Summary
Background
Statins increase the risk of new-onset type 2 diabetes mellitus. We aimed to assess whether this increase in risk is a consequence of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), the intended drug target.
Methods
We used single nucleotide polymorphisms in the HMGCR gene, rs17238484 (for the main analysis) and rs12916 (for a subsidiary analysis) as proxies for HMGCR inhibition by statins. We examined associations of these variants with plasma lipid, glucose, and insulin concentrations; bodyweight; waist circumference; and prevalent and incident type 2 diabetes. Study-specific effect estimates per copy of each LDL-lowering allele were pooled by meta-analysis. These findings were compared with a meta-analysis of new-onset type 2 diabetes and bodyweight change data from randomised trials of statin drugs. The effects of statins in each randomised trial were assessed using meta-analysis.
Findings
Data were available for up to 223 463 individuals from 43 genetic studies. Each additional rs17238484-G allele was associated with a mean 0·06 mmol/L (95% CI 0·05–0·07) lower LDL cholesterol and higher body weight (0·30 kg, 0·18–0·43), waist circumference (0·32 cm, 0·16–0·47), plasma insulin concentration (1·62%, 0·53–2·72), and plasma glucose concentration (0·23%, 0·02–0·44). The rs12916 SNP had similar effects on LDL cholesterol, bodyweight, and waist circumference. The rs17238484-G allele seemed to be associated with higher risk of type 2 diabetes (odds ratio [OR] per allele 1·02, 95% CI 1·00–1·05); the rs12916-T allele association was consistent (1·06, 1·03–1·09). In 129 170 individuals in randomised trials, statins lowered LDL cholesterol by 0·92 mmol/L (95% CI 0·18–1·67) at 1-year of follow-up, increased bodyweight by 0·24 kg (95% CI 0·10–0·38 in all trials; 0·33 kg, 95% CI 0·24–0·42 in placebo or standard care controlled trials and −0·15 kg, 95% CI −0·39 to 0·08 in intensive-dose vs moderate-dose trials) at a mean of 4·2 years (range 1·9–6·7) of follow-up, and increased the odds of new-onset type 2 diabetes (OR 1·12, 95% CI 1·06–1·18 in all trials; 1·11, 95% CI 1·03–1·20 in placebo or standard care controlled trials and 1·12, 95% CI 1·04–1·22 in intensive-dose vs moderate dose trials).
Interpretation
The increased risk of type 2 diabetes noted with statins is at least partially explained by HMGCR inhibition.
Funding
The funding sources are cited at the end of the paper.
Introduction
Statins reduce LDL cholesterol concentration by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), leading to a proportionate reduction in cardiovascular disease (CVD) risk.1–4 Consequently, statins have become the most widely prescribed drug class: over 25% of US adults aged at least 45 years (30 million individuals) received these drugs from 2005 to 20085 and an estimated 56 million might be eligible for statin treatment under new guidelines.6
A meta-analysis of randomised controlled trials of statins recently identified a higher risk of type 2 diabetes mellitus from statin treatment compared with placebo or standard care,7 which was dose related.8 These findings prompted a US Food and Drug Administration Drug Safety Communication in 20129 and a change to statin safety labelling. Subsequently, observational studies have also reported a higher risk of type 2 diabetes with statin treatment compared with individuals not taking statins.10–12 Although type 2 diabetes is a cardiovascular risk factor, there remains a net benefit of statin treatment for prevention of CVD3 including among patients with diabetes.4
The mechanism underlying the glucose-raising effect of statins is of interest. A potential explanation in observational studies is that statin users adopt a less healthy lifestyle than individuals not taking statins, but this explanation is unlikely in masked treatment trials, which suggests that the effect is pharmacological. However, whether the glucose-raising effect of statins is explained by the same mechanisms as for LDL cholesterol lowering (ie, HMGCR inhibition) or by one of the proposed pleiotropic effects of statins13,14 (eg, mediated through isoprenoid intermediates and G-protein signalling15) is uncertain.
To investigate the mechanism underlying the glucose-raising effect of statins, we used the mendelian randomisation principle,16,17 with common variants in the gene encoding a drug target as unconfounded, unbiased proxies for pharmacological action on that target.18 We identified single nucleotide polymorphisms (SNPs) in the HMGCR gene and examined their associations with bodyweight, body-mass index (BMI), waist circumference, plasma insulin and glucose, and risk of type 2 diabetes. Associations with these phenotypes would implicate a mechanism involving HMGCR inhibition. To test the correspondence of genetic and pharmacological effects, we updated a meta-analysis of the effect of statins on type 2 diabetes risk in randomised trials, and added new information on bodyweight.
Methods
Genetic studies
We selected as instruments two SNPs (rs17238484 and rs12916) in the HMGCR gene on the basis of genetic associations with LDL cholesterol in the Whitehall II study (n=4678)19 using the IBC HumanCVD BeadChip (Cardiochip; Illumina, San Diego CA, USA) (appendix).20 Both were subsequently associated with LDL cholesterol at a genome-wide level of significance,21 with strong associations in the largest genome-wide study of lipids so far (rs17238484 p=1·35 × 10−21; rs12916 p<1·00 × 10−30).22 Data were available for the greatest number of individuals for the rs17238484 SNP, and this was used for the principal analysis; a subsidiary analysis used the rs12916 SNP. To investigate potential confounding by linkage disequilibrium between our lead SNPs and others in nearby genes, we assessed the association of the HMGCR SNPs with hepatic genome-wide expression data (appendix). If the lead SNPs were in strong linkage disequilibrium with nearby loci, those genes might confound the noted effects of HMGCR genotype on measured phenotypes.23
In observational population studies (appendix) with genotype data for the rs17238484 SNP (or a proxy in strong linkage disequilibrium, r2>0·85), we included individuals of European descent for whom data were available on one or more phenotype of interest. In a secondary analysis, we included data from a subset of studies with data available on the rs12916 SNP or a suitable proxy.
