Abstract
This review is intended to provide an updated role of molecular genetics and various targeted therapies that have been developed to treat advanced stages of melanoma. Because of the declining success in melanoma therapy, the curative treatment for advanced stage melanoma has been a challenge for clinicians. Several mutations such as N-RAS, p53, BRAF including mutant-BRAF that lead to activation of kinase pathway, are implicated in the development of malignant melanoma. However, the current literature depicts that the prognostic role of BRAF mutation in disease progression is still controversial. While its higher level in advanced stage disease is associated with decreased overall survival (OS), some studies show that it failed to confer as an independent prognostic predictor of the disease. This has also led researchers to accomplish newer therapeutic strategies that lead to improved disease-response and grant survival benefits. Vemurafenib, a BRAF inhibitor agent, is one of the few available targeted therapies that is FDA approved and provides promising results in metastatic disease. However, its resistance at an early stage is of great concern. Recent implementation of combinational therapies including “targeted therapy”, immunotherapy, and biological agents has appealed many researchers to define the adjunctive role of available therapies and their limitations in advanced stage and metastatic melanoma. This commends the need for future multi-institutional studies to confirm the clinical validity of different therapeutic strategies on a large scale population.
Keywords: Malignant melanoma, disease progression, mutations, vemurafenib, immunotherapy
Introduction
A wide variety of BRAF mutations have been observed in human cancers. Over 30 somatic mutations in the BRAF gene have been identified in cancers such as melanoma, colorectal cancers, papillary thyroid cancer, breast and lung cancers (1,2). Inherited BRAF gene mutations cause birth defects of the heart and face and can affect cognitive development. In 2002, Davies et al. reported somatic BRAF as a mutated target that activates kinases. It was detected in 66% of malignant melanomas and other human cancers such as colorectal cancers, lung cancers and ovarian cancers (2). This finding prompted novel insights toward effective therapies against these cancers, including metastatic melanoma. Malignant melanoma, the most aggressive skin cancer, majorly affects young adults. Primary cutaneous melanoma can be cured by effective surgical resection. However, one of the major concerns occurs when the tumor displays visceral spread. Metastatic disease proves to be fatal, with long-term survival rates of less than a year (3,4). BRAF-mutated melanoma has recently cumulated a great interest in the field of oncology. One of the earliest trials with dacarbazine, an alkylating agent, showed no survival benefits in patients with advanced metastatic disease. The development of BRAF inhibitors may lead to a potential therapy to overcome resistance in advanced stages of melanoma.
Despite the proven involvement of BRAF in melanoma confirmed by various studies, its role as a prognostic marker remains unclear (5-7). Various clinicopathological parameters such as thickness, mitotic rate and ulceration are helpful in determining the risk of recurrence of the disease (8). With the aim to determine the overall survival (OS) and disease-free survival (DFS) in patients with advanced stage melanoma harboring BRAF mutations, a large number of clinical trials were conducted. Recently published series have compared BRAF V600E mutated melanoma with wild-type BRAF. They concluded that OS and DFS were lower for BRAF V600E than the wild-type BRAF (9). We conducted a review of literature to determine the prognostic role of BRAF mutations in advanced stage melanoma.
BRAF at the molecular level
Multiple factors combine to make the management of melanoma a great challenge to clinicians. Clinical factors, genetic aberrations and response to standard chemotherapeutic agents have not been able to definitively predict tumor recurrence or survival benefit (9). A wide variety of genomic aberrations are seen frequently in melanoma, such as N-RAS, p53 and p16INK4a, of which BRAF contributes to a majority of the mutations in the disease. It is estimated that BRAF mutation is present in approximately 50-60% of cutaneous melanomas. BRAF, a proto-oncogene, belongs to the family of growth signal transduction RAF kinases (6,7). It is responsible for regulation of the mitogen-activated protein (MAP) kinases pathway that mediates cell division, differentiation and secretion. Most BRAF mutations result from single point mutation with valine (V) being substituted for by glutamic acid (E) at codon 600 (BRAF V600E) (5,6,10). This substitution leads to elevated levels of BRAF that further stimulate extracellular regulated kinase (ERK) activity. The altered growth programming of cells results from accumulated genetic mutations which ultimately induce cancer formation (Figure 1). Mutant BRAF also produces a number of immunosuppressive factors that further favor tumor growth (11). The oncogenic role of the mutant form of BRAF in melanoma cell lines has been further confirmed by various authors (2,6,7). Moreover, circulating methylated DNA that carries BRAF mutations has been hypothesized to predict disease recurrence and response to chemotherapy (12) in melanoma patients.
