Abstract
Non-steroidal anti-inflammatory drugs prevent colorectal cancer by inhibiting cyclooxygenase (COX) enzymes that synthesize tumor-promoting prostaglandins. 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is a tumor suppressor that degrades tumor-promoting prostaglandins. Murine knockout of 15-PGDH increases susceptibility to azoxymethane-induced colon tumors. It also renders these mice resistant to celecoxib, a selective inhibitor of inducible COX-2 during colon neoplasia. Similarly, humans with low colonic 15-PGDH are also resistant to colon adenoma prevention with celecoxib. Here, we used aspirin and sulindac, which inhibit both COX-1 and COX-2, in order to determine if these broader COX inhibitors can prevent colon tumors in 15-PGDH knockout (KO) mice. Unlike celecoxib, sulindac proved highly effective in colon tumor prevention of 15-PGDH KO mice. Significantly, however, aspirin demonstrated no effect on colon tumor incidence in either 15-PGDH wild-type or KO mice, despite a comparable reduction in colonic mucosal Prostaglandin E2 (PGE2) levels by both sulindac and aspirin. Notably, colon tumor prevention activity by sulindac was accompanied by a marked induction of lymphoid aggregates and proximal colonic inflammatory mass lesions, a side effect seen to a lesser degree with celecoxib, but not with aspirin. These findings suggest that sulindac may be the most effective agent for colon cancer prevention in humans with low 15-PGDH, but its use may also be associated with inflammatory lesions in the colon.
Introduction
Colon cancer is the second leading cause of cancer-related death in the USA (1). The development of these tumors is driven by the cumulative effects of genetic and epigenetic alterations that occur over time resulting in gain-of-function oncogenic alterations and inactivation of tumor suppressor pathways (2,3). Among the most common of these changes is a marked induction of cyclooxygenase-2 (COX-2) expression. This enzyme catalyzes the initial step in the conversion of arachidonic acid into bioactive prostaglandins (3–6). Tumor promotion is further associated with suppression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) expression, an opposing tumor suppressor gene that catabolizes and inactivates prostaglandins through conversion of the prostaglandin 15-hydroxyl group to a keto-group (7). Thus, induction of COX-2 and inactivation of 15-PGDH combine to markedly increase levels of PGE2, the predominant prostaglandin detected in human colon tumors.
Human 15-PGDH is highly expressed in the normal colonic epithelium which is largely due to induction of 15-PGDH by transforming growth factor (TGF)-beta. In contrast, 15-PGDH expression is absent in colon cancer wherein the TGF-beta pathway is inactivated by mutations in the TGF-beta receptors and in SMAD genes encoding the postreceptor-signaling complex (3). Accordingly, 15-PGDH gene knockout renders mice markedly susceptible to induction of colon tumors, whereas COX-2 gene knockout confers resistance to colon tumor induction (8,9). Non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit COX-2 and/or its companion isoenzyme COX-1 demonstrate strong colon tumor prevention activity in humans (10–14). These drugs span a range of selectivity, with celecoxib best inhibiting COX-2, sulindac being slightly more potent as an inhibitor of COX-2 than COX-1, and aspirin, although preferentially a COX-1 inhibitor, can also inhibit prostaglandin production by COX-2 (15). In general, celecoxib, sulindac and aspirin have all been successful in preventing colon adenoma development, and/or colon cancer in high-risk human populations (10–14), and regular aspirin use has also been associated with a marked reduction in colon cancer development in observational studies of the general population (16).
The importance of NSAIDs in preventing colon tumors is, however, qualified by recent data showing that a substantial subset of humans demonstrate very low levels of 15-PGDH expression in their normal colon mucosa, imparting potential resistance to the colon tumor prevention activity of celecoxib in these individuals (17). These human observations were further supported by the finding that celecoxib was markedly less effective in preventing adenomas in 15-PGDH knockout (KO) mice, as compared with 15-PGDH wild-type (WT) mice (17).
The present study was undertaken to determine whether resistance to colon tumor prevention with the COX-2 selective inhibitor celecoxib in 15-PGDH KO mice, and by inference in humans who are low in 15-PGDH, could be circumvented by use of non-selective COX-1 and COX-2 inhibitors. Both aspirin and sulindac were chosen for this study, as these two agents along with celecoxib, are the best studied for colon tumor chemoprevention in man.
Materials and methods
Mouse azoxymethane/NSAID treatment
Mouse studies were performed in the Case Animal Resource Center under a protocol approved by the Institutional Animal Care and Use Committee. 15-PGDH KO mice on a FVB/N background were generated as described previously (17,18) and were further bred from generation F8 to generation F14 onto an FVB/N (Jackson Laboratory, Bar Harbor, ME) background, with genotyping done as described previously (8). Generation F14 15-PGDH +/− mice were intercrossed and siblings of 15-PGDH +/+ and −/− genotypes were selected for studies involving azoxymethane (AOM) and NSAID treatment. Eight- to 12-week-old mice were administered AOM by intraperitoneal injection once weekly for 6 weeks at 10 mg/kg dose (Sigma Chemical Co., St Louis, MO). Mice were euthanized 14 weeks after the last AOM injection. Concurrent with the first AOM injection, all mice were fed for the length of the study an AIN-76A diet (Harlan Teklad, Madison, WI) supplemented with or without NSAID. NSAID doses used were determined from the literature and were as follows: celecoxib (1250 p.p.m.; LKT laboratories, St Paul, MN) (17,19,20); sulindac (167 p.p.m.; DCP Repository, Germantown, MD) (21); aspirin (300 p.p.m.; LKT laboratories) (22). After euthanizing, the colons were opened longitudinally, rinsed with ice-cold phosphate-buffered saline and examined under a dissecting microscope to identify all tumors. Representative tumors, proximal colon lesions and lymphoid aggregates were resected, fixed in 10% neutral-buffered formalin and paraffin-embedded for histologic examination.