Biomarkers included in the genetic analysis were total cholesterol, LDL cholesterol, non-HDL cholesterol, bodyweight, BMI, waist and hip circumferences, waist:hip ratio, height, plasma glucose, and plasma insulin (appendix). The primary disease outcome was type 2 diabetes, including prevalent (occurring before study baseline) as well as incident cases (occurring subsequently; appendix). In the mendelian randomisation paradigm, the intervention is the naturally randomised allocation of genotype, which occurs at conception and exerts its effect from that point throughout the lifetime of the individual. Therefore, events prevalent at the time of recruitment to genetic studies are nevertheless incident from the perspective of the time of the genotypic randomisation and can be included in the genetic analysis. Thus, for the genetic analysis, both prevalent and incident cases were included to maximise power.
All studies contributing data to these analyses were approved by their local ethics committees, as described in the published findings of each study (appendix).
Meta-analysis of statin trials
We updated our two previous summary-level meta-analyses7,8 on the association of statin treatment with incident type 2 diabetes in cardiovascular prevention trials of at least 1000 participants, followed up for at least 1 year. The appendix contains details of the exclusion criteria and trials.
Investigators from 20 eligible trials with data on incident type 2 diabetes were contacted for information on bodyweight change during follow-up by treatment allocation, which was used as a coprimary outcome. 15 trials provided data on bodyweight at baseline and at the last visit attended among individuals free from type 2 diabetes at baseline. Two trials (ALLHAT24 and A to Z25) did not measure bodyweight sequentially, and bodyweight data were unavailable from the remaining three trials (appendix). Data were also analysed separately for participants not experiencing any primary cardiovascular outcome (according to trial-specific definitions) to exclude the possibility that the effect of statin treatment on bodyweight was limited to participants experiencing cardiovascular events.
Changes in LDL cholesterol in each treatment group at 1 year were available from the Cholesterol Treatment Trialists' Collaboration meta-analysis for 18 trials,1 whereas data for mean changes in LDL cholesterol during two trials were taken from the primary publications.26,27 Information about plasma glucose and insulin concentrations, BMI, waist circumference, and waist:hip ratio was unavailable from the trials.
Statistical analysis
For the genetic studies, we assessed study-specific associations of rs17238484 and rs12916 with each continuous trait using univariate linear regression models. Plasma glucose and insulin were analysed on the natural logarithmic scale because of their skewed distributions, and we present proportional differences in geometric means per allele. The rs17238484-G allele and rs12916-T allele were each associated with lower LDL cholesterol concentration and were designated the effect alleles, to facilitate direct comparison with statin treatment.
We assessed associations of the rs17238484 and rs12916 SNPs with type 2 diabetes risk using univariate logistic regression models to estimate the odds ratio (OR) per LDL-lowering allele. We combined within-study estimates using fixed-effects and random-effects meta-analyses, with heterogeneity quantified by the I2 statistic.28 Heterogeneity between subgroups was assessed using meta-regression. All genetic analyses were done using a prespecified routine in Stata version 12.1, which was translated for use in SPSS, SAS, and R where necessary.
To corroborate our genetic findings, we examined the associations of the two lead SNPs in a large genome-wide association study of BMI,29 a Metabochip analysis of plasma insulin,30 and a genome-wide association and Metabochip analysis of type 2 diabetes.31
In the meta-analysis of statin trial data, we synthesised within-trial ORs for type 2 diabetes during follow-up in participants free from type 2 diabetes at baseline and within-trial mean differences in bodyweight change between treatment groups, calculated as the difference from baseline to final visit, using random-effects and fixed-effects meta-analyses. We undertook meta-regression analyses of the associations of new-onset type 2 diabetes and bodyweight change with change in LDL cholesterol at 1 year and with follow-up duration. We assessed inter-study heterogeneity using the I2 statistic and used Stata version 10.1 for trial-related analyses.
Role of the funding source
The funding sources had no role in study design, data collection, data analysis, data interpretation, the writing of the report, or the decision to submit for publication. DIS, DP, ADH, and NS had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of 38 Cardiochip SNPs within 55 kb of the HMGCR gene, seven met prespecified criteria for instrument selection (appendix), of which all but the two selected, rs17238484 and rs12916, were in strong linkage disequilibrium (r2>0·9; appendix). Gene expression data for rs17238484 were unavailable, but the T allele of rs12916 was associated with lower hepatic HMGCR expression (p=1·30 × 10−5) but not with expression of adjacent genes (appendix).
Data for up to 195 444 individuals (43 studies) for the HMGCR rs17238484 SNP and 94 652 individuals (21 studies) for the rs12916 SNP (or suitable proxies in studies in which these were not directly measured) contributed to the analysis of genetic associations with biomarkers and outcomes. The mean age of study participants was 59 years (range 26–75; appendix).
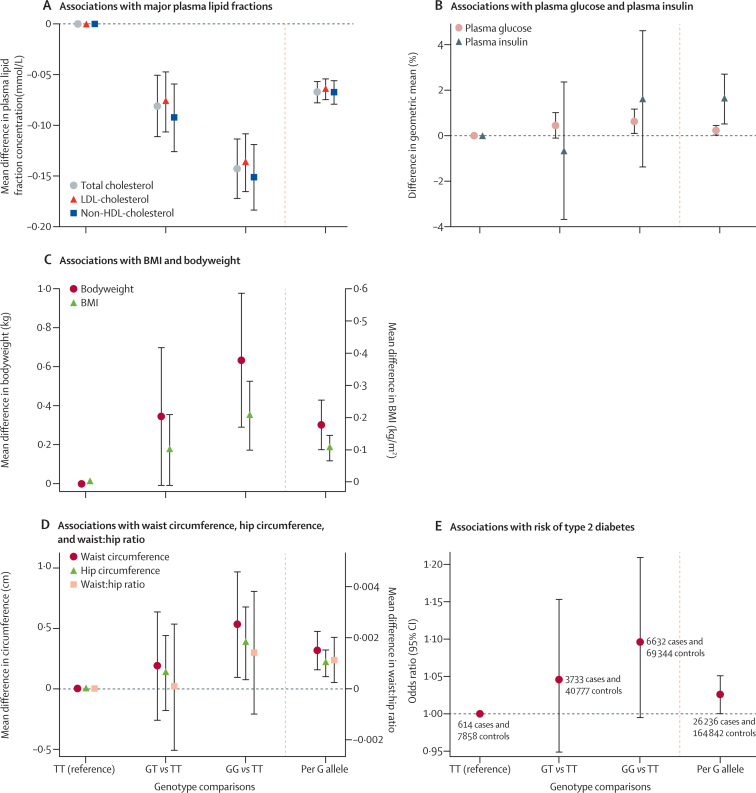
The association of the rs17238484 genotype with circulating concentrations of major lipid fractions followed an additive model in the meta-analysis of available data (figure 1A). Each additional rs17238484-G allele was associated with 0·06 mmol/L (95% CI 0·05–0·07) lower LDL cholesterol (p=1·34 × 10−35; 101 919 individuals, 26 studies), 0·07 mmol/L (0·06–0·08) lower total cholesterol (p=6·46 × 10−36; 117 545 individuals, 30 studies), and 0·07 mmol/L (0·06–0·08) lower non-HDL cholesterol (p=3·32 × 10−30; 103 375 individuals, 27 studies). The association of genotype with LDL cholesterol concentration was consistent between subgroups (data available in up to 29 studies, 116 327 individuals), with all meta-regression p values greater than 0·05 (appendix). Associations of rs12916 with plasma lipids were directionally concordant with rs17238484 and of similar magnitude (appendix).
