Abstract
Tick species distribution and prevalence of spotted fever group Rickettsiae (SFGR) in ticks were investigated in Zhejiang Province, China in 2010 and 2011. PCR was used to detect SFGR and positive amplicons were sequenced, compared to published sequences and phylogenic analysis was performed using MEGA 4.0. A total of 292 adult ticks of ten species were captured and 7.5 % (22/292) of the ticks were PCR-positive for SFG Rickettsia. The PCR-positive rates were 5.5 % (6/110) for Haemaphysalis longicornis, 3.6 % (1/28) for Amblyomma testudinarium and 16 % (15/94) for Ixodes sinensis, respectively. Phylogenetic analyses of gltA genes detected in ticks indicated that there are two dominating groups of SFGR. Sequences of group one were closely related to Rickettsia monacensis, whereas sequences of group two were closest related to Rickettsia heilongjiangensis and Rickettsia japonica, which are human pathogens. Our findings underline the importance of these ticks in public health surveillance in Zhejiang Province, China.
Keywords: Spotted fever group Rickettsiae, Public health, Rickettsia monacensis, Rickettsia heilongjiangensis, Rickettsia japonica, Ticks
Introduction
Rickettsia is a genus of non-motile, gram-negative, intracellular bacteria transmitted by ticks, fleas, lice and mites and cause diseases in humans such as typhus, Rickettsial pox, Boutonneuse fever, African tick bite fever, Rocky Mountain spotted fever, Flinders Island spotted fever and Queensland tick typhus (Raoult and Roux 1997; Nathan et al. 2007). The genus Rickettsia is traditionally classified into the conventionally well-defined typhus group (TG), the spotted fever group (SFG), ancestral group and transitional group, based mainly on phenotypic and serological features (Gillespie et al. 2008). SFG Rickettsiae (SFGR) are widely distributed throughout the world in foci of endemicity and cause sporadic outbreaks in areas such as Japan, southern China and eastern Russia (Choi et al. 2005). From about 30 SFGR described so far, at least 15 are known to be pathogenic for humans (Parola et al. 2005).
In China, many SFGR belong to Rickettsia sibirica group, including two subspecies, i.e., R. sibirica sibirica, the agent of North Asian tick typhus detected in Dermacentor silvarum and Dermacentor sinicus in northern China, and Rickettsia sibirica mongolitimonae, the agent of lymphangitis-associated rickettsiosis isolated from Hyalomma asiaticum in Inner Mongolia (Yu et al. 1993; Zhang et al. 2006). Rickettsia heilongjiangensis, firstly isolated from D. silvarum ticks in Heilongjiang Province, can cause spotted fever in humans (Fournier et al. 2003; Jiao et al. 2005). Additionally, ticks are widely distributed in Zhejiang Province where humans are frequently bitten by ticks. This stimulated us to explore the tick species distribution in different areas of Zhejiang Province and the prevalence of SFGR species in these ticks.
Materials and methods
Tick sampling
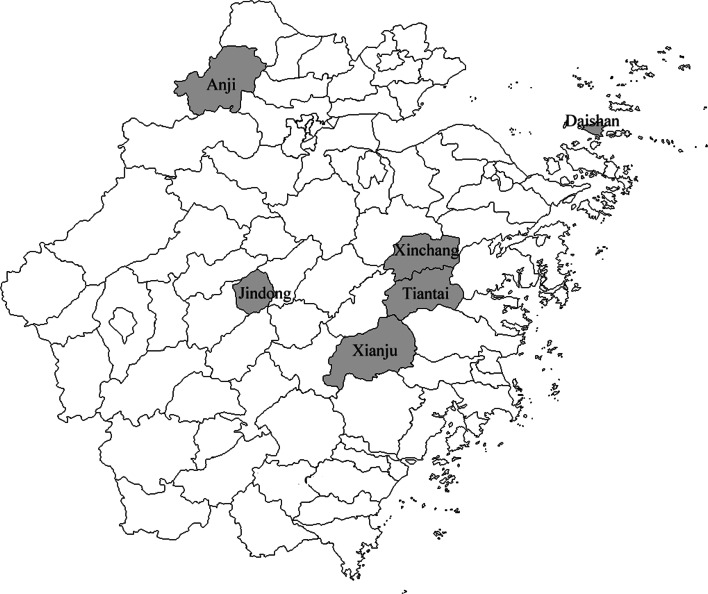
The investigated sites included Daishan, Xinchang, Jindong, Tiantai, Xianju and Anji which were randomly chosen based on their geographical and administrative locations (Fig. 1). Ticks were collected from sheep, cattle, hedgehogs, domestic dogs, wild boar and small mammals including Apodemus agrarius, Rattus niviventer, and Suneus murinus during January 2010 to December 2011. All ticks were identified to the species level by standard guides (Deng and Jiang 1991) according to morphology and were stored at −80 °C prior to DNA extraction.