Prostaglandin E2 (PGE2) analyses
Following euthanization after 2 weeks of control- or NSAID-supplemented diet, mouse colons were opened, washed with ice-cold phosphate-buffered saline and gently scraped using a glass slide to isolate the colonic mucosa, which was then snap frozen in liquid nitrogen and stored at −80°C. PGE2 levels were determined using a PGE2 ELISA kit from Enzo Life Sciences (Farmingdale, NY). Briefly, frozen tissue was homogenized in tissue lysis buffer (50mM Tris pH 7.2, 250mM sucrose, 2mM ethylenediaminetetraacetic acid, 2mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 50 μM NaF and 1% Triton) by an Ultrasonic Processor (Misonix, Farmingdale, NJ). An aliquot (100 μl) of supernatant, collected from centrifugation of homogenate at 16 000 r.p.m. for 10min at 4°C, was mixed with 100 μl of phosphate-buffered saline and acidified with 0.1 N citric acid (20 μl). The solutions were then applied to a Sep-Pak C18 cartridge (Waters Corp, Milford, MA) and prostaglandins were eluted with 3ml of hexane:ethyl acetate (1:1). The eluate was evaporated under stream of nitrogen and residue was reconstituted in 25 μl ethanol and 200 μl Assay buffer (Enzo Life Sciences). The level of PGE2 was quantified using an enzyme immunoassay kit (Enzo Life Sciences) and results are expressed as nanograms of PGE2 per milligram of protein (PGE2 ng/mg protein). Analysis of colonic PGE2 levels from vehicle control- or AOM-treated mice was similar as described above except that mice were injected at day 1 and day 8 with either vehicle control or 10mg/kg of AOM, with euthanization following 24h after the second AOM injection, and PGE2 levels were quantified using an enzyme immunoassay kit from R&D Systems. Control- or NSAID-containing diets were given concurrent with the first AOM injection and were present for the length of the study.
CD3, CD45R/B220, F4-80 and COX-2 immunohistochemical studies
The antibodies COX-2 (160106; Cayman Chemical, Ann Arbor, MI), CD3 (ab16669; Abcam, Cambridge, MA), CD45R/B220 (550286; BD Pharmingen, San Diego, CA) and F4-80 (MCA457GA; AbDSerotec, Raleigh, NC) were used for immunostaining. Briefly, 5 ìM sections were cut from formalin-fixed paraffin-embedded tissues and placed on glass slides. Sections were baked for 75min at 60°C, deparaffinized and rehydrated. Antigen retrieval was performed by either steaming at 98°C for 20min (CD45R/B220 and F4-80) or by pressure cooker for 30 s at 123°C (CD3 and COX-2) in 1x Rodent Decloaker (BioCare Medical, Concord, CA), plus a cool-down period of 20min. Reduction of peroxidases was accomplished by incubating in Peroxidazed 1 solution (BioCare Medical) for 8min. Non-specific protein blocking using Rodent Block M (BioCare Medical) was performed for 30min. Primary antibodies were diluted in Serum Free Protein Block (Dako, Carpenteria, CA) and the antibody dilution and room temperature incubation times were as follows, F4-80 1:200 for 30min, COX-2 1:400 for 1 h, CD3 1:200 for 1 h and CD45R/B220 1:100 for 1 h. After primary incubation, the slides were washed and the Rat-on-Mouse HRP-Polymer Detection Kit (F4-80 and CD45R/B220) or Rabbit-on-Rodent HRP-Polymer Detection Kit (COX-2 and CD3) (BioCare Medical) was used in combination with the Betazoid DABKit (BioCare Medical) for development, following manufacturer’s protocol. Development times were 5min for all immunostains.
Statistical analyses
Data were analyzed using the GLIMMIX procedure of SAS (Version 9.2; SAS Institute, Cary, NC). The normal distribution was used in determining the differences in PGE2 levels; the Poisson distribution was used in determining the difference between count data (number of tumors, number of lymphoid aggregates or number of inflammatory mass lesions). The model predictor variables included mouse type (WT or KO) and/or type of diet. The following comparisons were carried out: (i) Each diet for WT versus KO mice, (ii) Control diet versus each NSAID diet for the WT mice, (iii) Control diet versus each NSAID diet for the KO mice, (iv) WT mice on control diet versus KO mice on each of the NSAID diets, (v) WT mouse on celecoxib versus WT mouse on sulindac, and (vi) KO mouse on celecoxib versus KO mouse on sulindac, (vii) WT mouse on aspirin versus WT mouse on celecoxib/sulindac for PGE2 levels only, (viii) KO mouse on aspirin versus KO mouse on celecoxib/sulindac for PGE2 levels only. Contrast statements were used to obtain the P value for each pairwise comparison. Results were considered significant if the P value was ≤0.05. Differences in PGE2 levels from vehicle- or AOM-treated mice on control- or NSAID-supplemented diets were determined using an unpaired two-sided Student’s t-test with a P value of ≤0.05 considered significant.
Results
Effect of NSAID treatment on colonic PGE2
FVB/N mice were selected for study as this strain is highly sensitive to colon tumor induction by AOM (17,23). Doses of celecoxib, sulindac and aspirin for use in treating mice were largely determined on the basis of human dose equivalent and from past studies from our laboratory (17,19–22). WT and KO mice were placed on an NSAID-free control diet or a diet containing either 1250 p.p.m. celecoxib, 167 p.p.m. sulindac or 300 p.p.m. aspirin for 2 weeks, after which colon epithelial scrapes were collected and colonic PGE2 levels measured.
