Abstract
Purpose
The optimal chemotherapy regimen to use with radiotherapy in stage III non–small-cell lung cancer is unknown. Here, we compare the outcome of patents treated within the Veterans Health Administration with either etoposide-cisplatin (EP) or carboplatin-paclitaxel (CP).
Methods
We identified patients treated with EP and CP with concurrent radiotherapy from 2001 to 2010. Survival rates were compared using Cox proportional hazards regression models with adjustments for confounding provided by propensity score methods and an instrumental variables analysis. Comorbidities and treatment complications were identified through administrative data.
Results
A total of 1,842 patients were included; EP was used in 27% (n = 499). Treatment with EP was not associated with a survival advantage in a Cox proportional hazards model (hazard ratio [HR], 0.97; 95% CI, 0.85 to 1.10), a propensity score matched cohort (HR, 1.07; 95% CI, 0.91 to 1.24), or a propensity score adjusted model (HR, 0.97; 95% CI, 0.85 to 1.10). In an instrumental variables analysis, there was no survival advantage for patients treated in centers where EP was used more than 50% of the time as compared with centers where EP was used in less than 10% of the patients (HR, 1.07; 95% CI, 0.90 to 1.26). Patients treated with EP, compared with patients treated with CP, had more hospitalizations (2.4 v 1.7 hospitalizations, respectively; P < .001), outpatient visits (17.6 v 12.6 visits, respectively; P < .001), infectious complications (47.3% v 39.4%, respectively; P = .0022), acute kidney disease/dehydration (30.5% v 21.2%, respectively; P < .001), and mucositis/esophagitis (18.6% v 14.4%, respectively; P = .0246).
Conclusion
After accounting for prognostic variables, patients treated with EP versus CP had similar overall survival, but EP was associated with increased morbidity.
INTRODUCTION
Nearly one in four patients with non–small-cell lung cancer (NSCLC) has stage III disease at presentation.1 The outcome of these patients remains poor, with median survival times of only 15.3 to 21.7 months.2–4 However, approximately 20% of patients achieve durable disease control, arguing for treatment with curative intent in those able to tolerate aggressive therapy.1,5
Randomized controlled trials (RCTs) have demonstrated that concurrent chemoradiotherapy improves overall survival (OS) but increases toxicity, compared with sequential chemoradiotherapy.2,6 Such chemotherapy generally involves a platinum doublet; however, no large RCT directly compares specific combinations in this setting.2,7 The regimen used in several of the RCTs was etoposide-cisplatin (EP), at systemic doses, adequate to address micrometastatic disease.2,7–9 Because of the toxicity associated with EP, a competing regimen of dose-reduced weekly carboplatin-paclitaxel (CP) has emerged as an alternative choice.10
There is considerable concern that CP, although better tolerated than EP, may be inferior in terms of disease control.11 To gain insight into the relative efficacy of these regimens, we examined outcomes of patients with newly diagnosed stage III NSCLC using the Department of Veterans Affairs (VA) Central Cancer Registry (VACCR).
METHODS
Patient Selection
Using the VACCR, we identified patients diagnosed with stage III NSCLC (see Appendix, online only, for histologies included) between October 2001 and December 2010. The VACCR stages patients using the contemporaneous International Association for the Study of Lung Cancer/American Joint Committee on Cancer staging classification; thus, version 6 was used through 2009 and version 7 in 2010. We used the VA Decision Support System Pharmacy file to identify chemotherapy drugs received during the 120 days after diagnosis.
We included patients who were classified by the VACCR as receiving radiotherapy as part of primary treatment within 7 days of the start of chemotherapy and received either CP or EP as the chemotherapy backbone. We excluded patients who the VACCR classified as receiving surgery as part of initial treatment or received other anticancer agents in addition to CP or EP as part of their initial treatment. We characterized chemotherapy as induction when it was prescribed as part of the initial 6 weeks of treatment and as consolidation when it was prescribed between weeks 7 and 16. We identified patients who received lung resection using International Classification of Diseases Ninth Revision (ICD-9) or Current Procedural Terminology procedure codes. We augmented the VACCR database with demographic, diagnostic, and laboratory data from several other VA databases. We provide details of this process in the Appendix. The study was approved by the institutional review boards of the Milwaukee and Durham VAs.
Statistical Analysis
The primary outcome was OS, defined by days from VACCR diagnosis until death. We censored OS at 5 years after diagnosis to avoid excessive statistical impact by patients with long survival diagnosed early in the study period. We grouped year of diagnosis into 2001 to 2004, 2005 to 2007, and 2008 to 2010. We used clinical records to obtain baseline values for estimated glomerular filtration rate (eGFR), serum albumin, platelet count, and hemoglobin, as well as weight loss before treatment. We defined anemia as hemoglobin less than 12 g/dL, hypoalbuminemia as serum albumin less than 3.5 g/dL, and chronic kidney disease as eGFR less than 60 mL/min/1.73 m2.