Figure 1.
Association of rs17238484 genotype with type-2 diabetes-related traits
Association of the rs17238484 genotype with (A) major plasma lipids fractions; (B) plasma glucose and insulin; (C) BMI and bodyweight; (D) waist and hip circumference and waist:hip ratio; and (E) risk of type 2 diabetes. Bars are 95% CIs. BMI=body-mass index.
The rs17238484-G allele was associated with 1·62% (95% CI 0·53–2·72; p=0·004) higher plasma insulin concentration (37 453 individuals, 12 studies) and with higher plasma glucose concentration (0·23%, 0·02–0·44; p=0·03; 73 490 individuals, 23 studies; figure 1B). Each rs17238484-G allele was also associated with 0·30 kg higher bodyweight (95% CI 0·18–0·43; p=3·15 × 10−6; 143 113 individuals, 30 studies) and 0·11 kg/m2 higher BMI (0·07–0·14; p=1·77 × 10−7; 152 004 individuals, 32 studies; figure 1C), but not with height (p=0·23; 77 291 individuals, 23 studies; appendix). Each additional rs17238484-G allele was associated with greater waist circumference (0·32 cm, 95% CI 0·16–0·47; p=8·32 × 10−5; 69 163 individuals, 19 studies), hip circumference (0·21 cm, 0·10–0·32; p=1·67 × 10−4; 69 159 individuals, 19 studies), and waist:hip ratio (0·001, 0·0003–0·002; p=0·01; 95 496 individuals, 23 studies; figure 1D). The rs12916 SNP showed directionally concordant associations with these biomarkers (appendix). Additive association patterns were noted with all these traits, and no differences in the rs17238484 SNP effect occurred between subgroups (all meta-regression p values >0·05; appendix). The appendix shows estimates from random-effects meta-analyses.
Public domain data from a meta-analysis of genome-wide association studies of BMI29 and an Illumina Metabochip-based32 analysis of plasma insulin30 revealed directionally concordant associations of the rs17238484 and rs12916 SNPs or suitable proxies with both these traits: log plasma insulin rs12916 β 0·007 (95% CI 0·002–0·012; p=4·72 × 10−3) and rs17238484 β 0·01 (0·004–0·016; p=5·92 × 10−4); and BMI rs17238484 p=9·28 × 10−6 and rs12916 p=1·45 × 10−4. Associations of both SNPs with fasting insulin were attenuated to the null after adjustment for BMI in the same datasets (rs17238484 p=0·74; rs12916 p=0·63).
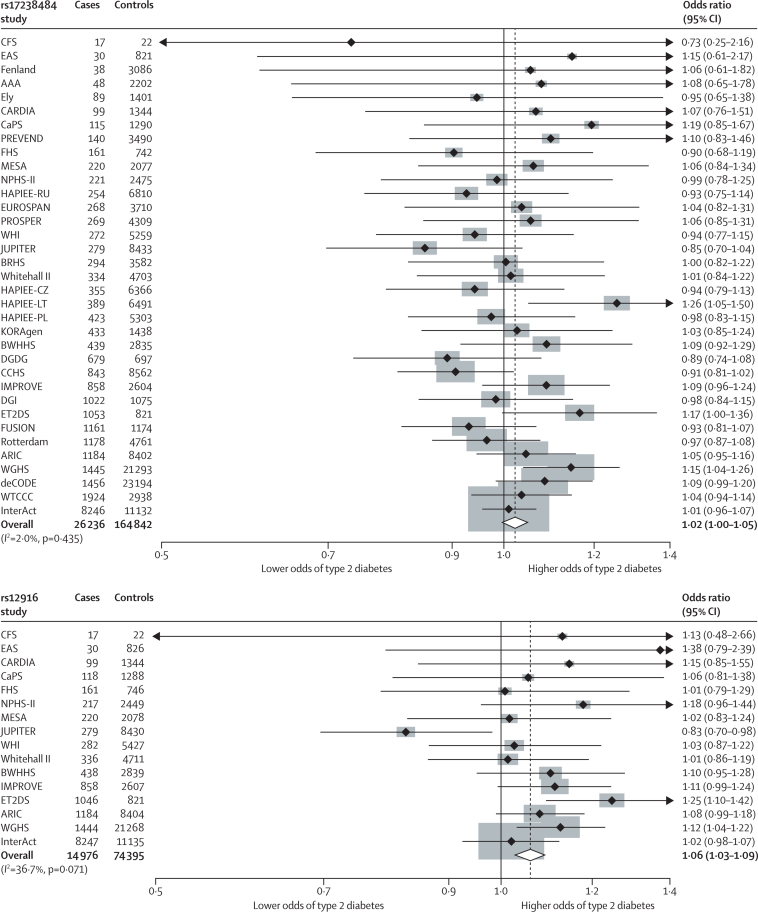
In 26 236 cases and 164 842 controls in 35 population studies, the HMGCR rs17238484-G allele, which was associated with lower LDL cholesterol and higher bodyweight and BMI, seemed to be associated with increased risk of type 2 diabetes (OR per allele 1·02, 95% CI 1·00–1·05; p=0·09; figures 1E and 2). Data on the association between HMGCR rs12916 and type 2 diabetes were available for 14 976 cases and 74 395 controls (16 studies). The OR per rs12916-T allele was 1·06 (95% CI 1·03–1·09; p=9·58 × 10−5). The associations of both SNPs were confirmed when our data were combined in a meta-analysis with those from a large genome-wide association and Metabochip study of risk of type 2 diabetes (rs17238484 OR 1·03, 95% CI 1·01–1·06; rs12916 1·02, 1·00–1·04; appendix).31
Figure 2.