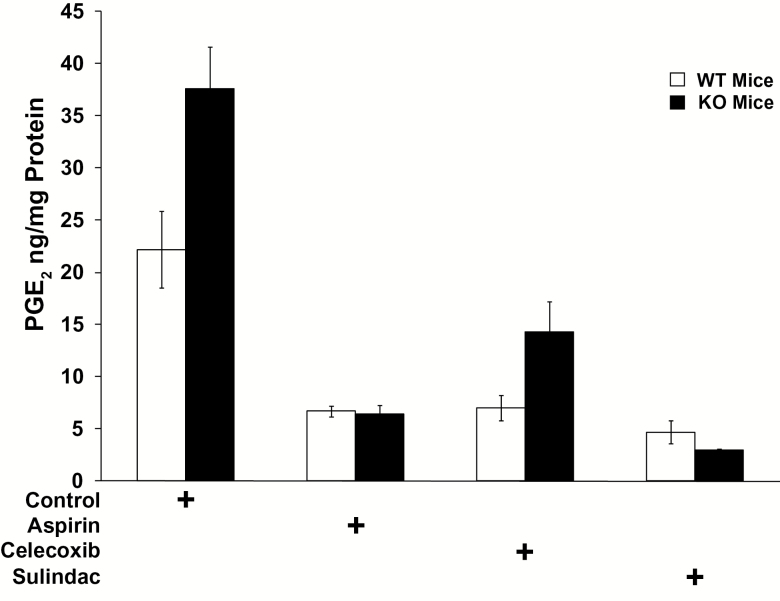
Treatment of WT mice with either celecoxib, aspirin or sulindac resulted in a significant 3.2- to 4.7-fold decrease in colonic mucosal PGE2 levels ranging from 22.2 ng/mg protein in control mice versus 6.7ng/mg protein for aspirin (P < 0.0001), 7.0ng/mg protein for celecoxib (P < 0.0001) and 4.7ng/mg protein for sulindac (P < 0.0001) (Figure 1 and Supplementary Table 1B, available at Carcinogenesis Online), with no statistically significant difference in the efficacy among the three different agents (P > 0.42, Supplementary Table 1B, available at Carcinogenesis Online).
Figure 1.
Colonic mucosal PGE2 levels (in ng/mg protein) in 15-PGDH WT FVB mice (open bars) that are: untreated (control, n = 5), or treated with celecoxib (n = 5), aspirin (n = 5) or sulindac (n = 5); versus FVB 15-PGDH KO mice (black bars) that are: untreated (control, n = 7), or treated with celecoxib (n = 5), aspirin (n = 4) or sulindac (n = 3). Error bars designate standard error of the mean.
As shown previously (17), colonic PGE2 levels are about double in 15-PGDH KO compared with WT mice fed a control diet (37.6ng/mg protein in KO versus 22.2ng/mg protein in WT; P = 0.0214; Figure 1 and Supplementary Table 1A, available at Carcinogenesis Online). Similar to our previous findings (17), while celecoxib lowered colonic PGE2 levels in the 15-PGDH KO mice [decreasing PGE2 from the initial level of 37.6ng/mg protein down to 14.3 ng/mg protein in mice on drug (P < 0.0001)] the colonic PGE2 levels of celecoxib-treated KO mice still remained twice that of celecoxib-treated WT mice (14.3 versus 7.0 ng/mg protein; P = 0.0499; Figure 1 and Supplementary Table 1A and D, available at Carcinogenesis Online). In contrast, aspirin and sulindac both lowered colonic PGE2 levels in 15-PGDH KO mice to levels shown in treated WT mice (Figure 1 and Supplementary Table 1A, available at Carcinogenesis Online). Thus, while aspirin, celecoxib and sulindac all lowered colonic PGE2 in 15-PGDH WT mice to essentially the same level, colonic PGE2 in 15-PGDH KO mice remained higher in the celecoxib treated than in aspirin- or sulindac-treated animals. The apparent resistance of 15-PGDH KO mice to celecoxib was not due to under dosing of the mice with this drug, as dosing with 1250 p.p.m. of celecoxib for 20 weeks was associated with a 38% mortality rate in the celecoxib-treated mice, as compared with a 7% mortality in control diet mice (P = 0.0004). This reflected the development of cecal perforations in some celecoxib-treated animals. In summary, while all three drugs were administered at pharmacologically active and equivalent doses, 15-PGDH KO mice demonstrated a selective resistance to the PGE2-lowering activity of celecoxib, suggesting in these mice a greater efficacy of agents that inhibit COX-1 as well as COX-2.
Differential NSAID effectiveness in preventing colon adenoma formation in 15-PGDH KO mice
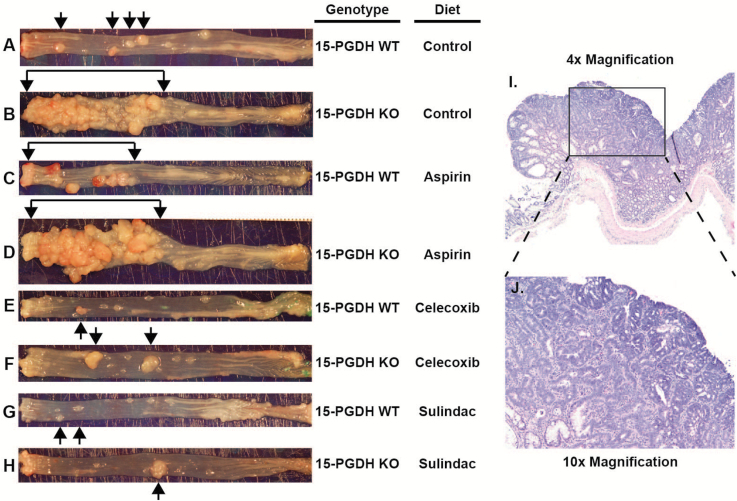
Previous studies from our laboratory have shown that 15-PGDH-deficient mice (and humans) are resistant to the colon tumor prevention activity of celecoxib. In view of the increased efficacy of aspirin and sulindac, versus celecoxib in decreasing colonic PGE2 in 15-PGDH KO mice, we next compared the activity of these agents in preventing colon tumor induction in 15-PGDH KO and WT mice. Mice were treated with AOM weekly for 6 weeks, along with concurrent administration of an NSAID. The NSAID was then continued for 14 more weeks (20 weeks total), at which time, mice were killed for inspection of the large bowel for colon tumors. In all experimental groups, gross examination of the colons identified typical AOM-induced tumors of the distal colon (Figure 2A–H, black arrows), which upon histologic examination proved to be colonic adenomas, often with high-grade dysplasia/carcinoma in situ (Figure 2I and J). These raised adenomatous lesions were easily separable by location and/or gross morphology from a second class of more proximal lesions, which as discussed below, proved to be inflammatory in nature.
Figure 2.