Fig. 1.
Geographical distribution of investigated sites in Zhejiang Province
DNA extraction
Each adult tick was subjected individually to DNA extraction. Ticks were washed using 70 % ethanol once; then they were washed three times with sterile deionized water to decontaminate the surface. Individual ticks were placed into different sterilized mortars and crushed with corresponding sterile pestles with liquid nitrogen. DNA was prepared from the crushed ticks using the QIAamp Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
Polymerase chain reaction amplification
All tick samples were screened for SFGR infection through testing them individually by polymerase chain reaction (PCR) amplification with the use of primer (5′-GGGGGCCTGCTCACGGCGG-3′; 5′-ATTGCAAAAAGTACAGTGAACA-3′) designed to amplify a fragment of the citrate synthase gene (gltA) of SFGR as described previously (Regnery et al. 1991; Roux et al. 1997).
The reaction mixture contained 10 mM Tris–HCl, 1.5 mM MgCl2, 50 mM KCl (pH 8.3), 200 mM each dNTP, 1.25 U Taq polymerase, and 0.5 mM each respective primer. PCR products were electrophoresed in a 1.5 % agarose gel, stained with gold view, and visualized using ultraviolet light. To avoid cross contamination, all steps were performed in separate rooms; mastermix was prepared under a laminar air flow bench. In each PCR, at least two negative controls contained mastermix and sterile water instead of DNA template were used.
Cloning and sequencing of PCR products
After electrophoresis, all positive DNA amplicons were purified using the Promega Wizard PCR Preps Kit (Promega, Madison, WI, USA) and then cloned into the PGEM-T Easy vector system (Promega) following the manufacturer’s protocol. The white recombinant clones were selected for sequencing. Bidirectional sequencing of positive PCR products were commercially conducted by Shanghai Sangon Biotechnology (Shanghai, China).
Database DNA comparisons
Our sequences were submitted to GenBank and compared to published sequences using the BLAST program from the National Center for Biotechnology Information Website (http://www.ncbi.nlm.nih.gov/BLAST/), and phylogenic analysis was performed using MEGA 4.0. For each gene analyzed, a phylogram was constructed by the neighbor-joining method. Confidence values for individual branches of the resulting tree were determined by bootstrap analysis with 1,000 replicates.
Data analysis
A Chi-square test or Fisher’s exact test, as appropriate, was used to compare SFGR prevalence among different species of ticks and different sampling sites. The difference was considered statistically significant when P < 0.05. Statistical analysis was performed with 16.0 (SPSS, Chicago, IL, USA).
Results
Tick samples
A total of 292 adult ticks of ten species were captured (Table 1). The species of ticks from different areas varied significantly (χ2 = 408.915, P < 0.001). The dominant tick species differed at various study areas. Haemaphysalis longicornis was dominant in Daishan (91.38 %) and Xinchang (80.00 %); H. longicornis (21.43 %), Rhipicephalus haemaphysaloides (21.43 %), and Ixodes sinensis (22.86 %) in Jindong; I. sinensis (100 %) in Tiantai; H. longicornis (58.14 %) and Rhipicephalus microplus (41.86 %) in Xianju; Amblyomma testudinarium (23.96 %) and I. sinensis (65.63 %) in Anji.