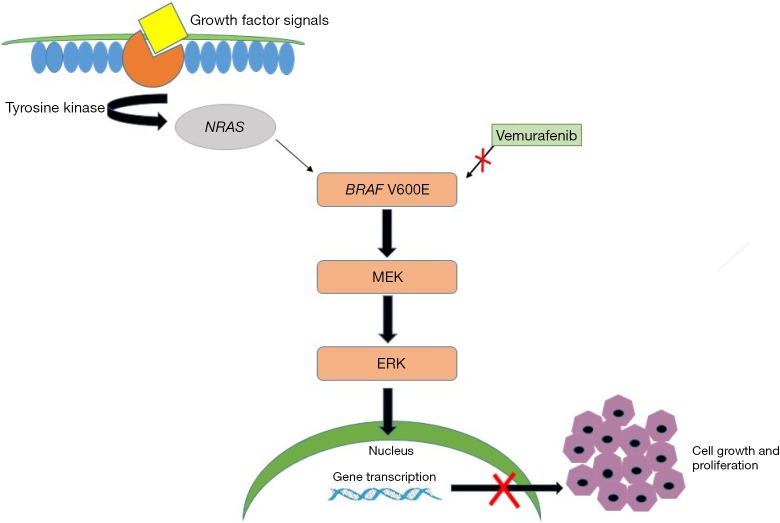
Figure 1.
Image displaying signaling pathways triggered by binding of growth factors to tyrosine kinase receptor that triggers RAS, RAF, MAPK and ERK pathways leading to cell growth and proliferation. Mutations in BRAF (V600E) which can lead to accelerated cell growth and cancer formation of melanoma cells. Inhibition of mutant BRAF by Vemurafenib (red cross) in the melanoma cells shuts down the signaling pathway causing tumor regression following cell apoptosis, tumor antigen expression and decreased release of cytokines and VEGF. MAPK, mitogen-activated protein kinase; ERK, extracellular-signal regulated kinase; EGF, vascular endothelial growth factor.
Targeted chemotherapy in melanoma
After initially frustrating results to target BRAF in melanoma, extensive clinical trials led to the advent of BRAF inhibitors. The discovery of targeted chemotherapy for melanoma has emerged as a milestone development in oncological research. One of the first drugs that was developed in RAF-targeted agents in melanoma was sorafenib (13). However, the responses were suboptimal due to its principal inhibitory effect on tyrosine kinase and limited ability to target RAF-1. This led to the innovation of newer drug therapies that could selectively inhibit mutant forms of BRAF (14-17). Vemurafenib, a drug that selectively acts on mutant-BRAF, inhibits ERK phosphorylation, leading to programmed cell death in melanoma cell lines. In 2011, because of the potential efficacy of Vemurafenib (earlier known as PX4032) towards melanoma, it received US Food and Drug Administration (FDA) approval (18). Other selective inhibitors such as dabrafenib and trametinib also selectively target BRAF. This novel discovery acted as an impetus to understand the detailed underlying molecular and genetic alterations that further impart resistance to anti-cancer therapy. A phase III clinical trial was conducted to compare vemurafenib with dacarbazine in metastatic melanoma patients with the BRAF V600E mutation. In addition to overall and progression-free survival, response rate, response duration and safety of the drug were also evaluated. They concluded that vemurafenib produced a significantly increased OS rate of 84%, with a 63% relative reduction in the risk of death from disease, while dacarbazine was associated with an OS of 64% (14). A newer concept has been established recently where liver-X nuclear hormone receptor (LXR) acts as a therapeutic target in malignant melanoma. LXRβ works by enhancing transcription of tumor and stromal Apo-lipoprotein E (apoE) which further suppresses the progression and metastatic activity of the disease (17). Pencheva et al. investigated the therapeutic role of LXR agonists in a genetically driven mouse model. Administration of oral LXR agonist agents was shown to decrease lymph node metastases and significantly increase OS in melanoma cell lines (17).