We used ICD-9 codes from health care encounters during the year preceding diagnosis to calculate a summary burden of comorbidity using the National Cancer Institute (NCI) combined index.12 ICD-9 codes from encounters during the 4 months after chemotherapy were used to identify adverse effects of treatment.
Outcome Analysis
We tested differences in baseline characteristics between groups using the Pearson χ2 or t test for categorical and continuous variables, respectively. Survival curves were prepared using the Kaplan-Meier method. We used the following three complementary approaches to adjust our comparison of OS among patients receiving EP and CP for differences in baseline characteristics: a standard Cox proportional hazards model, a propensity score adjustment, and an instrumental variables technique.
In our standard Cox model, we included variables that were associated with OS in univariable analysis with a significance level of P < .10. The proportional hazards assumption for each covariate in the final model was tested, and the assumption was appropriate for all. We tested the interaction between chemotherapy regimen and each covariate for significance and found none.
Propensity score analysis adjusts for the bias induced by nonrandom treatment assignment by comparing patients who had a similar likelihood of receiving a treatment but who received different treatments. For this analysis, we used multiple logistic regression to predict the likelihood that a given patient would receive treatment with EP (ie, their propensity score). The model included the following variables, regardless of their individual statistical significance: histology (squamous v nonsquamous), stage (IIIA v IIIB), weight loss, number of prior hospitalizations, hemoglobin level, platelet count, serum albumin level, age, eGFR, NCI combined index, and geographic region.
We estimated the effect of treatment on survival using the following two approaches: matching and weighting by inverse probability of treatment. Prior work has demonstrated that both approaches allow for the estimation of marginal hazard ratios (HRs) with minimal bias.13 For the matched-pair analysis, we matched each patient who received EP with one who received CP using exact treatment year category and the logit of the propensity score, using calipers of width equal to 0.2 standard deviations of the logit of the estimated propensity score.14 To compare the groups, a marginal Cox model was applied using maximum partial likelihood estimates of regression parameters and a robust sandwich covariance matrix.15,16 The main limitation of this methodology is that it limits the analysis to matched pairs only, thus reducing power. Another frequently used strategy that does not carry this limitation is the use of inverse probability of treatments weights; for this analysis, each observation was first weighted by the inverse probability of receiving the treatment the patients received, based on the propensity score. A Cox proportional hazards model was then fitted with chemotherapy regimen as the only predictor variable. A robust sandwich variance estimator was used to account for the weighted nature of the sample.17
