Summary
Background
Stenting is an alternative to endarterectomy for treatment of carotid artery stenosis, but long-term efficacy is uncertain. We report long-term data from the randomised International Carotid Stenting Study comparison of these treatments.
Methods
Patients with symptomatic carotid stenosis were randomly assigned 1:1 to open treatment with stenting or endarterectomy at 50 centres worldwide. Randomisation was computer generated centrally and allocated by telephone call or fax. Major outcomes were assessed by an independent endpoint committee unaware of treatment assignment. The primary endpoint was fatal or disabling stroke in any territory after randomisation to the end of follow-up. Analysis was by intention to treat ([ITT] all patients) and per protocol from 31 days after treatment (all patients in whom assigned treatment was completed). Functional ability was rated with the modified Rankin scale. This study is registered, number ISRCTN25337470.
Findings
1713 patients were assigned to stenting (n=855) or endarterectomy (n=858) and followed up for a median of 4·2 years (IQR 3·0–5·2, maximum 10·0). Three patients withdrew immediately and, therefore, the ITT population comprised 1710 patients. The number of fatal or disabling strokes (52 vs 49) and cumulative 5-year risk did not differ significantly between the stenting and endarterectomy groups (6·4% vs 6·5%; hazard ratio [HR] 1·06, 95% CI 0·72–1·57, p=0·77). Any stroke was more frequent in the stenting group than in the endarterectomy group (119 vs 72 events; ITT population, 5-year cumulative risk 15·2% vs 9·4%, HR 1·71, 95% CI 1·28–2·30, p<0·001; per-protocol population, 5-year cumulative risk 8·9% vs 5·8%, 1·53, 1·02–2·31, p=0·04), but were mainly non-disabling strokes. The distribution of modified Rankin scale scores at 1 year, 5 years, or final follow-up did not differ significantly between treatment groups.
Interpretation
Long-term functional outcome and risk of fatal or disabling stroke are similar for stenting and endarterectomy for symptomatic carotid stenosis.
Funding
Medical Research Council, Stroke Association, Sanofi-Synthélabo, European Union.
Introduction
Atherosclerotic stenosis of the internal carotid artery causes 10–15% of all strokes. Carotid endarterectomy lowers the long-term risk of stroke in patients with symptomatic carotid stenosis.1,2 Carotid artery stenting has emerged as an alternative to endarterectomy. In randomised trials comparing stenting with endarterectomy for symptomatic carotid stenosis, stenting was associated with a higher risk of procedure-related stroke, particularly in elderly patients, but with lower risks of myocardial infarction, cranial nerve palsy, and access site haematoma.3–6 A systematic review showed that the increase in procedure-related risk was driven by non-disabling stroke, with no evidence for a difference in rates of major or disabling stroke or death between the treatments.7 The International Carotid Stenting Study (ICSS) was the largest of the trials assessed that compared stenting with endarterectomy in patients with symptomatic stenosis, and we reported an interim safety analysis of outcomes within 120 days of randomisation in 2010.5 At that time the efficacy of stenting and endarterectomy in preventing stroke and maintaining patency of the carotid artery beyond 2–4 years after treatment and long-term functional outcome was unclear. Here we report the primary analysis of ICSS long-term outcomes up to 10 years after randomisation.
Methods
Study design and patients
ICSS was an international, multicentre, randomised clinical trial of endarterectomy versus stenting for the treatment of symptomatic carotid stenosis. Details on eligibility criteria for centres and patients, randomisation, and treatment have been reported previously.5,8 In brief, patients aged older than 40 years with symptomatic atherosclerotic carotid stenosis causing at least 50% reduction in carotid artery lumen diameter and who were deemed equally suited for both treatments were eligible. Patients who had had a major stroke or previous treatment with carotid stenting or endarterectomy, had contraindications to stenting or surgery, stenosis caused by non-atherosclerotic disease or life expectancy of less than 2 years, or who were scheduled for major surgery within 1 month were excluded. ICSS was approved by the Northwest Multicentre Research Ethics Committee in the UK. Individual participating centres obtained site-specific approval from their local ethics committees. All patients provided written informed consent.
Randomisation and masking
Patients were assigned to undergo stenting or endarterectomy in a 1:1 ratio. Randomisation was computer generated centrally by the Oxford Clinical Trials Service Unit, Oxford, UK, and allocations were obtained by telephone or fax from staff who were not involved in other parts of the trial. Randomisation was stratified by centre with minimisation for sex, age, side of stenosis, and occlusion of the contralateral carotid artery. Patients and investigators were not masked to treatment assignment.
Procedures and follow-up
Stents and cerebral protection devices were chosen at the discretion of the interventionist, but had to be CE marked and approved by the steering committee. Surgeons were free to perform standard or eversion endarterectomy under local or general anaesthesia, with or without the use of shunts and patches. All patients received medical care, including antiplatelet therapy, or anticoagulation if indicated, and medical risk factors, such as hypertension, smoking, and hyperlipidaemia, were monitored.
Patients attended follow-up visits 30 days after treatment (end of the procedural period), 6 months after randomisation, and annually thereafter. Functional ability was measured with the modified Rankin scale at the time of randomisation, at each follow-up visit, and 30 days after any stroke outcome event.9 Duplex carotid ultrasonography was used at each follow-up visit to assess the degree of carotid artery stenosis. Follow-up was initially planned for 5 years but was extended to 10 years in patients who were able and willing to continue.