Meta-analyses of the associations of 3-hydroxy-3-methylglutaryl-CoA reductase variants rs17238484 and rs12916 with risk of type 2 diabetes
Data were analysed by fixed-effects meta-analysis.
Data from 129 170 participants free from type 2 diabetes at baseline were available from 20 statin trials (table). At 1-year of follow-up, mean LDL cholesterol reduction was 0·92 mmol/L (95% CI 0·18–1·67) across all 20 trials, 1·07 mmol/L (0·44–1·70) in the 15 placebo-controlled and standard care-controlled trials (96 418 individuals), and 0·50 mmol/L (0·25–0·76) in the intensive-dose versus moderate-dose trials (32 752 individuals).
Table.
Baseline data for participants without diabetes in 20 large statin trials
| Number of patients (statin vs control) | Treatment (active vs control) | Follow-up (years) | Trial population | Age (years) | Diabetes diagnostic criteria* | Weight change data available | Absolute LDL cholesterol lowering at 1 year (%)† | Number of cases of type 2 diabetes on statin (or intensive statin) | Number of cases of type 2 diabetes on control (or low-dose statin) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 4S (1994) | 4242 (2116 vs 2126) | S 10–40 mg vs placebo | 5·2 | Angina or previous MI | 59 | I, II, III | Yes | −1·77 (−37%) | 198 | 193 |
| WOSCOPS (1995) | 5974 (2999 vs 2975) | P 40 mg vs placebo | 4·8 | Male, hypercholesterolaemia, no history of MI | 55 | II, III | Yes | −1·07 (−24%) | 75 | 93 |
| AFCAPS TexCAPS (1998) | 6211 (3094 vs 3117) | L 20–40 mg vs placebo | 5·2 | Average cholesterol concentrations, no CVD | 58 | I, II, III | Yes | −0·94 (−27%) | 72 | 74 |
| LIPID (1998) | 6997 (3496 vs 3501) | P 40 mg vs placebo | 5·9‡ | Hospital admission for unstable angina or previous MI | 62‡ | II, III | Yes | −1·03 (−25%) | 126 | 138 |
| GISSI-Prevenzione (2000) | 3460 (1743 vs 1717) | P 20 mg vs standard care | 1·9 | Recent MI | 59 | III | Yes | −0·35 (−12%) | 96 | 105 |
| LIPS (2001) | 1475 (724 vs 751) | F 80 mg vs placebo | 3·9‡ | Recent percutaneous coronary intervention | 60 | I | No | −0·92 (−27%) | 17 | 14 |
| HPS (2002) | 14 573 (7291 vs 7282) | S 40 mg vs placebo | 5·0 | CVD or diabetes | 65 | I, II | No | −1·29 (−29%) | 335 | 293 |
| PROSPER (2002) | 5023 (2510 vs 2513) | P 40 mg vs placebo | 3·2 | Age 70–82 years with CVD or risk factors | 75 | II, III | Yes | −1·04 (−31%) | 165 | 127 |
| ALLHAT-LLT (2002) | 6087 (3017 vs 3070) | P 40 mg vs no treatment | 4·8 | CHD or CHD risk factors | 66 | III | No | −0·54 (−18%) | 238 | 212 |
| ASCOT-LLA (2003) | 7773 (3910 vs 3863) | A 10 mg vs placebo | 3·3‡ | Hypertension, no CHD | 63 | IV | Yes | −1·07 (−35%) | 154 | 134 |
| PROVE-IT TIMI 22 (2004) | 3395 (1707 vs 1688) | A 80 mg vs P 40 mg | 2·0 | Recent hospital admission for ACS | 58 | I, II, III | Yes | −0·65 (−22%) | 101 | 99 |
| A to Z (2004) | 3504 (1768 vs 1736) | S 40–80 mg vs Placebo −S 20 mg | 2·0‡ | Recent hospital admission for ACS | 60 | I, II | No | −0·30 (−15%) | 65 | 47 |
| TNT (2005) | 7595 (3798 vs 3797) | A 80 mg vs A 10 mg | 5·0 | Stable CHD | 61 | I, II, III | Yes | −0·62 (−22%) | 418 | 358 |
| IDEAL (2005) | 7461 (3737 vs 3724) | A 80 mg vs S 20–40 mg | 4·8‡ | Previous MI | 62 | I, II, III | Yes | −0·55 (−16%) | 240 | 209 |
| SPARCL (2006) | 3803 (1905 vs 1898) | A 80 mg vs placebo | 4·4 | Recent stroke or transient ischaemic attack | 63 | I, II, III§ | Yes | −1·43 (−42%) | 166 | 115 |
| MEGA (2006) | 6086 (3013 vs 3073) | P 10–20 mg vs no treatment | 5·3 | Hypercholesterolaemia, no previous CHD or stroke | 58 | I, II, III | Yes | −0·67 (−17%) | 172 | 164 |
| CORONA (2007) | 3534 (1771 vs 1763) | R 10 mg vs placebo | 2·5 | Systolic heart failure | 73 | I | Yes | −1·63 (−45%) | 100 | 88 |
| JUPITER (2008) | 17 802 (8901 vs 8901) | R 20 mg vs placebo | 1·9‡ | No CVD, no diabetes, hsCRP ≥2·0 mg/L | 66‡ | I, II | Yes | −1·09 (−50%) | 270 | 216 |
| GISSI-HF (2008) | 3378 (1660 vs 1718) | R 10 mg vs placebo | 3·6 | Chronic heart failure | 67 | III | Yes | −0·92 (−35%) | 225 | 215 |
| SEARCH (2010) | 10 797 (5398 vs 5399) | S 80 mg vs S 20 mg | 6·7 | Previous MI | 64 | I | No | −0·39 (−12%) | 625 | 587 |
| Total | 129 170 (64 558 vs 64 612) | .. | 4·2 (1·6) | .. | .. | .. | .. | .. | 3858 | 3481 |
A=atorvastatin. CHD=coronary heart disease. CVD=cardiovascular disease. F=fluvastatin. L=lovastatin. P=pravastatin. MI=myocardial infarction. R=rosuvastatin. S=simvastatin.