Instrumental Variables and Near/Far Analysis
Although propensity scores can address biases caused by observed variables, there may be unknown or unmeasured variables that also affect the choice of treatment and outcomes. Instrumental variable analyses address such unmeasured confounders by using an instrument that is strongly associated with the treatment but is not expected to affect outcomes. The variability among VA medical centers in the use of EP versus CP provided an instrument for our analysis.
We used a novel technique, near/far matching, that unites propensity score matching with classical instrumental variables analysis.18,19 We identified EP-encouraging centers, in which more than 50% of patients received EP, and EP-discouraging centers, where less than 10% of the patients received EP. We matched an EP-encouraging center to one or more EP-discouraging centers based on overall hospital volume, oncology service volume, and the number of facility oncologists. Using the propensity score obtained in the prior analysis and treatment year, we matched each patient treated in an EP-encouraging center with a patient treated in a matching EP-discouraging center. Thus, the patients were a near match (nearest match) on patient and facility characteristics and a far match (farthest match) based on the instrument. The analysis proceeds as a Cox proportional hazards model with a single predictor variable (encouraged v discouraged); matched patient pairs define the strata.20
RESULTS
Patient Characteristics
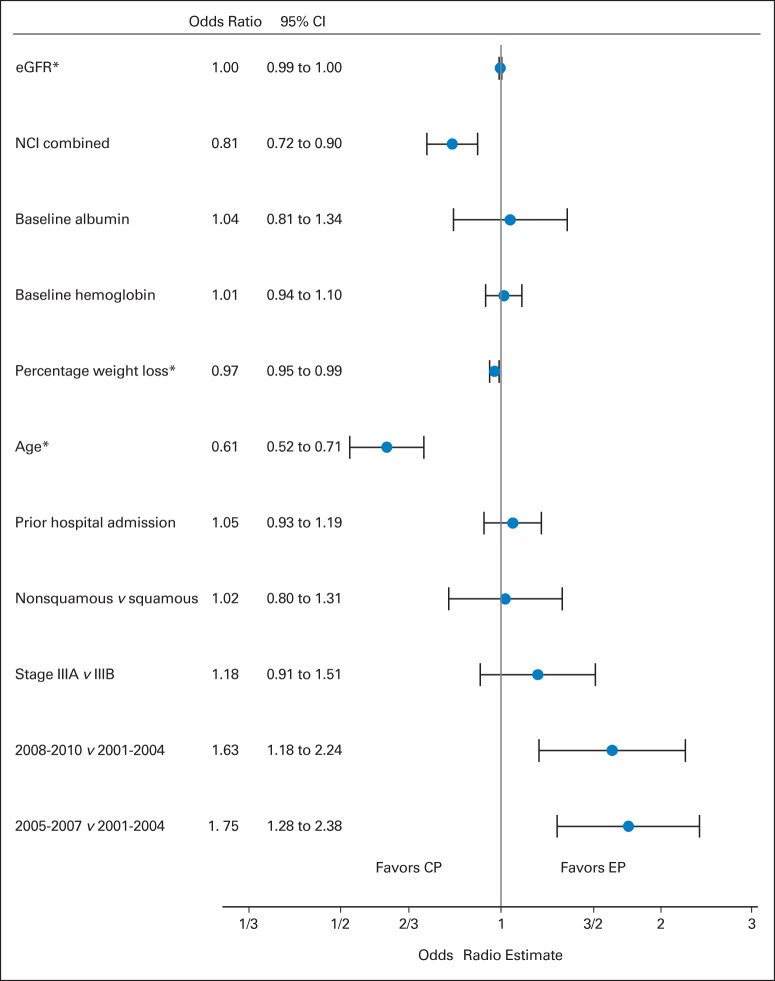
From an initial sample of 17,010 patients with stage III disease, 1,842 patients fulfilled our eligibility criteria (Fig 1). Most of our patients (98%) were men. EP was used in 27% of the patients (n = 499) and CP in 73% (n = 1,343; Table 1). Patients treated with EP, compared with patients treated with CP, were younger (mean age, 61.3 v 65.5 years, respectively; P < .001) and had higher hemoglobin (mean, 12.9 v 12.7 g/dL, respectively; P < .0378), higher albumin levels (mean, 3.6 v 3.5 g/dL, respectively; P = .0586), lower NCI combined index (mean score, 1.1 v 1.4, respectively; P < .001), and less weight loss (mean, 2.6% v 4%, respectively; P < .001). In multiple logistic regression analysis, treatment at a later era, younger age, less weight loss, and lower NCI combined index were associated with the decision to use EP (Fig 2).
Fig 1.
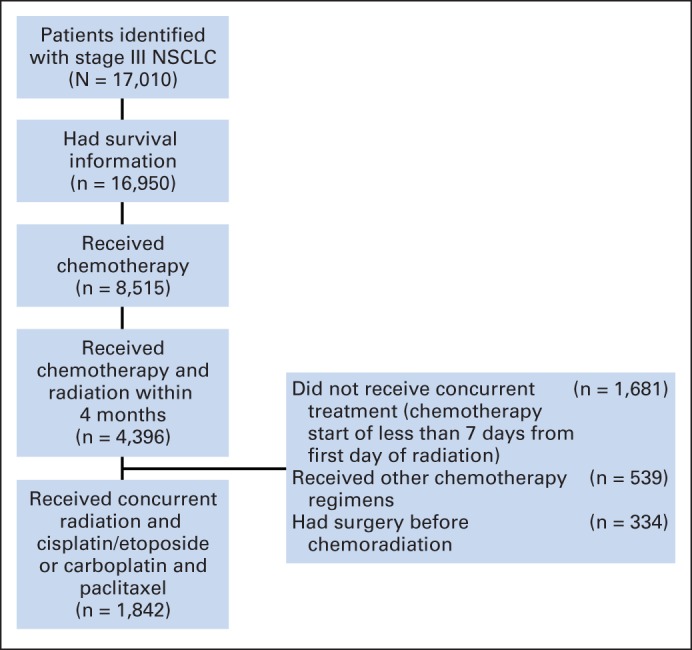
Identification of included and excluded patients with metastatic non–small-cell lung cancer (NSCLC).
Table 1.