Gross morphology of colon adenoma tumors (black arrows) in colons from AOM-treated 15-PGDH WT (A, C, E and G) and 15-PGDH KO mice (B, D, F and H) administered control (A and B), aspirin (C and D), celecoxib (E and F) or sulindac (G and H) containing diets. (I and J) Hematoxylin and eosin staining of a representative colon adenoma at ×4 magnification (I) and ×10 magnification (J).
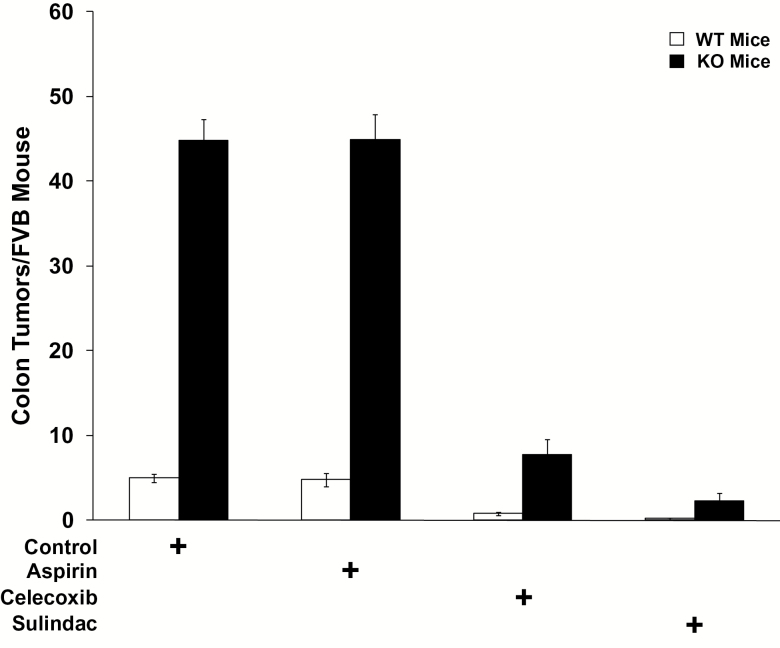
PGDH WT mice on control diets developed an average of 5.0 colon adenomas (Figures 2A and 3). Consistent with our previous studies, celecoxib treatment effectively prevented colon tumors in PGDH WT mice, lowering colon tumor numbers by 6-fold to only 0.8 tumors per mouse (P < 0.0001) (Figures 2E and 3; Supplementary Table 2B, available at Carcinogenesis Online). Sulindac proved even more active than celecoxib in preventing colon adenomas in PGDH WT mice, with only ~0.2 tumors per mouse (Figures 2G and 3), a significant reduction versus mice on control diet (P < 0.0001), as well as versus mice on celecoxib (P = 0.0185) (Supplementary Table 2B, available at Carcinogenesis Online). Quite unexpectedly, although aspirin was equally effective as celecoxib and sulindac in lowering PGE2 in 15-PGDH WT mice, it did not prevent colon tumor induction in these animals, as aspirin-treated 15-PGDH WT mice developing 4.8 tumors per mouse, indistinguishable from mice on control diet (P = 0.81) (Figures 2D and 3; Supplementary Table 2B, available at Carcinogenesis Online). In this regard, the dose of aspirin used in these studies was the metabolic equivalent to 167mg/day in humans (24) which was selected to exceed the 81 mg/day ‘low dose’ aspirin regime that has been shown to be colon tumor preventive in human interventional studies (25), and also to exceed the 325 mg twice weekly aspirin dose that has been associated with colon tumor prevention in observational studies (26). The lack of response to aspirin was not due to any compensatory downregulation of 15-PGDH, as western analysis of colon mucosa from identically aspirin-treated mice demonstrated levels of 15-PGDH protein that were similar or even slightly increased compared with mice on control diets (27).
Figure 3.
AOM induced colon tumor development in 15-PGDH WT FVB mice (open bars) that are: untreated (control, n = 19) or treated with celecoxib (n = 16), aspirin (n = 17) or sulindac (n = 19); versus FVB 15-PGDH KO mice (black bars) that are: untreated (control, n = 19), or treated with celecoxib (n = 20), aspirin (n = 16) or sulindac (n = 18). Error bars designate standard error of the mean.
We next examined the effectiveness of these agents on colon tumor prevention in 15-PGDH KO mice. Consistent with our previous findings, PGDH KO mice develop markedly more AOM-induced colon tumors, with 44.8 tumors per KO mouse versus 5 per WT mouse (P < 0.0001) (Figures 2B and 3; Supplementary Table 2A, available at Carcinogenesis Online). Consistent with the increased PGE2 in celecoxib-treated 15-PGDH KO (versus WT) mice (Supplementary Table 1A, available at Carcinogenesis Online), celecoxib’s ability to prevent colon tumors was significantly impeded in KO mice. Thus, PGDH KO mice treated with celecoxib developed 7.8 tumors per mouse, a number that was significantly higher than the 0.8 tumors arising in celecoxib-treated WT animals (P < 0.0001) (Figures 2F and 3; Supplementary Table 2A, available at Carcinogenesis Online), and a number that remained significantly higher than the 5.0 adenomas arising in untreated WT mice (P = 0.0013) (Supplementary Table 2D, available at Carcinogenesis Online). This impeded activity of celecoxib in protecting 15-PGDH KO mice was similar to prior findings from our group (17). In contrast, sulindac showed robust antitumor activity in the 15-PGDH KO mouse, reducing colon tumors down to 2.3 tumors per mouse, a reduction significantly below the 7.8 tumors arising in celecoxib-treated PGDH KO mice (P < 0.0001) (Figures 2H and 3; Supplementary Table 2C, available at Carcinogenesis Online), and also significantly below the 5.0 colon adenomas seen in WT mice on a control diet (P = 0.0001) (Supplementary Table 2D, available at Carcinogenesis Online). Thus, sulindac was more effective than celecoxib in reversing the increased colon tumor sensitivity shown by 15-PGDH gene knockout.