Diagnostic criteria: I=adverse event report or physician report; II=glucose lowering therapy; III=raised fasting plasma glucose (≥7·0 mmol/L) on at least one occasion.
Change in lipid values at 1 year except for SPARCL (average difference during trial) and CORONA (difference at 3 months).
Median values.
Included criterion that diagnostic raised fasting plasma glucose must be at least 2·0 mmol/L higher than baseline glucose.
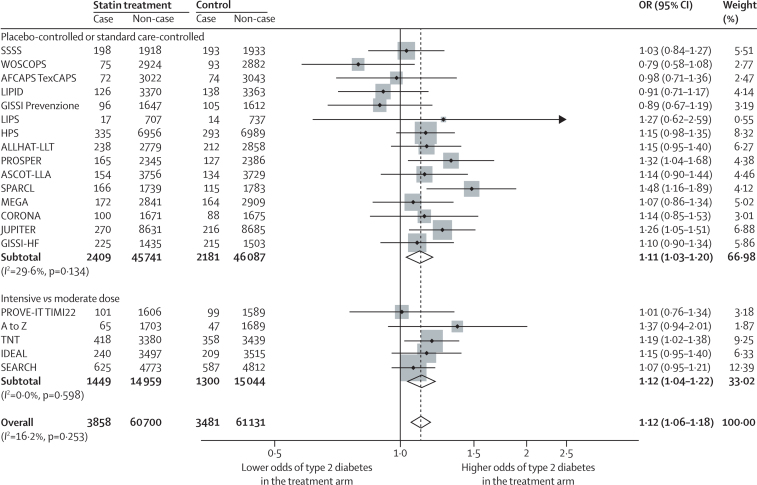
Mean follow-up across all 20 trials was 4·2 years (range 1·9–6·7). Over this time, 3858 individuals allocated to statin or intensive-dose statin and 3481 allocated to placebo, standard care, or moderate-dose statin were diagnosed with new-onset type 2 diabetes. The OR for new-onset type 2 diabetes with statin treatment was 1·12 (95% CI 1·06–1·18; figure 3), with little heterogeneity between trial-specific ORs (I2 16·2%, 95% CI 0·0–50·7). The appendix provides fixed-effects meta-analysis estimates. There was no association between LDL cholesterol lowering at 1 year and within-trial ORs for new-onset type 2 diabetes (log-odds per 1% reduction in LDL cholesterol 0·004, 95% CI −0·001 to 0·009; p=0·10; appendix), or between duration of follow-up and risk of type 2 diabetes in either univariate meta-regression (log odds per year increase in trial duration −0·021, 95% CI −0·058 to 0·017; p=0·26), or after adjustment for trial type (ie, placebo-controlled and standard care-controlled or intensive vs moderate statin dose) and percent LDL cholesterol change (log odds −0·006, −0·051 to 0·039; p=0·77).
Figure 3.
Effect of statin treatment on new-onset type 2 diabetes
Data were analysed by random-effects meta-analysis. OR=odds ratio. Case=developed type 2 diabetes. Non-case=did not develop type 2 diabetes.
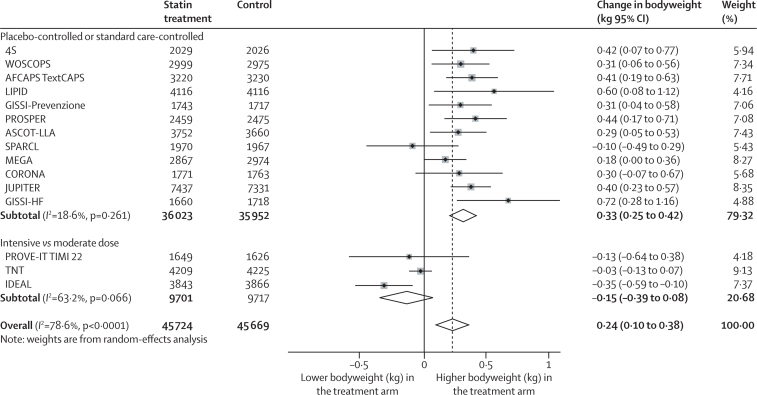
Data on the effect of statin treatment on bodyweight were available from 15 trials, including 91 393 participants free from type 2 diabetes at baseline. Mean follow-up was 3·9 years (range 1·9–5·9). Recipients of statin treatment or intensive-dose statin treatment were 0·24 kg (95% CI 0·10–0·38) heavier by the end of follow-up than were control recipients in a random-effects meta-analysis (figure 4), although there was substantial heterogeneity between trials (I2 78·6%, 95% CI 65·3–86·8). The appendix provides fixed-effects meta-analysis estimates. When limited to individuals not experiencing a cardiovascular event, estimates were similar (0·21 kg, 95% CI 0·08–0·35; 83 959 individuals). The effect on bodyweight change was noted only in trials comparing statin treatment with placebo or standard care (0·33 kg, 95% CI 0·25 to 0·42; I2 18·6%), but not in trials comparing moderate-dose with intensive-dose statin treatment (−0·15 kg, 95% CI −0·39 to 0·08; I2 63·2%). No association was noted between relative LDL cholesterol reduction and within-trial bodyweight change (meta-regression β 0·004, 95% CI −0·012 to 0·021; p=0·58; appendix). There was no relation between duration of follow-up and bodyweight change in either univariate meta-regression (β −0·028 kg/year, 95% CI −0·147 to 0·092; p=0·63) or multivariate meta-regression analysis (β −0·009, 95% CI −0·091 to 0·073; p=0·81) after adjustment for relative LDL cholesterol change and trial type. No relation was noted between bodyweight change and risk of new-onset type 2 diabetes across the trials (log-odds per 1 kg bodyweight increase −0·14, 95% CI −0·41 to 0·13; p=0·29).
Figure 4.
Effect of statin treatment on bodyweight
Data were analysed by random-effects meta-analysis. In most trials, the total number of participants without type 2 diabetes at baseline for whom bodyweight data were available was smaller than the total number for whom data were available for the analysis of new-onset type 2 diabetes.