Table 1.
Prevalence of spotted fever group Rickettsiae (SFGR) infection among tick species from different areas in Zhejiang Province, China
| Daishan | Xinchang | Jindong | Tiantai | Xianju | Anji | Total (n) | SFGR positive (n) | Positive rate (%) | |
|---|---|---|---|---|---|---|---|---|---|
| H. longicornis | 53 | 8 | 15 | 0 | 25 | 9 | 110 | 6 | 5.45 |
| R. haemaphysaloides | 3 | 2 | 15 | 0 | 0 | 0 | 20 | 0 | 0 |
| A. testudinarium | 0 | 0 | 5 | 0 | 0 | 23 | 28 | 1 | 3.57 |
| I. sinensis | 0 | 0 | 16 | 15 | 0 | 63 | 94 | 15 | 15.96 |
| R. microplus | 1 | 0 | 0 | 0 | 18 | 0 | 19 | 0 | 0 |
| I. granulatus | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| H. yeni | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 0 |
| D. taiwanensis | 0 | 0 | 9 | 0 | 0 | 0 | 9 | 0 | 0 |
| H. hystricis | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 0 |
| H. asiaticum | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Total (n) | 58 | 10 | 70 | 15 | 43 | 96 | 292 | 22 | 7.53 |
| SFGR positive (n) | 1 | 1 | 7 | 0 | 4 | 9 | 22 | ||
| Positive rate (%) | 1.72 | 10.00 | 10.00 | 0 | 9.30 | 9.38 | 7.53 |
Prevalence of SFGR infection
Overall, 7.53 % (22/292) of the ticks were PCR-positive to SFGR. The PCR-positive rates were 5.5 % (6/110) for H. longicornis, 3.8 % (1/28) for A. testudinarium, and 16 % (15/94) for I. sinensis, respectively (Table 1). No Rickettsia DNA was detected in R. haemaphysaloides, R. microplus, I. granulates, Haemaphysalis yeni, D. taiwanensis, Haemaphysalis hystricis, and Haemaphysalis asiaticum. The prevalence of Rickettsia was not significantly different among species (χ2 = 15.776, P = 0.072).
Prevalences in Daishan, Xinchang, Jindong, Tiantai, Xianju, and Anji were 1.7, 10, 10, 0, 9.3, 9.4, and 7.5 %, respectively. The prevalence was similar among different areas (χ2 = 5.391, P = 0.37).
Phylogenetic analysis
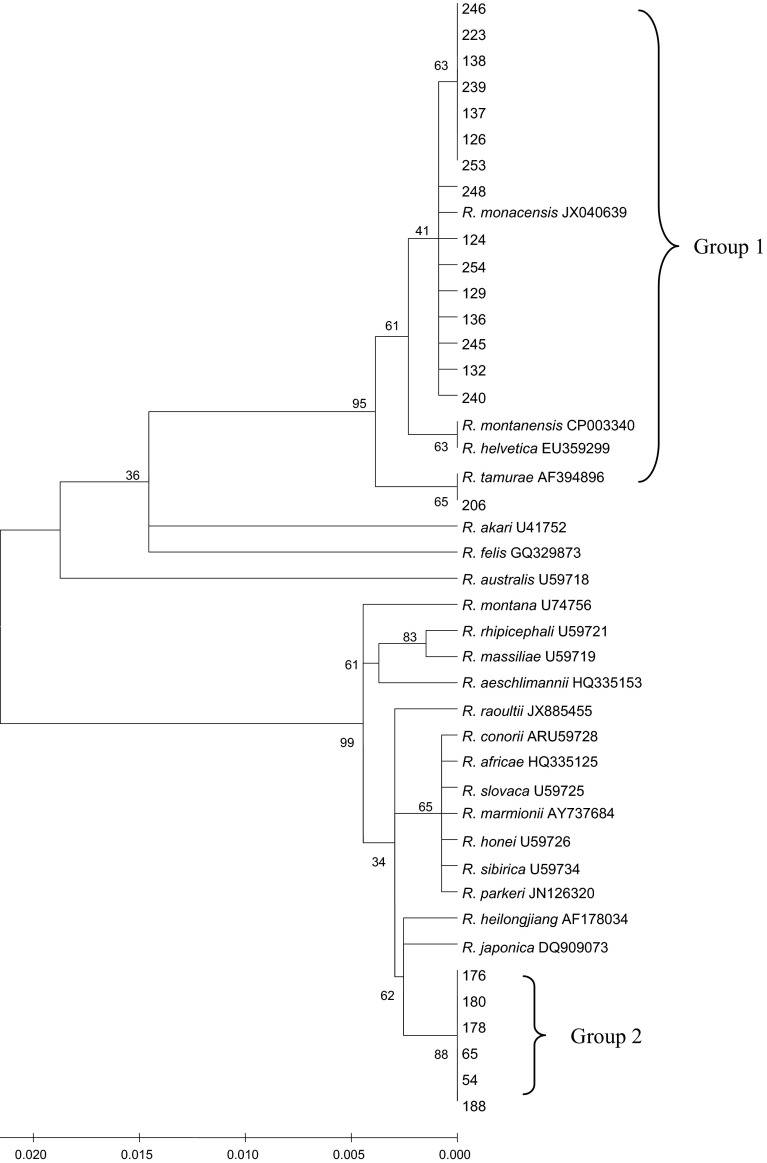
All sequences were submitted to GenBank, and the accession numbers were from KM886876 to KM886897. Phylogenetic analyses of gltA genes (340 bp) detected in ticks indicated that there were two dominating groups of SFGR (Fig. 2).
Fig. 2.
Phylogenetic analyses of partial gltA genes of Rickettsia species identified in ticks from Zhejiang Province, China