Outcome events and endpoints
Major outcome events were adjudicated by an independent endpoint committee that was unaware of treatment allocations. Stroke was defined as a rapidly developing clinical syndrome of focal disturbance of cerebral function that lasted more than 24 h or led to death within 24 h with no other apparent non-vascular cause. Stroke was classified as fatal if the patient died within 30 days of onset and as disabling if the modified Rankin scale score had increased to 3 or more at 30 days after onset. Ipsilateral stroke was defined as infarction or haemorrhage in the territory of the anterior or middle cerebral artery on the side of the randomised artery. A procedural event was defined as one that occurred at any time from the start of surgery or stenting (day 0) to day 30 after treatment.
The database manager at the trial office (RLF) monitored procedural outcome events. If two consecutive major events or a cumulative major event rate of more than 10% within 30 days of treatment were seen in the same arm of the study within a study centre, an assessment of the events was triggered. The anonymised records of the relevant outcome events were submitted to the chairman of the data monitoring committee who recommend further action, such as suspending randomisation at the centre.
The degree of carotid stenosis during follow-up was determined centrally from ultrasound flow velocity data, on the basis of predefined criteria (peak systolic velocity and end-diastolic velocity of the internal carotid artery and peak systolic velocity of the common carotid artery).10 The assessor (LHB) was unaware of treatment allocations and dates of ultrasound follow-up. Results of carotid imaging studies ordered outside regular follow-up at the discretion of the treating clinicians, for instance for recurrent symptoms, were also assessed centrally. Restenosis was defined as any residual or recurrent stenosis of at least 70% or occlusion of the carotid artery during follow-up. No correction was made for the presence of a stent when measuring stenosis.
The prespecified primary endpoint was fatal or disabling stroke in any territory after randomisation. Secondary endpoints were all-cause death, any stroke, and combined procedural stroke in any territory, procedural death, and ipsilateral stroke during follow-up. The primary analysis was done in all patients assigned to either treatment, irrespective of treatment received (intention-to-treat [ITT] population). A per-protocol analysis of non-procedural events was done from 31 days after treatment to the end of follow-up in patients who started and completed their allocated treatment (per-protocol population). Rates of ipsilateral restenosis were analysed from the date of the treatment in patients who started and completed their allocated treatment.
Statistical analysis
We estimated that the primary endpoint would be seen in 5·7% of patients in the stenting and endarterectomy groups at 5 years' follow-up. We therefore calculated that a sample size of 1500 patients enrolled at experienced centres would be sufficient to assess the absolute risk difference between groups as measured with a 95% CI of 2·5 percentage points in either direction. In the interim safety analysis no significant difference in risk was seen between stenting and endarterectomy in supervised centres (n=199) and experienced centres (n=1511).5 Therefore, we combined the results from supervised and experienced centres in the long-term analysis.
Kaplan-Meier estimates of cumulative risk at 1 year and 5 years after randomisation (ITT population) or treatment (per-protocol population) and absolute risk differences between treatment groups, with 95% CIs, were calculated. Log-rank tests were used to compare the Kaplan-Meier cumulative incidence curves. Cox's proportional hazard models were used to calculate hazard ratios (HRs) and 95% CIs for outcomes, with endarterectomy as the reference group and using all data to the end of follow-up. The proportionality for Cox's models was tested with Schoenfeld residuals and confirmed no significant departures from the proportionality assumption. We did sensitivity analyses for the main outcomes, as prespecified, with adjustment for the variables used to condition randomisation (centre, age, sex, side of stenosis, and contralateral occlusion). As the number of events was larger in the ITT than in the per-protocol population, we additionally adjusted the ITT analysis for the prespecified variables treated hypertension, treated hyperlipidaemia, diabetes, degree of ipsilateral stenosis, and type of latest event before randomisation. Censoring was assumed to be non-informative.
The restenosis outcome was interval-censored and, therefore, was analysed with a generalised non-linear model that assumes proportional hazards and allows the treatment effect parameter estimate to be interpreted as a log HR.11 The p value for treatment effect was calculated with a likelihood ratio test. Life-table analyses were used to estimate the cumulative incidence of restenosis at 1 year and 5 years after treatment.