Discussion
HMGCR genetic variants in population studies and statin treatment in trials were associated with higher bodyweight and higher risk of type 2 diabetes, suggesting that these effects are a consequence of HMGCR inhibition. The association of HMGCR SNPs with risk of type 2 diabetes is new, as is the association of statin treatment and HMGCR SNPs with increased bodyweight.
Increased bodyweight plays a causal part in the development of type 2 diabetes,33 suggesting a possible mechanism for the dysglycaemic effect of statin treatment. However, whether the relation between HMGCR inhibition and type 2 diabetes is mediated exclusively by changes in body composition remains unknown. Statin treatment led to higher bodyweight and increased risk of type 2 diabetes, and both HMGCR SNPs studied were associated with higher bodyweight and waist circumference, and one with higher plasma insulin and glucose concentrations. Insulin resistance might accompany bodyweight gain and a central distribution of adipose tissue. However, we were unable to identify a specific association of statin treatment with insulin resistance in these analyses because the relevant measures were unavailable from trials. One small trial34 that was ineligible for the present study reported 2 months of atorvastatin treatment led to higher glycated haemoglobin (HbA1c) and insulin concentrations and lower insulin sensitivity than with placebo, and findings from a previous meta-analysis35 of statin trials suggested differential effects on insulin sensitivity between statins. In JUPITER36 and PROVE-IT TIMI 22,37 small increases in HbA1c were noted in individuals randomly assigned to statin treatment compared with control individuals, and in AFORRD,38 HbA1c also increased slightly in patients on atorvastatin compared with placebo after 4 months. Nevertheless, the association of one HMGCR SNP with fasting insulin and glucose concentrations, and its attenuation to the null after adjustment for BMI, support a bodyweight-mediated association between HMGCR inhibition and insulin resistance as a possible mechanistic explanation. Conversely, the magnitude of bodyweight gain we noted in both statin trials and genetic studies seems insufficient to account for the corresponding risk of type 2 diabetes. Intensive statin treatment also showed no greater effect on bodyweight than low-dose or moderate-dose treatment, although type 2 diabetes risk was greater with intensive statin treatment.
The anatomical site of the genetic and drug effects on energy metabolism that we report is not completely certain. The liver is a likely location, in view of its important involvement in lipid metabolism; however, the dysglycaemic phenotypes reported here might be caused by modulation of HMGCR function in skeletal muscle. Additional, off-target effects of statins might also make a further contribution to bodyweight gain.39
Inhibition of HMGCR by statins impairs hepatocyte cholesterol synthesis, upregulates hepatic LDL receptor expression, and reduces circulating LDL cholesterol concentrations. Although the genetic findings provide evidence that the effect of statins on bodyweight and type 2 diabetes risk is caused by HMGCR inhibition, whether this effect requires or is independent of reductions in circulating LDL cholesterol remains unclear. A meta-regression analysis of trial data did not provide evidence for an association between LDL cholesterol reduction and bodyweight or type 2 diabetes risk, but these analyses were done with summary-level data, which might have limited our ability to detect any such relation. Studies of genetic variants from other loci affecting LDL cholesterol22 or drugs lowering LDL cholesterol by other mechanisms would probably help to resolve this uncertainty.
An association with BMI has been identified for a SNP 350 kb from HMGCR at a genome-wide level of significance (p=2·17 × 10−13),29 although with no other variants within the HMGCR gene. In publicly available data from two genome-wide association studies,29–30 associations of the rs17238484 and rs12916 with BMI and plasma insulin concentration were noted at strong but sub-genome-wide levels of significance. This evidence, the consistent effect of both SNPs on LDL cholesterol, and a specific association with hepatocyte HMGCR mRNA expression for one of the SNPs (rs12916; appendix) supports their validity as genetic instruments in this analysis.
We used two HMGCR SNPs in the genetic analysis, one for the main (rs17238484) and another (rs12916) for a subsidiary analysis. Although the findings were broadly consistent, the small differences in effect estimates between the two variants could be caused by the different allele frequencies, available sample size for each, and the association of each with a functional variant or variants that were not identified.
This study has some limitations. Not all phenotypes measured in genetic studies were available in the statin trials—notably plasma glucose and insulin, waist and hip circumference, and waist:hip ratio. Moreover, not all studies in the genetic analysis measured glucose in fasting samples. In view of the wide age range of participants included in these analyses, survival bias might have affected our findings; however, this is unlikely and any such effect, if present, would probably have been limited. The HMGCR variants might affect the odds of being prescribed lipid-lowering drugs and thus introduce bias to the association between HMGCR and risk of type 2 diabetes. However, we found no evidence of an interaction between genotype, lipid-lowering drug use at study baseline, and risk of type 2 diabetes (appendix). The source of the heterogeneity between the statin trials that provided bodyweight data, particularly for dose-comparison trials, remains uncertain. Reductions in LDL cholesterol between arms in the dose comparison trials was smaller than that achieved in the placebo-controlled trials. Our analysis was restricted to participants without type 2 diabetes at baseline. However, we did not have access to data on within-trial death, withdrawal, or loss to follow-up. Although observational pharmacoepidemiological studies have also examined the association of statin prescription with the development of type 2 diabetes, studies of this type can be prone to confounding and bias. For this reason, and to permit more direct comparison with the genetic analysis, we focused on data from randomised trials. Finally, trial analyses were done with summary-level data, which limited power for meta-regression.
Our findings pertain to the mechanism by which statins slightly increase the risk of type 2 diabetes—an association that has already been established. Findings from recent analyses of trials have shown that, although this association is robust, the absolute risk of developing type 2 diabetes is greatly offset by the benefits of statin treatment for CVD risk.3,40 Indeed, the efficacy of statin treatment to reduce the risk of CVD has been shown conclusively in several large primary and secondary prevention randomised controlled trials, including in individuals with type 2 diabetes, with a favourable risk:benefit profile.1,3,4 For this reason, our findings provide mechanistic insight, but should not alter present guidance on prescription of statins for prevention of CVD. Nevertheless, our results, including the new finding of increased bodyweight with statin treatment, suggest lifestyle interventions such as bodyweight optimisation, healthy diet, and adequate physical activity should be emphasised as important adjuncts to prevention of CVD with statin treatment to attenuate risks of type 2 diabetes. The reason why bodyweight change does not seem to be greater with intensive statin treatment compared with moderate-dose treatment needs further investigation.