The ability of melanoma to effectively respond to distinct modes of immunotherapy has attracted a broad audience for combinational therapy. In general, immunotherapy alone with high-dose interleukin-2, adoptive cell transfer therapy (ACT) and anti-CTLA4 agents have been proven beneficial in advanced stages of melanoma (19-21). However, their association with an increased number of adverse events precludes their use in all but a small subset of patients. The rapidly metastasizing nature of malignant melanoma can be disastrous. Therefore, combination therapy of BRAF inhibitors and immunotherapy can be of paramount importance in achieving durable destruction of tumor cells and prolonging survival in patients. There has been an increasing trend to perform trials of this combination therapy in metastatic disease (11,22-25). The addition of MEK inhibition to BRAF prevents MAPK reactivation, up regulates melanocyte differentiation antigen expression, and increases recruitment of tumor-infiltrating lymphocytes (TILs). Furthermore, this inhibitory mechanism makes the tumor niche favorable to T-lymphocytes by decreasing the immunosuppressive factors IL-6 and -10, and vascular endothelial growth factor. It also leads to a reduction in the number of drug toxicities and decreased development of secondary cutaneous malignancies linked to use of these agents. An added advantage of this strategy is the reduction in paradoxical activation of BRAF-wild type. However, side effects such as hepatotoxicity and severe skin rash have been observed with this combination (26). Flaherty and colleagues conducted phase 1 trials using combination therapy with dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) for metastatic melanoma. They compared this combination to monotherapy with dabrafenib. Progression-free survival with combination and monotherapy were 9.4 and 5.8 months, respectively. Moreover, it was also observed that the response rate with combination treatment (76%) was significantly higher than monotherapy (54%) (26).
Prognostic implications of BRAF
The bleak prognosis of advanced stage melanoma has led various researchers to determine the factors that result in failure of targeted therapy, decreased response rate, and recurrence of disease. In addition to various other prognostic factors such as age, gender, ECOG status, metastatic sites, and LDH levels, the detection of BRAF status post-chemotherapy plays a critical role in determining prognosis. Though this topic is disputable, authors are still in search of finding profound clinical utility with respect to disease progression. Shinozaki and colleagues studied the prognostic effect of mutant-BRAFV600E in patients receiving chemotherapy for melanoma (12). Their study concluded that circulating mutant-BRAF was significantly associated with decreased OS of 13 months compared to 30.6 months in those who did not possess mutant-BRAF. They also showed that 70% of patients in the non-responder group retained BRAF mutations compared to 10% in the responder group. Another study by Ardekani and colleagues revealed similar results (27). It was observed that higher BRAF expression was associated with significantly poor OS in primary melanoma patients. Additionally, high BRAF expression showed a significant correlation with thickness and ulceration of the tumor and higher AJCC stages. In contrast, some authors concluded that although BRAF was observed in a higher proportion of tumors, it failed to influence OS in melanoma (8,28). Results of Ugurel and colleagues were in concordance where mutant-BRAF was associated with decreased OS; however, the results were insignificant. Thus, it was concluded that BRAF did not confer an independent prognostic factor of OS (29).
Although BRAF does not show significant correlation with other prognostic markers of disease progression, its role in the determination of survival benefits still warrants continued interest. It is important to clarify the distinct genetic mechanisms that are associated with disease progression rendering the potential therapy ineffective in controlling metastatic disease. To validate the efficacy of BRAF mutation in the determination of poor survival outcomes, it becomes imperative to identify other biochemical markers in order to confirm the prognostic role of BRAF.
Current trends and limitations of targeted therapy
Currently, adjuvant biological agents such as interferon-α (IFN-α) have outsourced remote therapies such as radiotherapy and immune-modulator agents in the treatment of advanced stage melanoma (25). This transitioned use of adjuvant therapy has provided significant survival benefits to melanoma patients. However, the related toxicities and cost of the therapy impede their use in many countries. The use of chemotherapeutic agents has yielded optimal benefits in metastatic melanoma. Targeted inhibition of the MAP/ERK cascade has gained great popularity in the field. Despite the beneficial performance of chemotherapeutic agents against melanoma, their limitations have impacted a large group of the population. The use of BRAF inhibitors is associated with a diverse side effect profile, most commonly nausea, fatigue, rash, arthralgia, and alopecia. It has also been reported to cause a photosensitivity reaction that can be prevented by following sun-exposure protective measures (26). Another major toxicity that is of critical concern with administration of drugs like vemurafenib is the development of keratocanthomas and/or squamous cell carcinoma. Authors have reported an 18-20% incidence of cutaneous malignancies that were treated with simple surgical resection (14,22). Another obstacle with the use of Vemurafenib is its acquired secondary resistance, which has been well established in various clinical trials (30). There have been several proposed mechanisms of resistance to BRAF inhibitors. The general concept of a “gatekeeper” mutation that prevents binding of the drug to the targeted oncogene does not confer resistance to BRAF targeted agents. Though this question remains unanswered, various studies have revealed distinct mechanisms that play critical roles in tumor progression (Figure 2).