Interaction tests were done with Cox's proportional hazard models to investigate whether the treatment effect for the primary endpoint and for procedure-related stroke, death, or ipsilateral stroke during follow-up differed between subgroups of patients on the basis of baseline characteristics. Functional ability was compared in the ITT population at 1 year, 5 years, and final follow-up across the entire range of the modified Rankin scale scores, with the permutation test described by Howard and colleagues.12 Drug treatments and blood pressure at 1 year and 5 years were compared, respectively, with the χ2 test and t tests (Satterthwaite approximation) at each timepoint. All reported p values are two-sided, with values less than 0·05 taken to be significant. No adjustment was made for multiple comparisons. This study is registered, number ISRCTN25337470.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
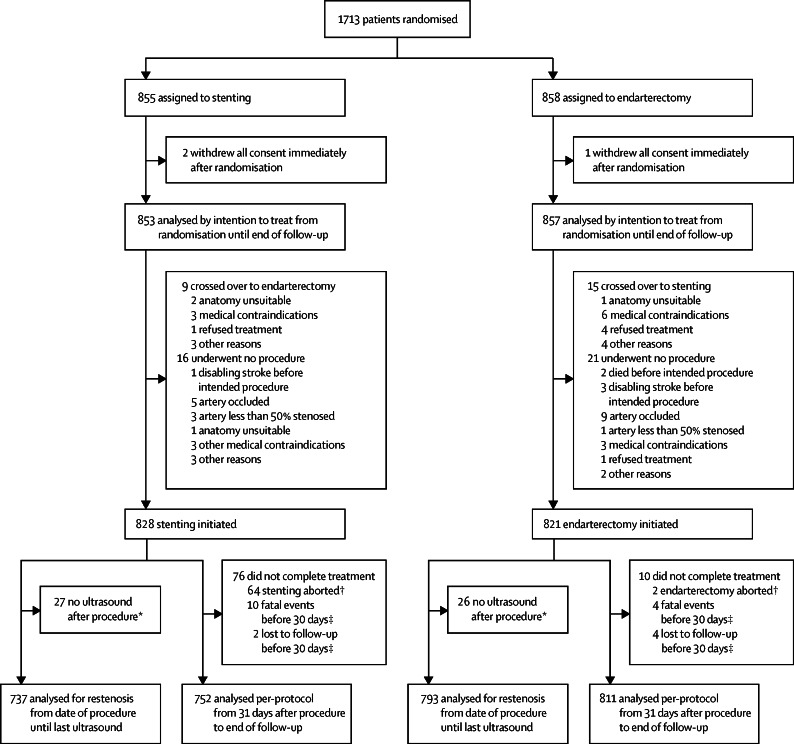
1713 patients were recruited at 50 centres in Europe, Australia, New Zealand, and Canada (appendix) between May, 2001, and October, 2008. 855 patients were randomly assigned to stenting and 858 to endarterectomy. Three patients withdrew before treatment and, therefore, 1710 patients comprised the ITT population (figure 1). The trial was terminated in 2011. Patients were followed up for a median of 4·2 years (IQR 3·0–5·2, maximum 10·0) after randomisation, yielding 7354 patient-years of follow-up. Length of follow-up did not differ between treatment groups (appendix).
Figure 1.
Trial profile
Numbers at screening and excluded before enrolment were not recorded. ITT=intention to treat. *Excluded from the restenosis analysis. † Excluded from per-protocol analysis and restenosis analysis. ‡Excluded from per-protocol analysis.
Patients' characteristics at baseline were similar in the two groups (table 1). Technical information for the stenting and endarterectomy procedures is provided in the appendix. The proportions of patients taking antiplatelet agents or lipid-lowering medications were similar in the two treatment groups for most of the trial period. At 1 year slightly more patients in the endarterectomy group than in the stenting group were taking antihypertensive medications (75% vs 71%, p=0·088) and had lower systolic and diastolic blood pressures (144 vs 147 mm Hg, p=0·011 and 78 vs 79 mm Hg, p=0·035, respectively; appendix). At 5 years of follow-up more patients in the stenting group were receiving antihypertensive treatment (83% vs 76%, p=0·017) and blood pressures no longer differed.
Table 1.
Baseline characteristics
| Stenting (n=853) | Endarterectomy (n=857) | ||
|---|---|---|---|
| Age (years) | 70 (9) | 70 (9) | |
| Male sex | 601 (70%) | 606 (71%) | |
| Vascular risk factors | |||
| Treated hypertension | 587 (69%) | 596 (70%) | |
| Systolic blood pressure (mm Hg) | 147 (24) | 146 (24) | |
| Diastolic blood pressure (mm Hg) | 79 (12) | 78 (13) | |
| Cardiac failure | 23 (3%) | 47 (5%) | |
| Angina in past 6 months | 83 (10%) | 77 (9%) | |
| Previous myocardial infarction | 151 (18%) | 156 (18%) | |
| Previous CABG | 109 (13%) | 116 (14%) | |
| Atrial fibrillation | 57 (7%) | 59 (7%) | |
| Other cardiac embolic source | 19 (2%) | 16 (2%) | |
| Type 2 diabetes | 134 (16%) | 147 (17%) | |
| Type 1 diabetes | 50 (6%) | 41 (5%) | |
| Peripheral artery disease | 139 (16%) | 136 (16%) | |
| Current smoker | 205 (24%) | 198 (23%) | |
| Ex-smoker | 408 (48%) | 424 (49%) | |
| Treated hyperlipidaemia | 522 (61%) | 563 (66%) | |
| Total serum cholesterol (mmol/L) | 4·8 (1·3) | 4·9 (1·3) | |
| Degree of symptomatic carotid stenosis* | |||
| 50–69% | 92 (11%) | 76 (9%) | |
| 70–99% | 761 (89%) | 781 (91%) | |
| Degree of contralateral carotid stenosis* | |||
| <50% | 565 (66%) | 561 (65%) | |
| 50–69% | 128 (15%) | 142 (17%) | |
| 70–99% | 105 (12%) | 110 (13%) | |
| Occluded | 49 (6%) | 37 (4%) | |
| Unknown | 6 (1%) | 7 (1%) | |
| Most recent ipsilateral event† | |||
| Ischaemic hemispheric stroke | 393 (46%) | 376 (44%) | |
| Transient ischaemic attack | 273 (32%) | 303 (35%) | |
| Retinal infarct | 26 (3%) | 23 (3%) | |
| Amaurosis fugax | 148 (17%) | 142 (17%) | |
| Unknown | 13 (2%) | 13 (2%) | |
| Event <6 months before randomisation | 826 (97%) | 816 (95%) | |
| Event 6–12 months before randomisation‡ | 27 (3%) | 36 (4%) | |
| Modified Rankin score at randomisation | |||
| 0–2 | 756 (89%) | 744 (87%) | |
| 3–5§ | 81 (10%) | 99 (12%) | |
| Unknown | 16 (2%) | 14 (2%) | |
Data are mean (SD) or number (%). Some totals do not add up to 100% because of rounding. CABG=coronary artery bypass graft.