In conclusion, both statin treatment in randomised trials and carriage of common SNPs in the HMGCR gene in population studies were associated with bodyweight gain and higher risk of type 2 diabetes. Bodyweight gain is physiologically linked to insulin resistance and is one of the strongest risk factors for type 2 diabetes, which might partly explain the higher risk of type 2 diabetes in statin-treated patients.
Acknowledgments
Acknowledgments
DIS is supported by a Medical Research Council (MRC) Doctoral Training Award and a grant from the Rosetrees Trust. MVH is supported by a MRC Population Health Scientist Fellowship (G0802432). JELE is supported by grants from the National Institutes of Health (NIH) and the MRC. RS has been supported by a British Heart Foundation (Schillingford) Clinical Training Fellowship (FS/07/011). SS is supported by the Danish MRC (grant no. 10-083788), the Research Fund at Rigshospitalet, Copenhagen University Hospital, and Chief Physician Johan Boserup and Lise Boserup's Fund. CC and APe are supported by grants from the Wellcome Trust (064947/Z/01/Z and 081081/Z/06/Z), a grant from the National Institute on Aging (1R01 AG23522-01), and a grant from MacArthur Foundation. SGB was supported by an award from the National Institute on Minority Health and Health Disparities of the NIH (award number P20MD006899). NGF is supported by a grant from the MRC. JAH is supported by the project (Ministry of Health, Czech Republic) for the development of research organisation 00023001 (IKEM, Prague, Czech Republic). APa is supported by grants from the Wellcome Trust (064947/Z/01/Z and 081081/Z/06/Z) and National Institute on Aging (1R01 AG23522-01). BS is supported by the Magnus Bergvall Foundation and the Foundation for Old Servants. RGJW is supported by the National Institute for Healthy Ageing (Grant 05060810). JFP is supported by the British Heart Foundation; Chest, Heart and Stroke Scotland; the Wellcome Trust; and the MRC. DAL, GDS, and NJT work in a unit that receives funding from the MRC and University of Bristol. EJB's research is supported by a British Heart Foundation programme grant (RG/13/2/30098) and the MooDFOOD Collaborative Project (FP7 grant 613598). RMK is supported by the NIH (grant NIH U19 HL065797). SEH and PJT are supported by the British Heart Foundation (BHF RG 08/008, PG/07/133/24260), MRC, and NIH (grant NHLBI 33014). MKi is supported by the National Institute on Aging (AG034454); the MRC (K013351); the National Heart, Lung and Blood Institute (HL036310); and the Academy of Finland. NJT is supported by the MRC (grant G0600705). FWA is supported by a clinical fellowship from the Netherlands Organisation for Health Research and Development (ZonMw grant 90700342). ADH is supported by University College London NIHR Biomedical Research Centre. NS's research is supported by the British Heart Foundation, Diabetes UK, and the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant 115372). We thank Merck and Novartis for contributing data to the statin trials analysis. Details of grants or other support for included studies are provided in the appendix.
Contributors
DIS, DP, ADH, and NS conceived the project, established and coordinated the consortium of studies, designed and executed the analysis, interpreted the findings, and wrote and revised the first and subsequent drafts of the manuscript. KBK contributed to analysis design and execution. MVH and FDr contributed to data analysis, interpretation of findings, and manuscript preparation. JELE, YG, AAm, YRL, MCWS, SGB, RT-M, US, PJS, MF, AW, LAL, and HH contributed to data collection and preparation. TS and RS contributed to analysis design. SS and AT-H contributed to data collection, data analysis, interpretation of findings, and manuscript preparation. PCDJ and SJS contributed to data preparation and analysis. RAS, ML, and NV contributed to data analysis and manuscript preparation. CG contributed to data analysis, interpretation of findings, and manuscript preparation. CC and JAC contributed to data collection, preparation, and analysis. APe, NGF, NCO-M, RBS, BS, IF, and OHK contributed to data collection and preparation, and manuscript preparation. KWL, JP, and PH contributed to genotyping, data collection, and preparation. GL contributed to data collection and interpretation of findings. JG, YTvdS, APa, SM, UdF, AAl, PAD, JK, JRD, ACK, GT, DDW, PA, WMMV, SRP, TRG, and JC contributed to data collection and manuscript preparation. IT contributed to data collection and analysis, and interpretation of findings. DLvdA, RK, MBa, AT, WS, DD, PSS, NRP, HN, CD, DK, RH, BAC, MGM, and MT contributed to data collection. JAH, JWJ, GJdB, PAdJ, PHW, RWM, DAL, GDS, YB-S, DSS, MC, and MV contributed to data collection and preparation, interpretation of findings, and manuscript preparation. DB and ET contributed to data collection, preparation, and analysis, and manuscript preparation. FV, RMK, and FDu contributed to interpretation of findings. RGJW, AHM-vdZ, CBE, JGR, AMG, RM, TRP, JJVM, JDL, PMR, APM, LT, KKR, JEM, JFP, JCW, JAD, SK, and PvdH contributed to data collection, interpretation of findings, and manuscript preparation. AdB contributed to manuscript preparation. DIC contributed to data collection and preparation, data analysis, interpretation of findings, and manuscript preparation. SRKS and RCH contributed to interpretation of findings and manuscript preparation. MKu, NJW, and SR contributed to data collection and preparation, and interpretation of findings. AH and EJB contributed to data collection and interpretation of findings. BMP contributed to data collection, study design, and manuscript preparation. SEH, PJT, and MKi contributed to data collection and preparation, study design, interpretation of findings, and manuscript preparation. NJT, CL, FWA, MBo, and HP contributed to data collection and preparation, data analysis, study design, interpretation of findings, and manuscript preparation. JGW, APR, and BJK contributed to data collection and preparation, coordination of consortium, study design, interpretation of findings, and manuscript preparation.