Group one consisted of 16 detected sequences, which were from Anji (n = 9) and Jindong (n = 7). Moreover, all sequences were detected in I. sinensis except that one sequence was detected in A. testudinarium. The majority of sequences of this group were closely related to R. monacensis (JX040639), which was detected in I. ricinus ticks from Romania. One sequence was most similar to R. tamurae (AF394896) that came from A. testudinarium in Japan.
Group two included six detected sequences, which were from three areas (Daishan, Xianju and Xinchang). Of note, these sequences were all from H. longicornis and were most similar to R. heilongjiangensis (AF178034) and R. japonica (DQ909073).
Discussion
In our study, ten species of ticks were found in six areas and the tick species varied significantly at different areas. This may be due to the different animal hosts from which they were removed, geographic sites, unbalanced number of ticks in different areas. The positive rate of SFGR was found to be 7.5 % among ticks, a percentage similar to the recently reported percentage (6.9 %) recorded in the Hebei Province, China (Zou et al. 2011). However, ticks were pooled prior to DNA extraction and the pools consisted of 2–10 ticks collected from the same site in their study.
Although no SFGR DNA was detected in Tiantai and infection rates varied at different survey areas, we could not determine the geographic diversity of SFGR in ticks. The number of ticks examined was limited and the species of ticks varied significantly; therefore, infection rates in the current study might be biased. In addition, because intensity of circulation of any vector-borne agent fluctuates dramatically throughout the year and from year to year, even at the same location (Bown et al. 2003; Wielinga et al. 2006), we could not justify comparing infection rates between different areas on the basis of unsynchronized single collections over a 2-year period. A randomized sampling scheme and further collection of ticks are required to clarify this issue.
Usually, for SFGR, ticks are both the reservoir and the vector of the bacteria and the geographical distribution of the disease is superimposed upon that of the tick (Raoult and Roux 1997). Among ten tick species collected in this study, only three were infected with SFGR. This demonstrated that these three tick species were the main carriers of SFGR. But it is not excluded that other ticks might act as vectors for SFGR because of small sample sizes.