Degree of stenosis reported by randomising centre according to the measure used in the North American Symptomatic Carotid Endarterectomy Trial1 or a non-invasive equivalent.
If two events were reported on the same day, that higher up in the order of events as listed was counted.
In three patients the event was more than 12 months before randomisation and in two the date was unknown.
Some modified Rankin scores ≥3 were caused by non-stroke disability.
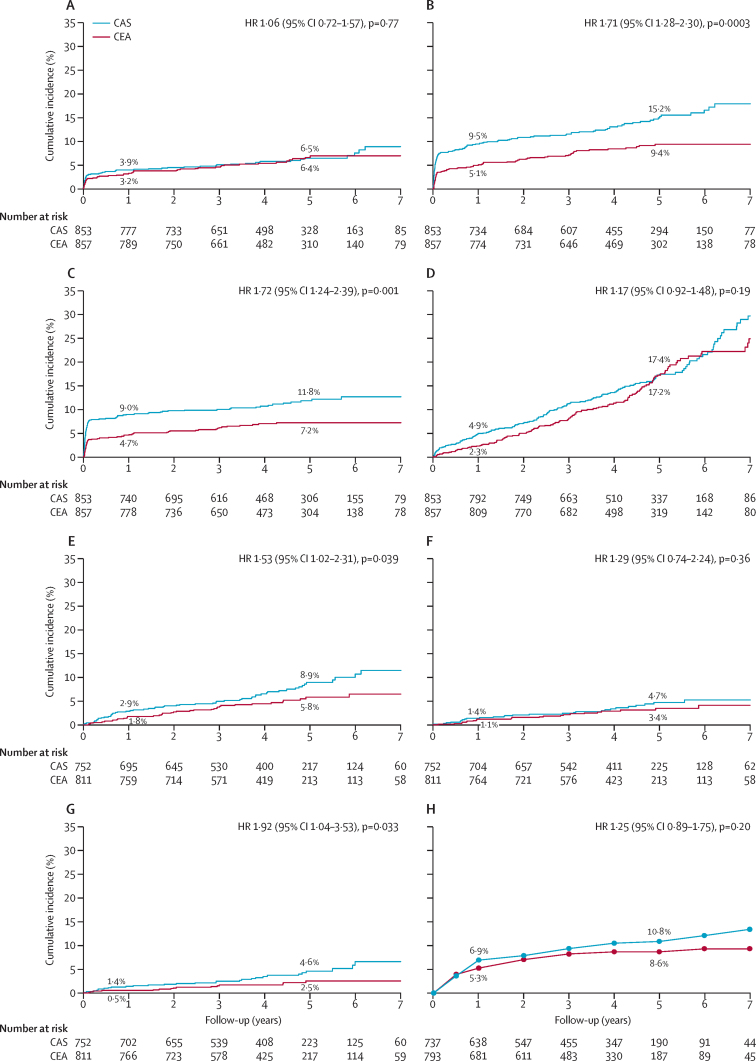
In the ITT population, the primary endpoint of fatal or disabling stroke between randomisation and end of follow-up was seen in 52 of 853 patients in the stenting group (cumulative 5-year risk 6·4%), and in 49 of 857 patients in the endarterectomy group (cumulative 5-year risk 6·5%). No difference was seen between groups in time to first event (table 2, figure 2). In the comparison of secondary outcome events in this population, significantly more patients in the stenting group had any stroke than in the endarterectomy group (p=0·0003, table 2), but the difference was attributable mainly to an excess of non-disabling stroke (73 vs 27 events, appendix). The combined outcome of procedure-related stroke or procedure-related death or ipsilateral stroke during follow-up was also more frequent in the stenting group than in the endarterectomy group (p=0·001, table 2). All-cause mortality did not differ significantly between treatment groups (p=0·19, table 2). Other procedural events and those occurring during the follow-up period are described in the appendix).
Table 2.
Intention-to-treat analysis of cumulative risks and hazard ratios of main outcome events
|
Stenting (n=853) |
Endarterectomy (n=857) |
Hazard ratio*(95% CI) |
Absolute risk difference (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of events* | Cumulative 1-year risk (SE)† | Cumulative 5-year risk (SE)† | Number of events* | Cumulative 1-year risk (SE)† | Cumulative 5-year risk (SE)† | At 1 year | At 5 years | ||
| Fatal or disabling stroke (primary outcome measure) | 52 | 3·9% (0·7) | 6·4% (0·9) | 49 | 3·2% (0·6) | 6·5% (1·0) | 1·06 (0·72 to 1·57) | 0·7% (−1·0 to 2·5) | −0·2% (−2·8 to 2·5) |
| Any stroke | 119 | 9·5% (1·0) | 15·2% (1·4) | 72 | 5·1% (0·8) | 9·4% (1·1) | 1·71 (1·28 to 2·30)‡ | 4·4% (1·9 to 6·9) | 5·8% (2·4 to 9·3) |
| Procedural stroke or procedural death or ipsilateral stroke during follow-up | 95 | 9·0% (1·0) | 11·8% (1·2) | 57 | 4·7% (0·7) | 7·2% (0·9) | 1·72 (1·24 to 2·39)§ | 4·2% (1·9 to 6·6) | 4·6% (1·6 to 7·6) |
| All-cause death | 153 | 4·9% (0·7) | 17·4% (1·5) | 129 | 2·3% (0·5) | 17·2% (1·5) | 1·17 (0·92 to 1·48) | 2·6% (0·8 to 4·4) | 0·2% (−4·0 to 4·4) |
Calculated as the first relevant event between randomisation and the end of follow-up.