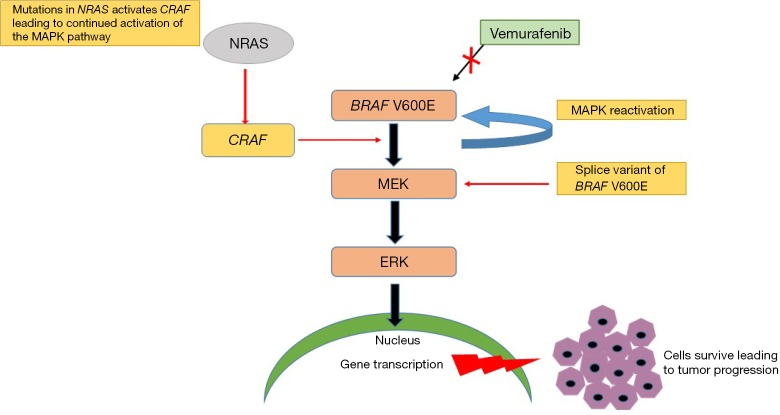
Figure 2.
Image displaying multiple hypotheses for acquired BRAF inhibition resistance to chemotherapeutic agents (BRAF inhibitors) and mechanisms causing MAPK pathway reactivation. This process further leads to tumor relapse thus suggesting trials of combinational therapy (BRAF inhibitors, MEK inhibitors and/or Immunotherapy) at different levels of the pathway to prevent tumor progression. MAPK, mitogen-activated protein kinase.
❖ Multiple studies have suggested that the primary mechanism of resistance is due to MAPK pathway reactivation. It has also been observed to be due to sustained mutant-BRAF presence during tumor progression (30);
❖ De novo activation of the MAPK pathway via oncogenic mutated NRAS has been observed in some trials (31,32). The continued activation of CRAS causes NRAS to evade BRAF inhibition, resulting in enhanced activation of MEK and ERK;
❖ A variant of BRAFV600E, splice BRAFV600E, also results in acquired resistance to selective BRAF inhibitors through RAF dimerization (15,16).
The presence of these factors can lead to tumor progression and decrease the efficacy of the drug. It has been observed that approximately 10% of patients will have tumor progression post-therapy, and a majority of those will lead to tumor prolapse within a year. Interestingly, recent results have emerged that also contribute to offering resistance to Vemurafenib in both ERK dependent and independent pathways. A recent study by Boussemart and group confirmed the role of the eIF4F complex in mutant-BRAF melanoma resistance and metastases (33). The eIF4F complex is a eukaryotic translational initiation complex, persistent formation of which is associated with resistance to treatment. Their findings also confirmed that the formation of this complex is increased in cases where metastasis has occurred, and decreased in tumors that respond well to Vemurafenib therapy. Therefore, it is suggested that these therapeutic targets can convene a rationale for treatment of metastatic as well as drug-resistant mutant-BRAF melanoma. Recently, Sun and colleagues have examined the idea of reversing acquired BRAF-resistance in melanoma (34). This is a strikingly new finding where authors have discussed the upregulated expression of epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor β (PDGFRB) only in the presence of anti-BRAF and anti-MEK drug treatment resistance. The acquired expression of EFGR is due to activation of TGF-β (due to suppressed levels of SOX10) after resistance to BRAF/MEK inhibitors in melanoma, which was observed in approximately 37% of EGFR-positive melanoma samples. However, it was noted that the higher expression of EGFR (low SOX10) was reversed on discontinuation of the drug. Thus, interruption in treatment causes an upsurge in SOX10 expression and, subsequently, increases drug sensitivity. This evidence indicates that BRAF-inhibitor resistant melanoma patients with EGFR expression can be conveniently re-treated with the same drugs after a “drug holiday” period.