Difference in Patient Characteristics Between Patients Treated With EP Versus CP in the Observational Data Set and in Patients Who Were Matched by a Propensity Score
| Characteristic | Observational Data Set (n = 1,842) |
Propensity Score–Matched Data Set (n = 762) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EP (n = 499) |
CP (n = 1,343) |
Standard Difference | P | EP (n = 381) |
CP (n = 381) |
Standard Difference | P | |||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |||||
| Age, years | 0.50 | < .001 | 0.06 | .3999 | ||||||||
| Mean | 61.3 | 65.5 | 62.0 | 62.4 | ||||||||
| SD | 7.6 | 9 | 7.4 | 7.9 | ||||||||
| Era of diagnosis | 0.24 | < .001 | 0 | 1 | ||||||||
| 2001-2004 | 129 | 25.9 | 477 | 35.5 | 94 | 50 | 94 | 50 | ||||
| 2005-2007 | 194 | 38.9 | 393 | 29.3 | 152 | 50 | 152 | 50 | ||||
| 2008-2010 | 176 | 35.3 | 473 | 35.2 | 135 | 50 | 135 | 50 | ||||
| Stage IIIB | 281 | 56.3 | 759 | 56.5 | < 0.01 | .9379 | 189 | 49.6 | 204 | 53.5 | 0.8 | .2769 |
| Histology | 0.11 | .2112 | 0.07 | .8158 | ||||||||
| Adenocarcinoma | 110 | 22 | 252 | 18.8 | 82 | 21.5 | 85 | 22.3 | ||||
| NOS | 146 | 29.3 | 378 | 28.1 | 107 | 28.1 | 112 | 29.5 | ||||
| Other | 3 | 0.6 | 15 | 1.1 | 3 | 0.8 | 5 | 1.3 | ||||
| Squamous cell | 240 | 48.1 | 698 | 52 | 189 | 49.6 | 179 | 48.3 | ||||
| Hemoglobin at baseline, g/dL | 0.11 | .0378 | 0.07 | .3164 | ||||||||
| Mean | 12.9 | 12.7 | 13.0 | 13.1 | ||||||||
| SD | 1.8 | 1.8 | 1.8 | 1.7 | ||||||||
| Platelets at baseline, × 109/L | 0.08 | .1393 | 0.03 | .7169 | ||||||||
| Mean | 331.2 | 321.2 | 324.4 | 321.1 | ||||||||
| SD | 124.0 | 123.3 | 118.9 | 126.4 | ||||||||
| eGFR at baseline, mL/min | 0.16 | .0024 | 0.01 | .9721 | ||||||||
| Mean | 87.3 | 83.7 | 85.7 | 85.7 | ||||||||
| SD | 22.0 | 23.1 | 22.1 | 21.8 | ||||||||
| Baseline serum albumin, g/dL | 0.11 | .0586 | 0.02 | .8084 | ||||||||
| Mean | 3.6 | 3.5 | 3.6 | 3.6 | ||||||||
| SD | 0.6 | 0.6 | 0.6 | 0.6 | ||||||||
| Weight loss, % | 0.25 | < .001 | 0.04 | .7438 | ||||||||
| Mean | 2.9 | 4.5 | 2.9 | 2.8 | ||||||||
| SD | 6.3 | 6.5 | 6.4 | 5.8 | ||||||||
| NCI combined index > 2 | 46 | 9.3 | 230 | 17.2 | 0.24 | < .001 | 39 | 10.2 | 30 | 7.9 | 0.08 | .2559 |
| No. of prior hospitalizations in the year before treatment | 0.07 | .1601 | 0.07 | .3563 | ||||||||
| Mean | 0.9 | 0.8 | 0.9 | 0.8 | ||||||||
| SD | 1.0 | 0.9 | 1.0 | 0.9 | ||||||||
Abbreviations: CP, carboplatin-paclitaxel; eGFR, estimated glomerular filtration rate; EP, etoposide-cisplatin; NCI, National Cancer Institute; NOS, not otherwise specified; SD, standard deviation.
Fig 2.
Odds ratio plot. This plot represents characteristics associated with the use of carboplatin-paclitaxel (CP) versus etoposide-cisplatin (EP). eGFR, estimated glomerular filtration rate; NCI, National Cancer Institute. (*) Multiples of 10.
Patients treated with CP received a median of five cycles (interquartile range, three to six cycles), with 46.8% of patients receiving six or seven cycles. In the EP group, 81.8% of patients received two cycles, and 18.2% received only one cycle. Patients who received CP were more likely to receive consolidation chemotherapy than patients who received EP (67.5% v 46.1%, respectively; P = .0026). The need for consolidation chemotherapy was put in question after the results of the Hoosier Oncology Group study were presented in 2007.3 In our database, before 2007, the rates of consolidation chemotherapy among patients receiving CP were similar to rates among those receiving EP. However, after 2007, the rate of consolidation in the CP group increased from 65.2% to 71.7%, whereas in the EP group, it decreased from 53.6% to 32.4%. Surgical resection after induction chemotherapy was uncommon but was more frequently used in the EP group 7% (n = 35) in contrast to only 2.4% (n = 32) in the CP group.
Survival Outcomes
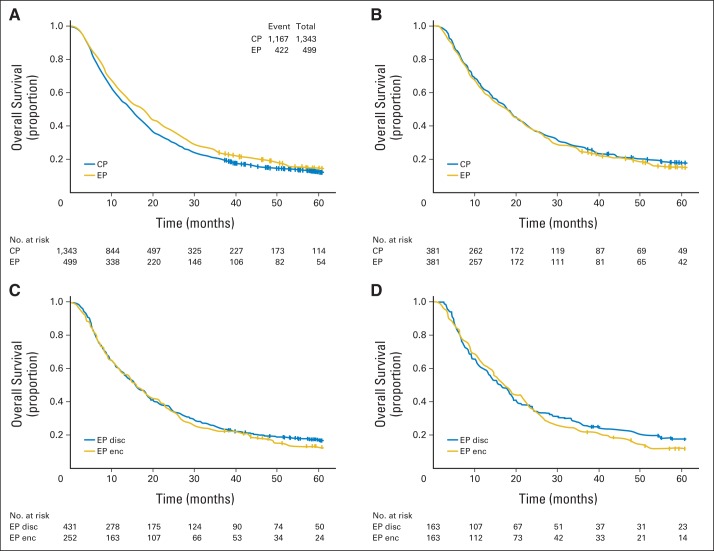
In an unadjusted two-group analysis, patients treated with EP had a better outcome compared with those treated with CP (median OS, 17.3 v 14.6 months, respectively; HR, 0.88; 95% CI, 0.79 to 0.99; P = .0209; Fig 3A). In a Cox proportional hazards model, patient age (HR, 1.08; 95% CI, 1.01 to 1.15; P = .0258), percentage of weight loss (HR, 1.04; 95% CI, 1.03 to 1.05; P < .001), baseline anemia (HR, 1.19; 95% CI, 1.05 to 1.36; P < .001), hypoalbuminemia (HR, 1.29; 95% CI, 1.14 to 1.46; P < .001), and treatment era were all independently associated with decreased survival, whereas the chemotherapy regimen received was not associated with any survival advantage (HR, 0.97; 95% CI, 0.85 to 1.10; P = .6327; Table 2).