Calculated from randomisation onwards.
p<0·001.
p<0·01.
Figure 2.
Kaplan-Meier estimates of cumulative incidence for major outcomes
(A) Fatal or disabling stroke. (B) Any stroke. (C) Procedural stroke or procedural death or ipsilateral stroke during follow-up. (D) All-cause death. (E) Any stroke more than 30 days after treatment. (F) Ipsilateral stroke more than 30 days after treatment. (G) Contralateral carotid or vertebrobasilar stroke more than 30 days after treatment. (H) Ipsilateral severe (at least 70%) carotid stenosis after completed treatment, generated by life-table analysis. Panels A–D show results for the intention-to-treat population, E–G for the per-protocol population from 30 days after treatment, and H for the per-protocol population from treatment. Percentage values are the estimated cumulative incidence at 1 year and 5 years. Graphs stop at 7 years' follow-up because numbers beyond that time were less than 100, but analyses were based on all follow-up data (maximum 10 years). HR=hazard ratio. CAS=carotid stenting. CEA=carotid endarterectomy.
Exploratory subgroup analyses showed that none of the patients' baseline characteristics significantly altered the risk of primary or secondary endpoints (appendix). The HR for a secondary endpoint (procedural stroke or procedural death, or ipsilateral stroke during follow-up) was lower for patients treated at larger centres (enrolling 50 or more patients in the trial) than for those treated at smaller centres (HR 1·27, 95% CI 0·83–1·93 vs 2·77, 1·60–4·77, pinteraction=0·024; appendix).
Monitoring of adverse events led to concern about the stenting results of two investigators at two different supervised centres. These investigators were stopped from treating further trial patients and the centres were suspended from randomisation. All the patients who had been allocated in these centres to stenting (n=11, five with disabling stroke or death) or endarterectomy (n=9, one with fatal stroke) were included in the analyses. Randomisation was restarted in one of the two centres when an interventionist experienced in stenting joined the team. Subgroup analysis showed no difference in treatment effects of stenting versus endarterectomy on primary and secondary endpoints between supervised centres (including the two stopped centres) and experienced centres (appendix).
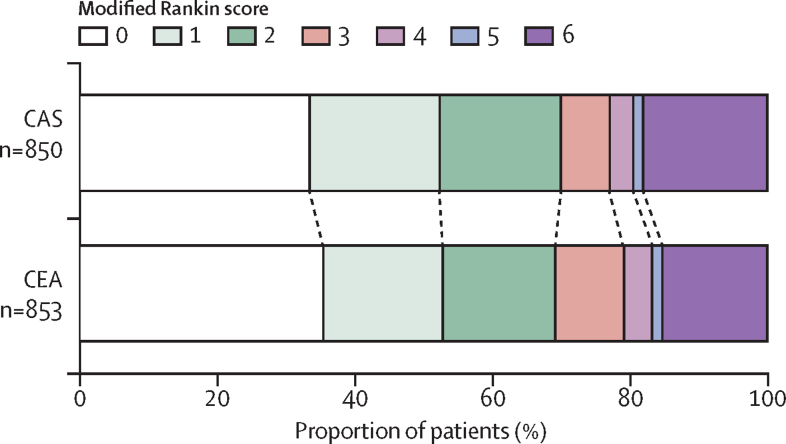
Functional outcome measured by the distribution of modified Rankin scale scores at the end of follow-up did not differ significantly between treatment groups (figure 3), nor was there any evidence of a difference at 1 year or 5 years after randomisation (appendix).
Figure 3.
Functional ability measured by the modified Rankin scale at the end of follow-up*
A permutation test12 was done to compare scores at the end of follow-up between the two groups: unadjusted, p=0·49; adjusted for baseline modified Rankin scale score, p=0·24. CAS=carotid stenting. CEA=carotid endarterectomy. *Excludes seven patients (three CAS and four CEA) who had no scores recorded during follow-up and were still alive at their final visit.
In the per-protocol population, no difference was seen between treatment groups in the rates of fatal or disabling stroke (table 3). Of the secondary endpoint events, no significant difference was seen between groups in ipsilateral stroke in the territory of the treated carotid artery, but stroke in any territory was more frequent in the stenting group than in the endarterectomy group (table 3, figure 2). This difference seemed to be driven by strokes occurring in the territory of the contralateral carotid artery or the vertebrobasilar circulation in patients treated with stents (cumulative 5-year risk 4·6% vs 2·5%, HR 1·92, 95% CI 1·04–3·53, p=0·033). Sensitivity analyses adjusted for the specified variables did not materially change the results.
Table 3.