Conclusions
After years of continued research, no single therapy has been found to improve the survival rate of metastatic melanoma. Although adjuvant therapy with IFN-α has provided survival benefit in high-risk cases, its adverse side effect profile is of great concern. Targeted BRAF chemotherapeutic agents have upstaged the management of cutaneous melanomas; however, these offer a palliative benefit to patients in advanced disease. Resistance to these agents disrupts management strategies. Because the role of BRAF has not been definitively correlated with the progression of the disease, it has become essential to clarify the mechanisms that are responsible for progression, relapse and recurrence. The drought of substantial evidence for the prognostic role of BRAF in metastatic melanoma opens areas of clinical trials to investigate newer prognostic markers. Moreover, it has been suggested that trials using combinational therapies such as BRAF inhibitors combined with biological agents such as IL-2, anti-angiogenic agents such as bevacizumab, and immunotherapy, could prove beneficial to halt the progression of metastatic melanoma.
Acknowledgements
Authors’ contributions: Bhatia P performed literature search and wrote the paper; Zakaria AE provided necessary analytical tools; Friedlander P and Kandil E analyzed and revised the content of the paper; Loula Burton edited and proofread the language.
Disclosure: The authors declare no conflict of interest.
References
- 1.Dadu R, Shah K, Busaidy NL, et al. Efficacy and Tolerability of Vemurafenib in Patients with BRAF (V600E) -Positive Papillary Thyroid Cancer: M.D. Anderson Cancer Center Off Label Experience. J Clin Endocrinol Metab 2015;100:E77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [DOI] [PubMed] [Google Scholar]
- 5.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 1997;9:180-6. [DOI] [PubMed] [Google Scholar]
- 6.Wellbrock C, Karasarides M, Marais R.The RAF proteins take centre stage. Nat Rev Mol Cell Biol 2004;5:875-85. [DOI] [PubMed] [Google Scholar]
- 7.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004;116:855-67. [DOI] [PubMed] [Google Scholar]
- 8.Edlundh-Rose E, Egyházi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471-8. [DOI] [PubMed] [Google Scholar]
- 9.Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol 2012;19:4314-21. [DOI] [PubMed] [Google Scholar]
- 10.Garnett MJ, Rana S, Paterson H, et al. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell 2005;20:963-9. [DOI] [PubMed] [Google Scholar]
- 11.Hu-Lieskovan S, Robert L, Homet Moreno B, et al. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol 2014;32:2248-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinozaki M, O'Day SJ, Kitago M, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res 2007;13:2068-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer 2006;95:581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArthur GA, Ribas A, Chapman PB, et al. Molecular analyses from a phase I trial of vemurafenib to study mechanism of action (MOA) and resistance in repeated biopsies from BRAF mutation–positive metastatic melanoma patients (pts). J Clin Oncol 2011;29:abstr 8502.
- 16.Nathanson KL, Martin A, Letrero R, et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol 2011;29:abstr 8501. [DOI] [PMC free article] [PubMed]
- 17.Pencheva N, Buss CG, Posada J, et al. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell 2014;156:986-1001. [DOI] [PubMed] [Google Scholar]
- 18.Bollag G, Tsai J, Zhang J, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 2012;11:873-86. [DOI] [PubMed] [Google Scholar]
- 19.Forget MA, Malu S, Liu H, et al. Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma. J Immunother 2014;37:448-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao MZ, Zhou WY, Du XL, et al. Novel anti-melanoma treatment: focus on immunotherapy. Chin J Cancer 2014;33:458-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pretto F, Elia G, Castioni N, et al. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother 2014;63:901-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberholzer PA, Kee D, Dziunycz P, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 2012;30:316-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salas Fragomeni RA, Chung HW, Landesman Y, et al. CRM1 and BRAF inhibition synergize and induce tumor regression in BRAF-mutant melanoma. Mol Cancer Ther 2013;12:1171-9. [DOI] [PubMed] [Google Scholar]
- 24.Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011;16:5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grazia G, Penna I, Perotti V, et al. Towards combinatorial targeted therapy in melanoma: from pre-clinical evidence to clinical application (review). Int J Oncol 2014;45:929-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol 2013;169:320-8. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski P, Gos A, Jurkowska M, et al. Molecular alterations in clinical stage III cutaneous melanoma: Correlation with clinicopathological features and patient outcome. Oncol Lett 2014;8:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugurel S, Thirumaran RK, Bloethner S, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS One 2007;2:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014;14:455-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 2008;68:4853-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan FM, Shao Y, Mayberry MM, et al. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene 2011;30:366-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boussemart L, Malka-Mahieu H, Girault I, et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 2014;513:105-9. [DOI] [PubMed] [Google Scholar]
- 34.Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014;508:118-22. [DOI] [PubMed] [Google Scholar]