Fig 3.
(A) Univariate analysis of overall survival of all patients by treatment with carboplatin-paclitaxel (CP) versus etoposide-cisplatin (EP). (B) Survival of a cohort of patients matched according to their propensity scores by treatment arm. (C) Survival of patients treated at EP-encouraging centers (enc) versus EP-discouraging centers (disc). (D) Survival of the near/far matched cohort.
Table 2.
Cox Proportional Hazards Model
| Variable | No. of Patients | Median Survival (months) | Cox Univariable Analysis |
Cox Multivariable Analysis |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P (n = 1,437) | |||
| Chemotherapy regimen | .0209 | .6327 | ||||||
| Carboplatin and paclitaxel | 1,343 | 14.6 | — | — | ||||
| Cisplatin and etoposide | 499 | 17.3 | 0.88 | 0.79 to 0.98 | 0.97 | 0.85 to 1.10 | ||
| Baseline hemoglobin, g/dL | < .001 | .0064 | ||||||
| ≥ 12 | 1,213 | 17.5 | — | — | ||||
| < 12 | 595 | 10.6 | 1.42 | 1.28 to 1.57 | 1.19 | 1.05 to 1.36 | ||
| Baseline albumin, g/dL | < .001 | < .001 | ||||||
| ≥ 3.5 | 958 | 17.7 | — | — | ||||
| < 3.5 | 694 | 10.9 | 1.49 | 1.35 to 1.66 | 1.29 | 1.14 to 1.46 | ||
| Stage | .0014 | .1845 | ||||||
| IIIA | 802 | 17.3 | — | — | ||||
| IIIB | 1,040 | 13.7 | 1.17 | 1.06 to 1.30 | 1.08 | 0.96 to 1.21 | ||
| NCI combined index score | .0035 | .0503 | ||||||
| < 2 | 1,559 | 15.4 | — | — | ||||
| ≥ 2 | 276 | 12.9 | 1.22 | 1.07 to 1.40 | 1.17 | 1.00 to 1.37 | ||
| Treatment era | < .001 | .0281 | ||||||
| 2001-2004 | 606 | 12.8 | — | — | ||||
| 2005-2007 | 587 | 15.2 | 0.84 | 0.74 to 0.95 | 0.89 | 0.77 to 1.02 | ||
| 2008-2010 | 649 | 17.4 | 0.78 | 0.69 to 0.88 | 0.83 | 0.72 to 0.95 | ||
| Age, in 10-year increments | 1,842 | 15.0 | 1.09 | 1.03 to 1.15 | .0037 | 1.08 | 1.01 to 1.15 | .0258 |
| % of weight lost in the year before diagnosis | 1,622 | 15.3 | 1.05 | 1.04 to 1.06 | < .001 | 1.04 | 1.03 to 1.05 | < .0001 |
Abbreviation: NCI, National Cancer Institute.
Propensity Score
For the matched analysis, 381 patients treated with EP were identified and matched based on their propensity score and era of treatment with an equal number of patients receiving CP. This analysis eliminated differences in age, hemoglobin, albumin level, percentage of weight loss, and comorbidity scores seen in the larger cohort (Table 1) and revealed no survival advantage for EP (HR, 1.07; 95% CI, 0.91 to 1.24; P = .4264; Fig 3C). Subsequently, a Cox proportional hazards model weighted on the inverse propensity for being treated with EP was fitted. This analysis also showed no survival advantage for EP (HR, 0.97; 95% CI, 0.85 to 1.10; P = .6212).
Instrumental Variables
In facilities that treated more than 20 patients, there were marked differences between facilities in EP and CP use: eight facilities used EP more than 50% of the time (range, 55% to 81%), whereas 11 centers used EP in fewer than 10% of the cases (range, 0% to 9%). Among these facilities, which were all affiliated with an academic medical center, there were no significant differences in oncologist full-time employment equivalent (median, 2.7 v 2.3; P = .8043), unique patients seen annually in oncology clinics (median, 1,898 v 1,714; P = .5915), or overall facility volume (mean, 58,037 v 65,151; P = .5357).