Per-protocol analysis of cumulative risks and hazard ratios of main outcome events
|
Stenting (n=752) |
Endarterectomy (n=811) |
Hazard ratio*(95% CI) |
Absolute risk difference (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of events* | Cumulative 1-year risk (SE)* | Cumulative 5-year risk (SE)* | Number of events* | Cumulative 1-year risk (SE)* | Cumulative 5-year risk (SE)* | At 1 year | At 5 years | ||
| Fatal or disabling stroke | 24 | 0·9% (0·4) | 3·4% (0·8) | 27 | 1·4% (0·4) | 4·3% (0·9) | 0·93 (0·53 to 1·60) | −0·5% (−1·5 to 0·6) | −0·9% (−3·2 to 1·4) |
| Any stroke | 56 | 2·9% (0·6) | 8·9% (1·2) | 39 | 1·8% (0·5) | 5·8% (1·0) | 1·53 (1·02 to 2·31)† | 1·1% (−0·4 to 2·6) | 3·1% (0·0 to 6·2) |
| Ipsilateral carotid stroke | 28 | 1·4% (0·4) | 4·7% (0·9) | 23 | 1·1% (0·4) | 3·4% (0·8) | 1·29 (0·74 to 2·24) | 0·2% (−0·9 to 1·3) | 1·2% (−1·1 to 3·6) |
| Contralateral carotid or vertebrobasilar stroke | 29 | 1·4% (0·4) | 4·6% (0·9) | 16 | 0·5% (0·3) | 2·5% (0·7) | 1·92 (1·04 to 3·53)† | 0·9% (−0·1 to 1·8) | 2·1% (−0·2 to 4·3) |
| Severe carotid restenosis (≥70%) or occlusion | 72/737 | 6·9% (1·0) | 10·8% (1·3) | 62/793 | 5·3% (0·8) | 8·6% (1·1) | 1·25 (0·89–1·75) | 1·7% (−0·8 to 4·1) | 2·2% (−1·1 to 5·4) |
Calculated from the end of the procedural period (30 days after completed treatment) for the first four outcomes and from immediately after completed treatment for the last outcome, until the end of follow-up.
p<0·05.
No significant difference was seen between groups in long-term rates of severe carotid restenosis (at least 70%) or occlusion (table 3, figure 2).
Discussion
The ICSS primary analysis shows that stenting is as effective as endarterectomy in preventing fatal or disabling stroke in patients with symptomatic carotid stenosis up to 10 years after treatment. Carotid stenting was associated with a higher procedure-related and long-term risk of non-disabling stroke than endarterectomy, but functional ability did not differ overall. Both treatments seemed to be equally preventive against ipsilateral stroke and severe restenosis of the treated carotid artery.
Two smaller randomised trials of stenting versus endarterectomy for symptomatic carotid stenosis have reported mid-term follow-up. The EVA-3S trial13 showed no significant differences in cumulative 4-year rates of fatal or disabling stroke between stenting and endarterectomy (6·3% vs 4·0%). In the SPACE trial,14 ipsilateral disabling stroke within 2 years or death or disabling stroke in any territory within 30 days of treatment were recorded in 5·7% patients who underwent stenting and 4·7% of patients who underwent endarterectomy. In CREST,6 which included patients with symptomatic and asymptomatic carotid stenosis, a slightly increased risk was seen for major ipsilateral stroke in the stenting group compared with that in the endarterectomy group up to 4 years after treatment (1·4% vs 0·5%; p=0·05). Our data are consistent with these findings and show that the long-term risk of having a severe stroke remains low after either treatment.
The increased risk of procedure-related stroke of any severity with stenting was reported in our interim report on short-term outcomes of ICSS (excess risk of 3·7% at 30 days after treatment).5 The current analysis showed that the risk of stroke of any severity occurring in any territory during follow-up was also increased in the stenting group (excess risk 1·1% compared with endarterectomy at 1 year, and 3·1% at 5 years), but strokes were mainly non-disabling events. In CREST,6 patients who had minor strokes were reported to have reduced physical and mental health when assessed with the SF-36 questionnaire. We used the modified Rankin scale, which does not directly capture subjective perception of wellbeing or subtle changes in physical or mental functioning. Assessment with the EQ-5D questionnaire in ICSS, however, showed no significant difference in quality of life between the two treatment groups (unpublished). Thus, while we cannot rule out any differences in long-term sequelae of stroke and other outcome events that are not captured by the modified Rankin scale or EQ-5D, the absolute difference in the risk of any stroke is small, with the 47 additional strokes in the stenting group translating to one extra stroke (typically non-disabling) for every 156 patient-years of follow-up.
The difference between treatment groups in risk of stroke after the procedural period was mainly attributable to strokes occurring in the contralateral carotid or vertebrobasilar territory in the stenting group. We have no conclusive explanation for this finding. In ACST,15 immediate endarterectomy reduced the number of strokes occurring in the ipsilateral and contralateral carotid territories. Patients treated by endarterectomy in CAVATAS16 also had fewer strokes in the contralateral carotid or vertebrobasilar territory than those who received endovascular treatment. Endarterectomy might, therefore, have a beneficial effect in preventing strokes occurring outside the territory of the revascularised artery, but the underlying mechanism is unclear. A small substudy in the ICSS population showed that at 1 month after revascularisation, stenting and endarterectomy had different effects on the diameters of segments of the circle of Willis.17 Whether this difference had any effect on collateral flow to the opposite hemisphere is unclear, as neither cerebral perfusion nor long-term differences were assessed. We cannot rule out the possibility that the difference in non-ipsilateral strokes in ICSS represents a chance finding because the number of events was small.
CAVATAS10 showed an excess risk of severe carotid restenosis or occlusion after endovascular treatment. The SPACE trial14 also reported higher rates of severe restenosis 2 years after treatment in the stenting group than in the endarterectomy group (10·7% vs 4·6%). By contrast, the rates of severe restenosis at 3 years did not differ between stenting and endarterectomy in the EVA-3S trial (3·3% vs 2·8%)18 or in CREST at 2 years (6·0% vs 6·3%).19 In ICSS we found no evidence of differences in the long-term rates of severe restenosis or occlusion after stenting compared with endarterectomy (cumulative 5-year risk 10·8% vs 8·6%). The value for stenting, however, might overestimate the rate of restenosis because we used flow velocity criteria to grade stenosis and made no correction for possible changes in elasticity of the stented artery.20 However, even if there were a true small difference of a few percentage points in restenosis rates, it might not be clinically relevant. Further analysis to compare the rate of moderate (at least 50%) restenosis between treatment groups and to investigate the association between restenosis and recurrent stroke is planned.