Phylogenetic analyses indicated that two groups of SFGR were detected in ticks, one identified in Jindong and Anji, another identified in Xinchang, Daishan and Xianju. Interestingly, sequences of group 1 were all from I. sinensis and A. testudinarium, sequences of group two were all from H. longicornis. Additionally, one sequence which was detected in A. testudinarium was most similar to one sequence, which was also detected in A. testudinarium in Japan. These indicate that SFGR species might be related to tick species.
Some sequences were most similar to R. monacensis which had been isolated from I. ricinus in Germany (Simser et al. 2002) and Hungary (Sreter et al. 2005). Rickettsia monacensis can cause spotted fever in humans, as the disease attributed to this agent has not received a specific name. The other sequences were most related to R. heilongjiangensis and R. japonica which were also human rickettsial pathogens. Rickettsia heilongjiangensis was first isolated in 1982 from D. silvarum in Heilongjiang Province in the northeast of China and subsequently demonstrated to cause human spotted fever in China and in the Russian Far East (Mediannikov et al. 2004). Rickettsia japonica was first isolated in 1984 in Japan (Mahara 1997) and is the agent of Japanese spotted fever (Uchida et al. 1992).
In comparable studies throughout Zhejiang Province, SFGR were detected in H. longicornis, R. haemaphysaloides (Cheng et al. 2010; Meng et al. 2008; Jiang et al. 2010). However, sequences detected in their study were most similar to R. rhipicephali and R. massiliae. This is the first identification of R. monacensis in ticks from Zhejiang Province which demonstrate the importance of epidemiological survey of R. monacensis infection in Zhejiang Province.
There were several limitations to our study. Firstly, the low numbers of ticks of each species in each region collected in different times and from different animals reduced the probabilities of getting useful information from this study. Secondly, the examined ticks were collected from animals rather than as questing specimens which brings into question whether SFGR would survive moulting of the ticks, especially for non-Ixodes ticks. Finally, tick species from different sites varied significantly, which may also influence infection rates of different sites.
In summary, we detected SFGR in diverse species of ticks from different areas suggesting that SFGR is widely prevalent in ticks in Zhejiang Province. SFGR detected were similar to R. monacensis, R. heilongjiangensis or R. japonica which can cause human infection. SFGR infections are largely unrecognized but may become more frequently diagnosed in Zhejiang Province.
Acknowledgments
We thank the physicians and staff at Daishan, Xinchang, Jindong, Tiantai, Xianju and Anji Center for Disease Control and Prevention for their support and assistance with this investigation. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. This research was supported by a grant from Zhejiang Province major science and technology program (2012C13016-2), the Project of the State Scientific and Technological Development of the 12th Five Year Plan grant (2012ZX10004219), the medical research program of Zhejiang Province (2012KYA045, 2014RCA002) and national Science Foundation of China (81201319).
Footnotes
Jimin Sun, Junfen Lin and Zhenyu Gong have contributed equally to this work.