For the instrumental variable analysis, patients treated at EP-encouraging centers, compared with patients treated at EP-discouraging centers, were younger (mean age, 62.1 v 65 years, respectively; P = .001) and had higher baseline albumin (mean, 3.8 v 3.5 g/dL, respectively; P = .0032) and hemoglobin levels (mean, 12.9 v 12.6, g/dL, respectively; P = .0346; Appendix Table A1, online only). Despite having patients who had better prognostic characteristics, no survival advantage was seen for patients treated in EP-encouraging centers in a univariable analysis (HR, 1.07; 95% CI, 0.90 to 1.26; P = .4653; Fig 3C). Furthermore, the near/far analysis, which equilibrated prognostic variables, did not demonstrate a survival advantage for matched patients treated in EP-encouraging centers (HR, 1.03; 95% CI, 0.75 to 1.40; P = .8736; Fig 3D).
Toxicity
In the original cohort without adjustments, patients treated with EP had a higher incidence of adverse events during the initial 4 months of treatment compared with patients treated with CP (Table 3). Patients treated with EP, compared with patients treated with CP, had more oncology clinic visits (mean, 17.6 v 12.6 visits, respectively; P < .001) and more hospitalizations (mean, 2.4 v 1.7 hospitalizations, respectively; P < .001).
Table 3.
Morbidity in Patients Treated With EP Versus CP
| Outcome* | EP |
CP |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| No. of hospitalizations | < .001 | ||||
| Mean | 2.4 | 1.7 | |||
| SD | 2.4 | 1.8 | |||
| Outpatient visits | < .001 | ||||
| Mean | 17.6 | 12.6 | |||
| SD | 11.8 | 6.7 | |||
| At least one encounter for any of the following complications | |||||
| Infectious complication | 236 | 47 | 529 | 39.4 | .0022 |
| Acute kidney injury/dehydration | 152 | 30.5 | 285 | 21.2 | < .001 |
| Nausea/vomiting | 65 | 13 | 110 | 8.2 | .0017 |
| Mucositis/esophagitis | 93 | 18.6 | 193 | 14.4 | .0246 |
| Any of the above | 321 | 64.4 | 739 | 55 | < .001 |
Abbreviations: CP, carboplatin-paclitaxel; EP, etoposide-cisplatin; SD, standard deviation.
Limited to the 4 months after the initiation of chemotherapy.
DISCUSSION
In a large cohort of patients with stage III NSCLC who received concurrent chemoradiotherapy at academically affiliated tertiary care VA hospitals, we found that EP offered no survival advantage over CP and was associated with more toxicity. This finding was consistent across multiple analytic approaches in a database that included a wide range of potential confounders.
After the establishment of concurrent chemoradiotherapy as the standard of care,2,6 little research has addressed the choice of chemotherapy. Because most of the trials that established this new standard of care used cisplatin-based chemotherapy and because of the theoretical advantage that its initial systemic doses are able to eradicate micrometastatic disease, EP is widely recommended for use.8,9 However, concerns about toxicity associated with EP have led oncologists to consider alternate regimens, most notably CP.10,21
The results of the Cancer and Leukemia Group B 39801 trial cast doubt on the use of CP as chemotherapy because of the low median survival in both arms (12 to 14 months), which was roughly similar to prior sequential chemoradiotherapy arms in RCTs and markedly inferior to that observed in phase III studies using EP.9,22 In our matched data set, patients treated with CP had an OS of 16.1 months (95% CI, 14 to 18 months; Fig 3). Although we used date of diagnosis, rather than start of treatment, as time zero, this OS is consistent with that seen in clinical trials using EP and better than that seen in the Cancer and Leukemia Group B 39801 trial. This suggests that the lower survival seen in the latter study is not a function of the CP regimen, but rather some characteristic of the population that was enrolled onto that study, because both arms did less well than other studies of similar stage NSCLC.
We are only aware of one published randomized prospective comparison of EP and CP. This was a study of 65 patients by Wang et al,23 which found that EP had superior OS but similar progression-free survival compared with CP. There are several possible explanations for our contradictory results. Most obviously, the study by Wang et al23 is small; promising data obtained through small phase II studies frequently are not confirmed when a larger population is studied.24 Another possibility is that the Chinese population studied by Wang et al23 has important differences in disease characteristics (eg, frequency of EGFR mutations) from our largely white male population. Finally, it could be that our adjustment was unable to eliminate biases that obscured a true benefit of EP.
To our knowledge, the current study is the largest study comparing the survival of patients receiving EP versus CP in this clinical setting. Its main strengths are the richness and robustness of the VA database and the number of patients it contains. We used multiple approaches to address biases that may be introduced by nonrandom assignment of the treatments (EP and CP) being compared. First, we included a wide range of clinically relevant variables in a multiple regression analysis to adjust for differences in the patients who received EP or CP. These variables included change in weight and key baseline laboratory values in addition to the staging, comorbidity, and demographic data.
Second, we used propensity score analysis, a method designed to achieve the same goal of eliminating bias caused by measured patient characteristics that affect both treatment and outcomes. Finally, we used an instrumental variables analysis to overcome bias caused by unmeasured or unknown variables associated with treatment and outcomes. The consistency of our results strengthens our conclusions.