Subgroup analyses showed no significant effects of patient-related baseline characteristics on the comparison of stenting and endarterectomy. However, ICSS was not powered to discover such effects. In a separate analysis of ICSS restricted to patients who were randomised to and received stent treatment, age was an independent predictor of the risk of stroke, myocardial infarction, or death within 30 days of stenting, with a relative increase in risk of 17% for every 5 years of increasing age (risk ratio 1·17, 95% CI 1·01–1·37; unpublished). A previous pooled analysis of stent trials by the Carotid Stenting Trialists' Collaboration (CTSC) also showed that the excess in procedural strokes associated with stenting for symptomatic carotid stenosis was limited to patients older than 70 years, whereas younger patients had very similar risks of stroke or death whether they were allocated stenting or endarterectomy.21 An update of those data, taking into account data from CREST,19 showed a positive interaction between age and the combined outcome of procedural stroke or death or ipsilateral stroke during follow-up, with the comparison showing no significant difference between treatments in patients younger than 70 years and a significant risk increase in the stenting group compared with the endarterectomy group in patients older than 70 years.22
An excess risk of myocardial infarction and wound-related complications associated with endarterectomy was shown in a systematic review of randomised trials.7 The long-term results of ICSS in conjunction with the existing evidence, therefore, provides reassurance that stenting can be offered as a durable procedure to patients with characteristics associated with similar or reduced risks of procedure-related stroke (eg, age younger than 70 years). Findings on brain imaging might be useful to consider, as we have shown previously that severe white-matter lesions on baseline CT or MRI are associated with an increased risk of stroke in patients who undergo carotid artery stenting.23 In patients who are suitable for both treatments, their preferences related to the different risks with the two procedures should also be taken into account.
Ongoing controversy surrounds the role of operator experience when comparing carotid stenting with endarterectomy. We found no difference in relative hazards between stenting and endarterectomy when comparing supervised and experienced study centres. The HR for the combined outcome of procedural stroke or procedural death or ipsilateral stroke during follow-up was lower for centres that enrolled 50 or more patients than for smaller centres, which might indicate some effect of procedural volume on technical expertise, but the point estimate still favoured endarterectomy in the larger centres. However, the previous pooled analysis of stent trials by the CSTC showed no significant difference between high-volume and low-volume recruiting centres in relative risks of stroke or in death between stenting and endarterectomy in the first 120 days after randomisation.21 Therefore, we cannot rule out a chance effect in the long-term findings of ICSS. A separate analysis by the CSTC showed that risks of procedure-related stroke or death decreased with increasing annual in-trial procedure volumes, which suggests that regular practice in carrying out the procedure matters more than individual total experience or centre volumes.24
This study has several limitations. First, carotid stenting was a relatively new procedure when ICSS started. Since that time experience with the procedure has increased and new cerebral protection systems exerting flow arrest or reversal have become available that might more effectively prevent procedural stroke than the distal filter-type devices that were mainly used in ICSS.25 Evidence also indicates, however, that the risks associated with endarterectomy have declined over the past few years.26 Second, we are unable to account for causes (eg, dementia) of functional decline over time, as measured by the modified Rankin scale, other than the endpoint outcomes events measured in the trial. Third, the modified Rankin scale is not a precise measure of the level of independence, and we cannot rule out subtle differences in functional outcome or subjective perception of wellbeing between the two treatment groups. Fourth, the trial was not powered to detect variations in treatment effects between subgroups of patients.
Overall we found that stenting and endarterectomy are durable procedures that are equally effective in preventing severe strokes that lead to disability or death (panel). Stenting has the disadvantage of causing more minor non-disabling strokes in the procedural period and possibly in the long term. This feature, however, must be weighed against the increased risk of procedural myocardial infarction, cranial nerve palsy, and access-site haematoma associated with endarterectomy.3–6 The modified Rankin scale scores suggested similar short-term and long-term functional outcomes with the two treatments. The choice between stenting and endarterectomy should take into account the different procedure-related risks in line with other characteristics of individual patients.
Panel. Research in context.
Systematic review
We updated our Cochrane Collaboration review of angioplasty and stenting treatment versus endarterectomy for symptomatic carotid artery stenosis with the long-term outcomes of ICSS and those of the EVA-3S trial.7 Only randomised trials of primary carotid stenting (ie, routine placement of a stent) and reporting outcomes in symptomatic patients were included. The systematic review assessed combined stroke (any) or death in the procedural period or ipsilateral stroke thereafter until the end of follow-up, which was reported in all large randomised trials. The procedural period was that between randomisation and 30 days after the procedure or 30 days after randomisation in patients who did not undergo carotid revascularisation, to match the definition in the seven reports assessed. The odds ratio of the combined outcome of any stroke or death in the procedural period or ipsilateral stroke thereafter was significantly greater in patients randomised to stenting than in those randomised to endarterectomy, with little evidence for heterogeneity between trials (odds ratio 1·47, 95% CI 1·16–1·85, p=0·001; I2=10%; appendix).27
Interpretation
ICSS contributes about a third of the total evidence from randomised trials of stenting versus endarterectomy for symptomatic carotid stenosis, measured by statistical weight (appendix). We found overall the balance between procedure-related risks and long-term efficacy in preventing stroke favours endarterectomy. At the individual level, however, the data show that this difference is caused by an excess in non-disabling procedural strokes associated with stenting,22 and did not seem to translate into lesser functional ability in the long term in patients who received stents. Moreover, this excess risk seemed to be limited to elderly patients (70 years or older).7,22 The choice between stenting and endarterectomy, therefore, should take into account the different procedure-related risks for the two approaches in individual patients.