Significantly, aspirin proved completely inactive in protecting 15-PGDH KO mice from colon tumor development, with 44.9 colon tumors arising in aspirin-treated KO mice versus 44.8 tumors in mice on a control diet (Figures 2D and 3; Supplementary Table 2C, available at Carcinogenesis Online). Thus, aspirin had no activity in preventing colon tumors in either 15-PGDH WT or KO mice. The failure of aspirin to reduce colon tumor development occurs in spite of aspirin’s ability to reduce colonic PGE2 levels in both WT and KO mice, particularly including aspirin’s reducing colonic PGE2 levels in 15-PGDH KO mice to well below the basal level of untreated WT mice (P = 0.0016) (Supplementary Table 1C, available at Carcinogenesis Online). One reason for aspirin’s failure to block tumor incidence in AOM-treated mice may be due to its inability to reduce the additional increased PGE2 levels in the colon induced by AOM. (Supplementary Figure 1, available at Carcinogenesis Online; Supplementary Table 3A and B, available at Carcinogenesis Online).
NSAIDs induce inflammatory lesions in the mouse colon
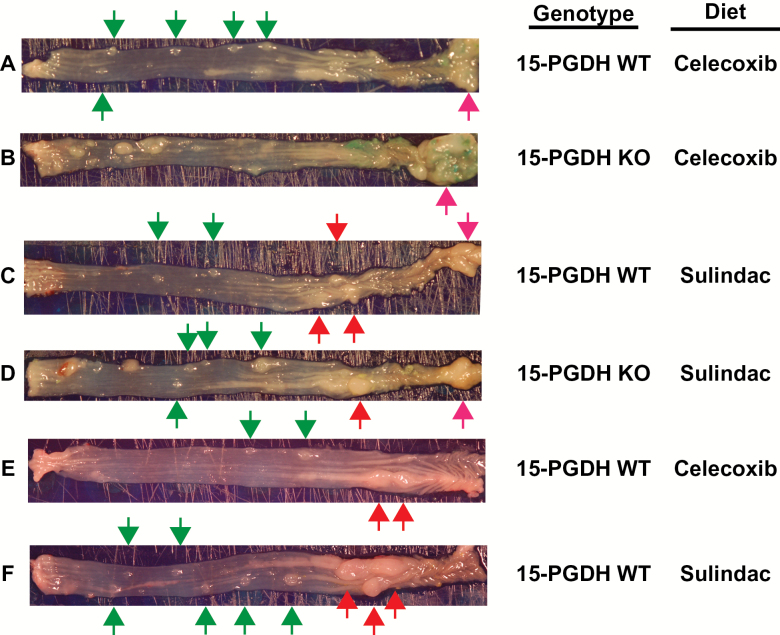
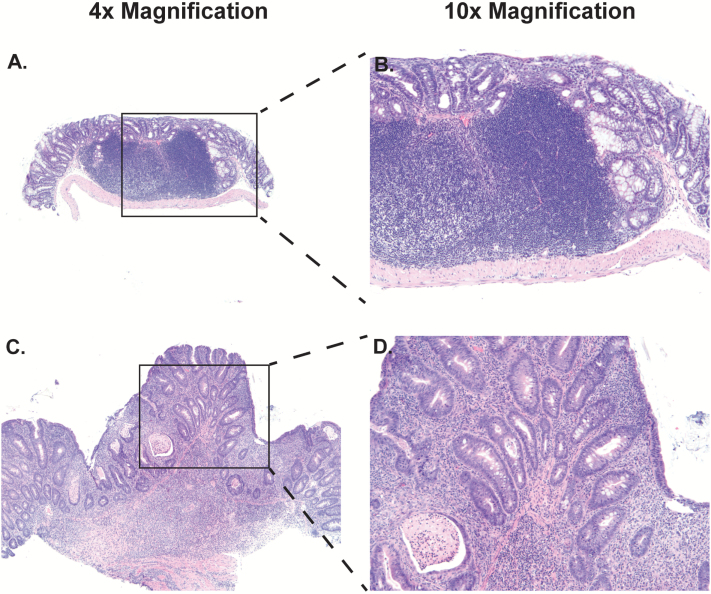
As previewed above, gross examination of the mouse colons demonstrated the presence of two additional types of lesions that were both visually and regionally distinct from adenomatous colon tumors. The first type of lesions were round, 1–3 mm annular or ‘donut-type’ excrescences (Figure 4A–F, green arrows) located principally in the mid colon region. The second type lesions were large, smooth, whitish masses arising chiefly in the proximal colon (Figure 4C–F, red arrows) and occasionally at the ileocecal junction (Figure 4A–D, pink arrows). Histologic analysis revealed that ‘donut-type’ lesions were comprised of large lymphoid aggregates (Figure 5A and B). The histology of proximal and ileocecal mass lesions revealed the presence of a more generalized and diffuse inflammatory infiltrate accompanied by extensive reparative changes in the epithelium (Figure 5C and D). The epithelial architecture while disordered could be clearly distinguished from adenomatous change (Figure 2I and J versus Figure 5C and D), except in one instance in which the disorder was so extreme as to preclude accurate histologic distinction.
Figure 4.
Gross morphology of representative annular lymphoid aggregates (green arrows), and inflammatory mass lesions of the proximal colon (red arrows) and cecal junction (pink arrows) in colons from AOM-treated 15-PGDH WT and KO mice administered celecoxib (A, B and E), or sulindac (C, D and F) containing diets.
Figure 5.
Hematoxylin and eosin staining of representative lymphoid aggregates (A and B) and inflammatory mass lesions (C and D) at ×4 (A and C) and ×10 (B and D) magnification.