Our study does have other limitations. First, it is not currently possible to establish the dose or duration of radiation treatment in our database. Second, there were other differences in treatment. Notably, patients receiving CP were more likely to receive consolidation chemotherapy. Although studies have not demonstrated a benefit from consolidation25–27 and its use remains controversial, the lower cumulative dose of chemotherapy in the EP arm might have obscured an advantage for EP. Similarly, our database does not include chemotherapy doses, although our record review (Appendix, online only) confirmed that data regarding which specific drugs were used is accurate. Because we did not collect data prospectively, we cannot comment on the proportion of patients treated as part of a clinical trial or on the initial treatment plan. Similarly, we rely on coded administrative data to identify treatment toxicity; this is a less comprehensive analysis of adverse events than can obtained in prospective trials. Moreover, our database includes only hospitalizations and outpatient visits occurring in the VA system. Thus, we likely underestimate the frequency of visits and adverse events after treatment. However, it is unlikely that this differentially affects patients receiving EP versus CP. We also acknowledge that our VA data set includes mostly older white men; extrapolation beyond this group may be problematic. However, patients treated at the VA for lung cancer have similar outcomes to patients treated in clinical trials28 as well as in the private sector.29 Next, although our sample size is comparatively large, it is still possible that a small benefit of EP was missed as a result of insufficient power. Finally, our study compared two specific platinum-based regimens; we cannot comment on the relative efficacy of other combinations, including the use of a platinum agent with vinorelbine or vinca alkaloids.
Despite these limitations, we believe this study shows that there is considerable equipoise regarding which regimen should be preferred. Given the prevalence of unresectable stage III lung cancer, we believe a phase III randomized control trial should be considered to definitively answer this question. Pending the availability of such data, our results may help guide clinicians and patients trying to decide which chemotherapy regimen to pair with radiotherapy.
Glossary Terms
- non–small-cell lung cancer (NSCLC):
a type of lung cancer that includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma.
Appendix
Potential Confounders
We augmented the Department of Veterans Affairs (VA) Central Cancer Registry database with data regarding laboratory results from the VA Decision Support System laboratory files, weight from the VA Corporate Data Warehouse, and comorbid conditions identified using International Classification of Diseases Ninth Revision (ICD-9) codes from VA Medical SAS inpatient and outpatient encounter files.
Laboratory values.
We defined baseline hemoglobin, platelet, creatinine, and albumin levels as the mean of tests obtained within 45 days before and 7 days after initial treatment. We excluded biologically implausible values (hemoglobin > 22 g/dL or < 5 g/dL; albumin > 8 g/dL), which made up less than 0.1% of observations. No implausible values for creatinine or platelets were identified. Plausible hemoglobin, albumin, and platelet levels were identified in 98.2% (n = 1,822), 89.8% (n = 1,666), and 92% (n = 1,878) of eligible patients, respectively. We determined the estimated glomerular filtration rate using the Modification of Diet in Renal Disease equation (Levey AS, et al: Ann Intern Med 145:247-254, 2006); estimated glomerular filtration rate was obtained in 97% of patients (n = 1,801).
Baseline patient characteristics.
We defined baseline weight as the median of documented weights obtained between 3 months and 2 years preceding the day chemotherapy started. We calculated change in weight by subtracting the median of weights obtained during the 15 days after the start of treatment from the baseline weight. Weight loss could be calculated in 88% (n = 1,634) of our patients.
Treatment Era
The cohort was divided into three treatment eras based on date of diagnosis (2001 to 2003, 2004 to 2007, and 2008 to 2010).
Record Review
Two of the authors (A.M. and R.S.-D.) reviewed the electronic records of a random sample of 158 patients (8.5%) to confirm that chemotherapy was administered concurrently with radiotherapy and that the chemotherapy regimen was correctly classified as etoposide-cisplatin (EP) or carboplatin-paclitaxel (CP). Both were confirmed in all patients.
Chemotherapy.
The Decision Support System pharmacy records were used to obtain chemotherapy information. We defined induction chemotherapy as that prescribed in the first 6 weeks after the initiation of treatment and consolidation as that administered after 6 weeks and until 16 weeks after initiation of treatment. During the induction phase, for patients treated with CP, all chemotherapy drugs identified every 7 days were counted as a cycle. For patients treated with EP, all chemotherapy drugs identified every 19 days were counted as a cycle. For the consolidation phase in both groups, cycles were identified as all drugs administered every 19 days.
Surgery.
Receipt of surgery was identified by looking at diagnostic and procedure codes in both the outpatient and inpatient fee basis and MedSAS files. We used ICD-9 Clinical Modification codes (32.09, 32.10, 32.50, 32.60, 32.40, 32.29, and 32.90) and Current Procedural Terminology codes (32440, 32442, 32445, 32480, 32482, 32484, 32486, 32500, and 32999).
