur Hausen H., Becker V. EB viral genomes in epithelial nasopharyngeal carcinoma cells. Nat New Biol. 1973 Aug 22;244(138):245–247. doi: 10.1038/newbio244245a0. [DOI] [PubMed] [Google Scholar]