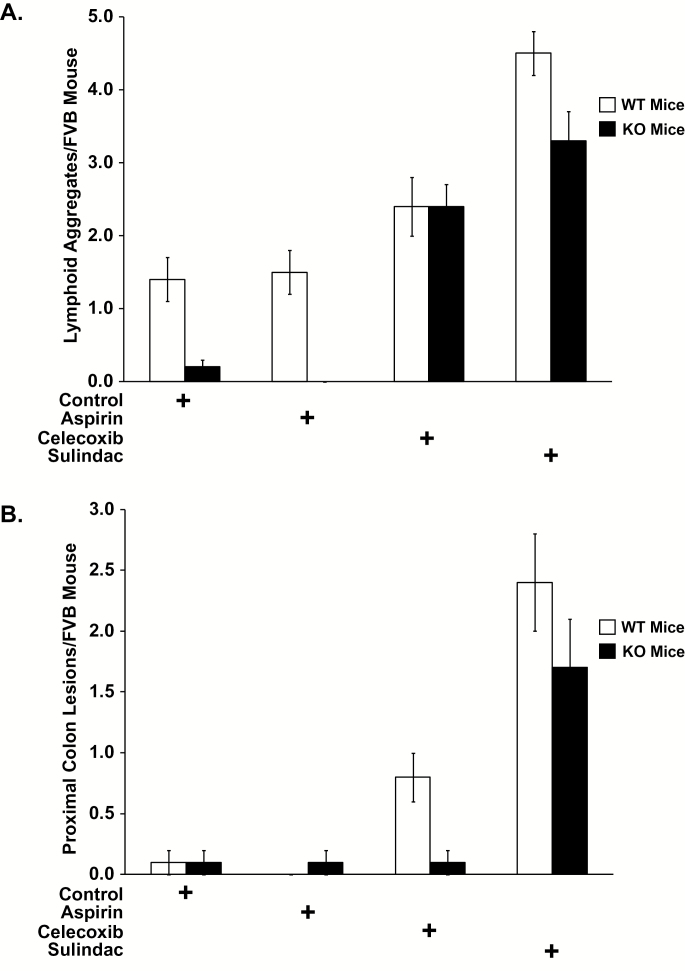
Intriguingly, the numbers of lesions proved notably different between mice treated with different agents, and agent’s induction of both donut-type lymphoid aggregates and of inflammatory mass lesions showed an inverse correlation with each agent’s potency in reducing adenoma numbers. Thus, aspirin, which demonstrated no effect in preventing colon adenomas, was indistinguishable from control diet in being inactive in promoting induction of either lymphoid aggregates or inflammatory mass lesions, as assessed in both 15-PGDH WT and KO mice. Accordingly, 15-PGDH WT mice treated with aspirin demonstrated 1.5 lymphoid aggregates per mouse, equivalent to the 1.4 lymphoid aggregates per mouse on control diets (P = 0.9021; Figure 6A; Supplementary Table 4B, available at Carcinogenesis Online). Aspirin-treated WT mice demonstrated 0.0 inflammatory masses per mouse, versus 0.1 inflammatory masses per control diet mouse (P = 0.9737; Figure 6B; Supplementary Table 5B, available at Carcinogenesis Online). Similarly, aspirin-treated 15-PGDH KO mice demonstrated 0.0 lymphoid aggregates per mouse and 0.1 inflammatory masses per mouse, indistinguishable from mice on control diets who demonstrated 0.2 lymphoid aggregates per mice and 0.1 inflammatory masses (P = 0.9737 for lymphoid aggregates; Figure 6A; Supplementary Table 4C, available at Carcinogenesis Online) (P = 0.8641 for inflammatory masses; Figure 6B; Supplementary Table 5C, available at Carcinogenesis Online).
Figure 6.
Lymphoid aggregate and inflammatory mass lesions of the proximal colon/cecal junction induction with celecoxib and sulindac treatment. (A) Lymphoid aggregate development in 15-PGDH WT FVB mice (open bars) that are: untreated (control, n = 19) or treated with celecoxib (n = 16), aspirin (n = 17) or sulindac (n = 19); versus FVB 15-PGDH KO mice (black bars) that are: untreated (control, n = 19), or treated with celecoxib (n = 20), aspirin (n = 16) or sulindac (n = 18). (B) Inflammatory mass lesions of the proximal colon/cecal junction development in 15-PGDH WT FVB mice (open bars) that are: untreated (control, n = 19) or treated with celecoxib (n = 16), aspirin (n = 17) or sulindac (n = 19), versus FVB 15-PGDH KO mice (black bars) that are: untreated (control, n = 19), or treated with celecoxib (n = 20), aspirin (n = 16) or sulindac (n = 18). Error bars designate standard error of the mean..
In contrast, celecoxib induced inflammatory mass lesions in a manner diametrically opposite to its activity in reducing colon adenomas. In 15-PGDH WT mice, in which celecoxib actively reduced adenoma numbers, celecoxib also induced an 8-fold increase of inflammatory mass lesions of the proximal colon (0.8 lesions per celecoxib-treated mouse versus 0.1 lesions per control diet mouse; P = 0.009; Figure 6B; Supplementary Table 5B, available at Carcinogenesis Online). However, in 15-PGDH KO mice, in which celecoxib showed attenuated reduction of adenoma numbers, celecoxib also showed no effect in inducing mass lesions, with 0.1 inflammatory mass lesions identically arising in both control diet and celecoxib-treated 15-PGDH KO mouse (P = 0.9592; Figure 6B; Supplementary Table 5C, available at Carcinogenesis Online). Celecoxib did promote increased lymphoid aggregates in both 15-PGDH WT and KO mice. Thus, compared with mice on control diets, celecoxib-treated 15-PGDH WT mice showed a 1.7-fold increase in lymphoid aggregates (2.4 versus 1.4; P = 0.0347; Figure 6A; Supplementary Table 4B, available at Carcinogenesis Online), and in 15-PGDH KO mice lymphoid aggregates increased 12-fold with celecoxib treatment (2.4 versus 0.2; P < 0.0001; Figure 6A; Supplementary Table 4C, available at Carcinogenesis Online).