References
- Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH. Seasonal dynamics of Anaplasma phagocytophilum in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. 2003;9:63–70. doi: 10.3201/eid0901.020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SY, Zheng SQ, Meng Z, Ye XD, Jiang LP, Wang ZQLuQY. Analysis of DNA sequences of spotted fever group Rickettsia detected from ticks in Zhejiang Province. Dis Surveill. 2010;25:466–468. [Google Scholar]
- Choi YJ, Lee SH, Park KH, Koh YS, Lee KH, Baik HS, Choi MS, Kim IS, Jang WJ. Evaluation of PCR-based assay for diagnosis of spotted fever group rickettsiosis in human serum samples. Clin Diagn Lab Immunol. 2005;12:759–763. doi: 10.1128/CDLI.12.6.759-763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng GF, Jiang ZJ (1991) Economic insect fauna of Chinese, 39, Acari Leach Science Press
- Fournier PE, Dumler JS, Greub Q, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, Ceraul SM, Dharmanolla C, Rainey D, Soneja J, Shallom JM, Vishnubhat ND, Wattam R, Purkayastha A, Czar M, Crasta O, Setubal JC, Azad AF, Sobral BS. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One. 2008;3:e2008. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LP, Meng Z, Cui QR, Tong WS, Ling F, Wang Z. Detection of rOmpA and gltA genes of spotted fever group Rickettsiae from tick specimens in Zhejiang Province. Chin J Vector Biol Control. 2010;21:350–352. [Google Scholar]
- Jiao YM, Wen BH, Chen ML, Niu DS, Zhang J, Qiu L. Analysis of immunoprotectivity of the recombinant OmpA of Rickettsia heilongjiangensis. Ann N Y Acad Sci. 2005;1063:261–265. doi: 10.1196/annals.1355.042. [DOI] [PubMed] [Google Scholar]
- Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis. 1997;3:105–111. doi: 10.3201/eid0302.970203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediannikov OY, Sideonikov Y, Ivanov L, Mokressova E, Fournier PE, Tarasevich I, Raoult D. Acute tick borne rickettsiosis, caused by Rickettsia heilongjiangensis variant in the Russian Far East. Emerg Infect Dis. 2004;10:810–817. doi: 10.3201/eid1005.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Jiang LP, Lu QY, Cheng SY, Ye JL, Zhan L. Detection of co-infection with Lyme spirochetes and spotted fever group Rickettsiae in a group of Haemaphysalis longicornis. Chin J Epidemiol. 2008;29:1217–1220. [PubMed] [Google Scholar]
- Nathan BU, Stenos J, Graves SR, Faa AG, Cox GE, Dyer JR, Boutlis CS, Lane AM, Shaw MD, Robson J, Nissen MD. Flinders Island spotted fever rickettsioses caused by “marmionii” strain of Rickettsia honei, Eastern Australia. Emerg Infect Dis. 2007;13:566–573. doi: 10.3201/eid1304.050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the Rickettsiae. Int J Syst Bacteriol. 1997;47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microb. 2002;68:4559–4566. doi: 10.1128/AEM.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter L, Sreter Z, Szelli T, Egyed ZL. Molecular evidence of Rickettsia helvetica and R. monacensis infections in Ixodes ricinus from Hungary. Ann Trop Med Parasitol. 2005;99:325–330. doi: 10.1179/136485905X28027. [DOI] [PubMed] [Google Scholar]
- Uchida T, Uchiyama T, Kumano K, Walker DH. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int J Syst Bacteriol. 1992;42:303–305. doi: 10.1099/00207713-42-2-303. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Gaasenbeek C, Fonville M, Deboer A, Vries AD, Dimmers W, Jagers GAO, Schouls LM, Borgsteede F, van der Giessen JWB. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl Environ Microb. 2006;72:7594–7601. doi: 10.1128/AEM.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Jin Y, Fan MY, Xu GM, Liu QH, Raoult D. Genotypic and antigenic identification of two new strains of spotted fever group Rickettsiae isolated from China. J Clin Microbiol. 1993;31:83–88. doi: 10.1128/jcm.31.1.83-88.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jin J, Fu X, Raoult D, Fournier PE. Genetic differentiation of Chinese isolates of Rickettsia sibirica by partial ompA gene sequencing and multispacer typing. J Clin Microbiol. 2006;44:2465–2467. doi: 10.1128/JCM.02272-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YX, Wang QY, Fu ZX, Liu PP, Jin HT, Yang HH, Gao HW, Xi Z, Liu Q, Chen LF. Detection of spotted fever group Rickettsia in Haemaphysalis longicornis from Hebei Province. J Parasitol. 2011;97:960–962. doi: 10.1645/GE-2751.1. [DOI] [PubMed] [Google Scholar]