Declaration of interests
JWJ has received research grants from and was speaker at CME-accredited meetings sponsored by Astellas, Anthera, AstraZeneca, Bayer, Biotronik, Boston Scientific, Correvio, Daiichi Sankyo, Lilly, Genzyme, Medtronic, Merck-Schering-Plough, Pfizer, Orbus Neich, Novartis, Roche, Servier, Sanofi Aventis, the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Community Framework KP7 Programme. JGR's institution has received grants for her work from Amgen, Daiichi Sankyo, Esperion, GlaxoSmithKline, Merck, Genentech/Hoffmann-La Roche, and Zinfandel/Takeda. AMG has received funds for board membership of Aegerion, Arisaph, DuPont, VascuVis, and Vatera; consultancy for Janssen, Kowa, Merck, and Roche; and manuscript preparation for AstraZeneca. ACK has received funds in the form of grants to his institution, consultancy fees, and travel support from Bristol-Myers Squibb; consultancy fees from AstraZeneca, Merck, Novartis, and Pfizer; grants paid to his institution from AstraZeneca, Merck, Novartis, and Pfizer; and fees for speaking engagements from AstraZeneca, Merck, Novartis, and Pfizer. RM has received funds for speaking engagements from Ferrer, Pronova BioPharma, Sigma-Tau, and Societa Prodotti Antibiotica; his institution has received funds from Sigma-Tau, Societa Prodotti Antibiotica, GlaxoSmithKline, Novartis, Amgen, Pronova BioPharma, and General Electric. PSS has received consultancy fees from Pfizer and Servier, fees for speaking engagements from Pfizer and Servier, and fees for development of educational presentations from Pfizer; his institution has received funds for his work from Pfizer and Servier. NRP has received fees for speaking engagements from Pfizer and fees for production of books from Servier; his institution has received grants from Pfizer and the Hypertension Trust. DDW has received consultancy fees from Merck-Schering Plough and Pfizer, and fees for speaking engagements from Pfizer. TRP has received consultancy fees, grants, and fees for speaking engagements from Merck; and fees for speaking engagements from AstraZeneca, Roche, and Amgen. PA has received funds for board membership, consultancy, grants, speaking engagements, and the development of educational presentations from Pfizer. JJVM has received reimbursement for travel from AstraZeneca, and his institution has received funds from AstraZeneca. LT's institution has received funds for his work from the ANMCO Foundation. KKR has received fees for advisory board membership from Pfizer; for involvement in trial management and advisory boards from Roche; for speaking engagements and advisory board membership from MSD; for speaking engagements, advisory board membership, and trial involvement from AstraZeneca; for advisory board membership and trial involvement from Sanofi; for advisory board membership from Aegerion, Regeneron, and Abbott; for speaking engagements from Menarini, Novo Nordisk, and theHeart.org; for trial involvement and steering committee membership from GlaxoSmithKline; and for advisory board membership from Novartis. JEM is a co-inventor on a patent held by the Brigham and Women's Hospital that relates to inflammatory biomarkers in diabetes prediction. JCW is an employee of and holds stock in GlaxoSmithKline. RMK has received funds for advisory board membership from Merck, consultancy fees from Celera and Genentech, and grants from Quest Diagnostics, and his institution receives funds resulting from a patent related to diagnostic use of a HMGCR spliced isoform. RCH has received funds for board membership of Liposcience, for speaking engagements for Denka Seiken, and in the form of grants to his institution from Merck/Schering-Plough, Diadexus, and Denka Seiken. All other authors declare no competing interests.
Contributor Information
Daniel I Swerdlow, Email: d.swerdlow@ucl.ac.uk.
David Preiss, Email: david.preiss@glasgow.ac.uk.
Supplementary Material
References
- 1.Baigent C, Blackwell L, Emberson J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy in stroke prevention: a meta-analysis involving 121 000 patients. Am J Med. 2008;121:24–33. doi: 10.1016/j.amjmed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists' (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney PM, Blackwell L, Collins R. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics . Health, United States 2010: with special feature on death and dying. National Center for Health Statistics; Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- 6.Pencina MJ, Navar-Boggan AM, D'Agostino RB., Sr Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Preiss D, Murray HM. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 8.Preiss D, Seshasai SRK, Welsh P. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration FDA Drug Safety Communication: important safety label changes to cholesterol-lowering statin drugs. 2012. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm (accessed April 28, 2012).
- 10.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarese EP, Buffon A, Andreotti F. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–1130. doi: 10.1016/j.amjcard.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Danaei G, García Rodríguez LA, Fernandez Cantero O, Hernán MA. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care. 2013;36:1236–1240. doi: 10.2337/dc12-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 14.Axsom K, Berger JS, Schwartzbard AZ. Statins and diabetes: the good, the bad, and the unknown. Curr Atheroscler Rep. 2013;15:299. doi: 10.1007/s11883-012-0299-z. [DOI] [PubMed] [Google Scholar]
- 15.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani A, Humphries S. Nature's randomised trials. Lancet. 2005;366:1906–1908. doi: 10.1016/S0140-6736(05)67767-7. [DOI] [PubMed] [Google Scholar]
- 18.The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmot MG, Smith GD, Stansfeld S. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 20.Keating BJ, Tischfield S, Murray SS. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kathiresan S, Melander O, Guiducci C. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Schmidt EM, Sengupta S. Discovery and refinement of loci associated with lipid levels. Nature Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008;5:e177. doi: 10.1371/journal.pmed.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 25.De Lemos JA, Blazing MA, Wiviott SD. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 26.Kjekshus J, Apetrei E, Barrios V. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 27.Amarenco P, Bogousslavsky J, Callahan A., 3rd High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speliotes EK, Willer CJ, Berndt SI. Association analyses of 249 796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott RA, Lagou V, Welch RP. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris AP, Voight BF, Teslovich TM. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voight BF, Kang HM, Ding J. The Metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmes MV, Lange LA, Palmer T. Causal effects of body mass index on cardiometabolic traits and events: a mendelian randomization analysis. Am J Hum Genet. 2014;94:198–208. doi: 10.1016/j.ajhg.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2010;87:98–107. doi: 10.1016/j.diabres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Danielson E, Fonseca FAH. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 37.Sabatine MS, Wiviott SD, Morrow DA, McCabe C, Cannon CP. High-dose atorvastatin associated with worse glycemic control: a PROVE-IT TIMI 22 substudy. Circulation. 2004;110(suppl III):834. [Google Scholar]
- 38.Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HAW. Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes (AFORRD): a randomised controlled trial. Diabetologia. 2009;52:50–59. doi: 10.1007/s00125-008-1179-5. [DOI] [PubMed] [Google Scholar]
- 39.Sattar N, Taskinen M-R. Statins are diabetogenic—myth or reality? Atheroscler Suppl. 2012;13:1–10. doi: 10.1016/j.atherosclerosissup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.