Acknowledgments
Acknowledgments
This study was supported by the Dutch Heart Foundation (2010T075; HBvdW), European Union, Kaethe-Zingg-Schwichtenberg-Fonds of the Swiss Academy of Medical Sciences (STE), Medical Research Council (MRC G0300411, which was managed by the National Institute for Health Research on behalf of the MRC-NIHR partnership), Sanofi-Synthélabo, the Stroke Association, Swiss Academy of Medical Sciences (STE), Swiss National Science Foundation (PBBSB-116873; LHB, STE), and the University of Basel (LHB). MMB's Chair in Stroke Medicine is supported by the Reta Lila Weston Trust for Medical Research. This work was done at University College London Hospital/University College London, which received a proportion of funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.
Contributors
LHB contributed to analysis and interpretation of data and wrote the first draft of the manuscript. JD did the statistical analyses and prepared the tables and figures. RLF maintained the trial database and prepared the data for analysis. JE contributed to data analysis. All authors contributed to revision of the manuscript for important intellectual content and made substantial contributions to the concept and design of the study and acquisition, analysis, and interpretation of data.
Declaration of interests
HBvdW has received speaker's fees from GlaxoSmithKline, Sanofi-Aventis, and Servier, and has served as a consultant to Bristol-Myers Squibb. STE has received funding for travel or speaker honoraria from Bayer, Boehringer Ingelheim, Pfizer, Sanofi-Aventis, and Shire, and has served on scientific advisory boards for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Pfizer. All other authors declare no competing interests.
Supplementary Material
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 3.Mas JL, Chatellier G, Beyssen B. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 4.The SPACE Collaborative Group 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 5.International Carotid Stenting Study investigators Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brott TG, Hobson RW, Howard G. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD000515.pub4. CD000515. [DOI] [PubMed] [Google Scholar]
- 8.Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69–74. doi: 10.1159/000078753. [DOI] [PubMed] [Google Scholar]
- 9.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 10.Bonati LH, Ederle J, McCabe DJ, for the CAVATAS Investigators Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:908–917. doi: 10.1016/S1474-4422(09)70227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collet D. Modelling survival data in medical research. 2nd edn. Chapman & Hall/CRC; London: 2003. pp. 286–296. [Google Scholar]
- 12.Howard G, Waller JL, Voeks JH. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke. 2012;43:664–669. doi: 10.1161/STROKEAHA.111.632935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mas JL, Trinquart L, Leys D, Albucher JF, for the EVA-3S investigators Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein HH, Ringleb P, Allenberg JR. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 15.Halliday A, Harrison M, Hayter E, for the Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ederle J, Bonati LH, Dobson J, for the CAVATAS Investigators Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:898–907. doi: 10.1016/S1474-4422(09)70228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bost RB, Hendrikse J, Algra A. Effects of carotid endarterectomy or stenting on arterial diameters in the circle of Willis. J Stroke Cerebrovasc Dis. 2014;23:699–705. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Arquizan C, Trinquart L, Touboul PJ. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. 2011;42:1015–1020. doi: 10.1161/STROKEAHA.110.589309. [DOI] [PubMed] [Google Scholar]
- 19.Silver FL, Mackey A, Clark WM, for the CREST Investigators Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) Stroke. 2011;42:675–680. doi: 10.1161/STROKEAHA.110.610212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nederkoorn PJ, Brown MM. Optimal cut-off criteria for duplex ultrasound for the diagnosis of restenosis in stented carotid arteries: review and protocol for a diagnostic study. BMC Neurol. 2009;9:36. doi: 10.1186/1471-2377-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carotid Stenting Trialists' Collaboration Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 22.Calvet D, Dobson J, Algra A. Long term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Cerebrovasc Dis. 2014;37(suppl 1):192. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 23.Ederle J, Davagnanam I, van der Worp HB, for the ICSS investigators Effect of white-matter lesions on the risk of periprocedural stroke after carotid artery stenting versus endarterectomy in the International Carotid Stenting Study (ICSS): a prespecified analysis of data from a randomised trial. Lancet Neurol. 2013;12:866–872. doi: 10.1016/S1474-4422(13)70135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvet D, Mas JL, Algra A. Carotid stenting: is there an operator effect? A pooled analysis from the carotid stenting trialists' collaboration. Stroke. 2014;45:527–532. doi: 10.1161/STROKEAHA.113.003526. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Afonso LH, Abud LG, Rolo JG. Flow reversal versus filter protection: a pilot carotid artery stenting randomized trial. Circ Cardiovasc Interv. 2013;6:552–559. doi: 10.1161/CIRCINTERVENTIONS.113.000479. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy F, Dobson J, Doig DD, Featherstone RL, Richards T, Brown MM, for the ICSS and CAVATAS Investigators The risks of carotid endarterectomy have declined: an analysis of two trials with similar protocols. Cerebrovasc Dis. 2013;35(suppl 3):463. [Google Scholar]
- 27.Higgins JPT, Altman DG, Sterne JAC, eds, for the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Assessing risk of bias in included Studies. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions: version 5.1.0. http://handbook.cochrane.org/front_page.htm (accessed Aug 20, 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.