The pattern of inverse correlation between agent’s activity in adenoma reduction versus its effect on induction of lymphoid aggregates and inflammatory masses was most clearly evident in study of sulindac, which was the most active of the agents in adenoma prevention, and also proved to be the most highly active agent in induction of inflammatory mass lesions and of lymphoid aggregates. Thus, sulindac increased inflammatory mass lesions of the proximal colon by a marked 24-fold in 15-PGDH WT mice (2.4 lesions per sulindac-treated mouse versus 0.1 lesions per mouse on control diet; P < 0.0001; Figure 6B; Supplementary Table 5B, available at Carcinogenesis Online) and by 17-fold in 15-PGDH KO mice (1.7 lesions per sulindac-treated mouse versus 0.1 lesions per mouse on control diet; P = 0.0003; Figure 6B; Supplementary Table 5C, available at Carcinogenesis Online). Sulindac similarly increased levels of macroscopic lymphoid aggregate lesions by 3.2-fold in 15-PGDH WT mice (4.5 lesions per sulindac-treated mouse versus 1.4 lesions per mouse on control diet; P < 0.0001; Figure 6A; Supplementary Table 4B, available at Carcinogenesis Online) and by 16.5-fold in 15-PGDH KO mice (3.3 lesions per sulindac-treated mouse versus 0.2 lesions per mouse on control diet; P < 0.0001; Figure 6A; Supplementary Table 4C, available at Carcinogenesis Online). Sulindac exceeded celecoxib in inducing colonic mass lesions and of lymphoid aggregates both in 15-PGDH WT and in 15-PGDH KO mice. The increased numbers of inflammatory lesions in sulindac-treated mice was highly statistically significant for all comparisons (P values ranging from 0.0002 to 0.0021, Supplementary Tables 4 and 5, available at Carcinogenesis Online), although the increase in lymphoid aggregates in sulindac- versus celecoxib-treated 15-PGDH KO mice had P value of 0.0933.
Immunostaining for CD3 (T cells), CD45R/B220B-cell (B cells), F4-80 (macrophages) and COX-2 performed on representative lymphoid aggregates from WT and KO mice fed control- or NSAID-supplemented diets revealed that all the aggregates were similar in immune cell composition and were comprised mainly of T and B cells, plus a few macrophages along the aggregate periphery (Supplementary Figure 2, available at Carcinogenesis Online). COX-2 staining was not detected in the lymphoid aggregates, whereas strong, focal staining was detected in subepithelial tumor stromal cells. There was no difference in tumor COX-2 staining expression between the different conditions (Supplementary Figure 2, inset, available at Carcinogenesis Online).
Taken together prevention of AOM-induced adenomatous tumors increased from aspirin (no effect), to celecoxib, to sulindac, an effect that was particularly marked in the 15-PGDH KO mouse which is highly more tumor susceptible. However, increased efficacy in tumor protection was accompanied by both macroscopic lymphoid aggregates and of proximal colonic inflammatory mass lesions, wherein the induction of these lesions was positively associated with each NSAIDs efficacy in preventing colonic tumors.
Discussion
In the present study, we have compared NSAIDs most often applied for colon tumor prevention in the clinical setting, namely celecoxib, sulindac and aspirin, leading to the following important findings.
First, we find that sulindac is markedly more effective in preventing AOM-induced colon tumors in tumor-susceptible 15-PGDH KO mice than are either celecoxib or aspirin. 15-PGDH KO mice treated with sulindac developed significantly less tumors than did 15-PGDH WT mice on a control diet. The advantage of sulindac in the 15-PGDH-deficient mouse model has clear implications for the treatment of individuals who express low colonic 15-PGDH, making sulindac the most likely potential agent of choice for colon tumor prevention in these individuals. Consistent with our previous findings, 15-PGDH KO mice were also resistant to the chemoprotective effects of celecoxib, developing significantly more tumors than WT mice on a control diet (17). Similar results have been seen in clinical trials wherein celecoxib has shown lesser activity in colon tumor prevention among individuals who express low levels of 15-PGDH (17).
Second, while aspirin was effective in lowering PGE2 levels in the colonic mucosa of 15-PGDH WT mice or KO mice, it showed no colon tumor prevention activity in these mice. These results were unexpected, since in general the murine model has otherwise faithfully captured the role of COX-2, 15-PGDH and PGE2 in human colon tumor development. Similarly, studies by Reddy et al. (28,29) demonstrate that 200–400 p.p.m. aspirin decreased colon tumors in AOM-injected F344 rats. The dose of aspirin used in our study was 300 p.p.m. which could be roughly equated with low-dose aspirin in humans (24), a formulation that is active in preventing colon tumors in man (10). Pharmacokinetic and pharmacodynamic differences between human, rats and mice may account for the disparity of aspirin’s lack of chemopreventive activity against murine colon tumors despite having a substantial effect on tissue prostaglandins. Alternatively, mice may capture a biology that is representative of that subset of human individuals who are aspirin resistant, i.e. those individuals who in aspirin trials have continued to develop colon tumors despite being on aspirin treatment. Or alternatively, in both mouse and man, the relationship between tumor prevention and lowering PGE2 may be context dependent.
A third significant observation is that NSAIDs may induce inflammatory lesions comprising macroscopic lymphoid aggregates and diffuse inflammatory lesions of the proximal colon. Our findings of sulindac-induced inflammation of the proximal colon are similar to those reported by Mladenova et al. (30) and are potentially related to observations by Itano et al. (31) of sulindac-induced cecal adenomas in Min mice. Moreover, celecoxib-induced colon inflammation has also been noted in Min mice models (32,33). We also note that observations in humans with inflammatory bowel disease show that NSAIDs can activate or exacerbate disease activity (34,35). These findings suggest that it will be important in future clinical studies with NSAIDs, particularly studies that use sulindac, to examine the mucosa of the proximal colon for evidence of subclinical induction of inflammatory lesions.
A fourth observation is that induction of inflammatory lesions by the agents studied was directly associated with their chemopreventive activity in both 15-PGDH WT and KO mice. We have no final resolution as to why this is the case, but note that it could suggest that targets other than COX-2, and/or mediators other than PGE2, may be important in both the chemoprevention activity of these agents and their activity in inducing colonic inflammation. One potential mediator of sulindac’s chemopreventive, yet inflammatory effects, could be the cyclin-dependent kinase inhibitor p21WAF1/cip1 that is increased with sulindac, inhibits colon tumor formation in ApcMin/+ and Apc1638+/− mice and that is a candidate regulator of inflammation (36–38).
In summary, this study demonstrates that sulindac shows the best efficacy in conferring colon tumor protection in 15-PGDH KO mice, and hence, may be of particular value for colon cancer chemoprevention in a subset of human individuals who have low levels of colonic 15-PGDH. Sulindac, however, was also the most potent agent in inducing inflammatory lesions of the proximal mouse colon. It will be important to determine if this side effect also occurs during clinical studies of individuals at risk of colorectal cancer.
Supplementary material
Supplementary Tables 1–5 and Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute contract (N01 CN-43302).
Supplementary Material
Acknowledgements
The authors thank Dr A.Dannenberg at Weill Cornell Medical College, Dr M.Bertagnolli at Brigham and Women’s Hospital for helpful discussions and L.Lei for expert assistance with mouse maintenance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- AOM
azoxymethane
- COX
cyclooxygenase
- KO
knockout
- NSAID
non-steroidal anti-inflammatory drug
- WT
wild-type.
References
- 1. Siegel R., et al. (2012). Cancer statistics, 2012. Cancer J. Clin., 62, 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Markowitz S.D. (2008). Colorectal neoplasia goes with the flow: prostaglandin transport and termination. Cancer Prev. Res. (Phila)., 1, 77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markowitz S.D., et al. (2009). Molecular origins of cancer: molecular basis of colorectal cancer. N. Engl. J. Med., 361, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown J.R., et al. (2005). COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol., 23, 2840–2855. [DOI] [PubMed] [Google Scholar]
- 5. Gupta R.A., et al. (2001). Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer, 1, 11–21. [DOI] [PubMed] [Google Scholar]
- 6. Markowitz S.D. (2007). Aspirin and colon cancer–targeting prevention? N. Engl. J. Med., 356, 2195–2198. [DOI] [PubMed] [Google Scholar]
- 7. Tai H.H., et al. (2006). NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr. Pharm. Des., 12, 955–962. [DOI] [PubMed] [Google Scholar]
- 8. Myung S.J., et al. (2006). 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl Acad. Sci. USA, 103, 12098–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oshima M., et al. (1996). Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell, 87, 803–809. [DOI] [PubMed] [Google Scholar]
- 10. Baron J.A., et al. (2003). A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med., 348, 891–899. [DOI] [PubMed] [Google Scholar]
- 11. Bertagnolli M.M., et al. (2006). Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med., 355, 873–884. [DOI] [PubMed] [Google Scholar]
- 12. Burn J., et al. (2011). Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet, 378, 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyskens F.L., Jr, et al. (2008). Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila)., 1, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandler R.S., et al. (2003). A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med., 348, 883–890. [DOI] [PubMed] [Google Scholar]
- 15. Marnett L.J. (2009). The COXIB experience: a look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol., 49, 265–290. [DOI] [PubMed] [Google Scholar]
- 16. Thun M.J., et al. (1991). Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med., 325, 1593–1596. [DOI] [PubMed] [Google Scholar]
- 17. Yan M., et al. (2009). 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proc. Natl Acad. Sci. USA, 106, 9409–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coggins K.G., et al. (2002). Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat. Med., 8, 91–92. [DOI] [PubMed] [Google Scholar]
- 19. Carothers A.M., et al. (2006). Changes in antitumor response in C57BL/6J-Min/+ mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res., 66, 6432–6438. [DOI] [PubMed] [Google Scholar]
- 20. Williams C.S., et al. (2000). Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res., 60, 6045–6051. [PubMed] [Google Scholar]
- 21. Beazer-Barclay Y., et al. (1996). Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis, 17, 1757–1760. [DOI] [PubMed] [Google Scholar]
- 22. Williamson S.L., et al. (1999). Intestinal tumorigenesis in the Apc1638N mouse treated with aspirin and resistant starch for up to 5 months. Carcinogenesis, 20, 805–810. [DOI] [PubMed] [Google Scholar]
- 23. Nambiar P.R., et al. (2003). Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int. J. Oncol., 22, 145–150. [PubMed] [Google Scholar]
- 24. Newmark H.L. (1987). Nutrient density: an important and useful tool for laboratory animal studies. Carcinogenesis, 8, 871–873. [DOI] [PubMed] [Google Scholar]
- 25. Chan A.T., et al. (2012). Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev. Res. (Phila)., 5, 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan A.T., et al. (2007). Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med., 356, 2131–2142. [DOI] [PubMed] [Google Scholar]
- 27. Fink S.P., et al. (2013). Colonic 15-PGDH levels are stable across distance and time and are not perturbed by aspirin intervention. Dig. Dis. Sci., 58, 2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy B.S. (2004). Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ. Mol. Mutagen., 44, 26–35. [DOI] [PubMed] [Google Scholar]
- 29. Reddy B.S., et al. (1993). Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis, 14, 1493–1497. [DOI] [PubMed] [Google Scholar]
- 30. Mladenova D., et al. (2011). The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut, 60, 350–360. [DOI] [PubMed] [Google Scholar]
- 31. Itano O., et al. (2009). Sulindac effects on inflammation and tumorigenesis in the intestine of mice with Apc and Mlh1 mutations. Carcinogenesis, 30, 1923–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carothers A.M., et al. (2010). Persistent cyclooxygenase-2 inhibition downregulates NF-{kappa}B, resulting in chronic intestinal inflammation in the min/+ mouse model of colon tumorigenesis. Cancer Res., 70, 4433–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davids J.S., et al. (2010). Chronic cyclooxygenase-2 inhibition promotes myofibroblast-associated intestinal fibrosis. Cancer Prev. Res. (Phila)., 3, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufmann H.J., et al. (1987). Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann. Intern. Med., 107, 513–516. [DOI] [PubMed] [Google Scholar]
- 35. Thiéfin G., et al. (2005). Toxic effects of nonsteroidal antiinflammatory drugs on the small bowel, colon, and rectum. Joint Bone Spine, 72, 286–294. [DOI] [PubMed] [Google Scholar]
- 36. Greenspan E.J., et al. (2010). Molecular alterations associated with sulindac-resistant colon tumors in ApcMin/+ mice. Cancer Prev. Res. (Phila)., 3, 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang W., et al. (2001). p21(WAF1/cip1) is an important determinant of intestinal cell response to sulindac in vitro and in vivo . Cancer Res., 61, 6297–6302. [PubMed] [Google Scholar]
- 38. Yao H., et al. (2008). Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am. J. Respir. Cell Mol. Biol., 39, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.