Comorbidities
We measured comorbidities using ICD-9 diagnosis and procedure codes from inpatient and outpatient encounters occurring between 13 months and 1 month before non–small-cell lung cancer diagnosis. We applied the Deyo algorithm for generating the Charlson comorbidity index from administrative data. We then excluded cancer diagnoses and applied the lung cancer–specific weights developed by Klabunde et al (Klabunde CN, et al: J Clin Epidemiol 53:1258-1267, 2000)12 to obtain the National Cancer Institute combined index. We identified VA hospitalizations during the year before the start of treatment, because prior hospitalizations are known to predict future hospitalizations and death (Anderson G, et al: JAMA 263:967-972, 1990). We used the 2009 Area Resource File to identify area-level surrogates for socioeconomic status (ie, proportion of adults with high school education, median household income, urban/rural status).
Outcomes
We obtained data regarding date of death from the VA's vital status file updated in February of 2014. This is created by combining mortality data from various VA databases and the Social Security Administration and provides data quality similar to that available from the National Death Index for veterans who use the VA for health care (Sohn MW, et al: Popul Health Metr 4:2, 2006).
We summarized the number of VA hospitalizations and outpatient visits during the 4 months after the start of treatment. We identified complications commonly associated with chemotherapy using the ICD-9 diagnosis codes associated with these encounters. When available, previously defined coding algorithms were applied; for other complications, we developed algorithms based on appropriate coding documents. Complications were categorized as infection or neutropenia; acute kidney injury or dehydration; nausea or vomiting; hemorrhage; or mucositis/esophagitis. We did not attempt to grade the severity of these complications, but note that they were significant enough to be coded.
Complications of Treatment
Hospital encounters and outpatients records were used to obtain coding information to identify complications from treatment that occurred within 4 months of the start of treatment. Infections or neutropenic complications were defined as having an inpatient or outpatient visit with a primary or secondary diagnosis of neutropenia (ICD-9 Clinical Modification 288.0), fever (780.6), or a list of infections previously used by other authors (Weycker D, et al: BMC Health Serv Res 13:60, 2013). Acute kidney disease or dehydration was defined as having a primary or secondary diagnosis of dehydration (276.5), hypernatremia (276.0), acute kidney failure (584.0), or renal failure, unspecified (586.0). Nausea/vomiting complications were identified with diagnosis for nausea and vomiting (787.0) or persisting vomiting (536.2). Hemorrhage was defined as having ICD-9 codes for extracranial hemorrhages (ie, GI, genitourinary, retroperitoneal) or primary and secondary diagnoses of intracranial hemorrhage, including intracerebral, subarachnoid, or subdural hemorrhages, as done by Fang et al (Fang MC, et al: J Am Coll Cardiol 58:395-401, 2011).
Facilities
We used 2012 VA Physician Workforce and Support Staff Data from the VA Office of Productivity, Efficiency, and Staffing to identify academic affiliation, number of oncologist full-time employment equivalents, and the number of unique patients seen at the facility, both overall and in oncology clinics.
Histologies
Histologies are listed in Appendix Table A2.
Table A1.
Differences in Patient Characteristics Between Centers Where the Majority of Patients Were Treated With EP (EP-encouraging centers) and Centers Where EP Was Used in Less Than 10% of Patients (EP-discouraging centers)
| Characteristic | Comparing All Patients |
Near/Far Match Analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EP-Encouraging Center (n = 252) |
EP-Discouraging Center (n = 431) |
Standard Difference | P | EP-Encouraging Center (n = 163) |
EP-Discouraging Center (n = 163) |
Standard Difference | P | |||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |||||
| Treatment | 1.83 | < .001 | 1.82 | < .001 | ||||||||
| EP | 173 | 68.7 | 16 | 3.7 | 107 | 65.6 | 3 | 1.8 | ||||
| CP | 79 | 31.3 | 415 | 96.3 | 56 | 34.4 | 160 | 98.2 | ||||
| Treatment era | 0.17 | .0964 | 0 | 1 | ||||||||
| 2001-2004 | 95 | 38.9 | 149 | 61.1 | 61 | 50 | 61 | 50 | ||||
| 2005-2007 | 92 | 40.2 | 137 | 59.8 | 60 | 50 | 60 | 50 | ||||
| 2008-2010 | 65 | 30.9 | 145 | 69.1 | 42 | 50 | 42 | 50 | ||||
| Age, years | 0.31 | < .001 | 0.02 | .7250 | ||||||||
| Mean | 62.1 | 65 | 64.5 | 64.2 | ||||||||
| SD | 8.8 | 8.8 | 8.5 | 7.8 | ||||||||
| Stage IIIB | 140 | 55.6 | 64.9 | 8.8 | 0.06 | .4576 | 89 | 54.6 | 102 | 62.6 | 0.17 | .1438 |
| Histology | 0.28 | .0065 | 0.04 | .0210 | ||||||||
| Adenocarcinoma | 54 | 21.4 | 69 | 16 | 32 | 19.6 | 24 | 14.7 | ||||
| NOS | 92 | 36.5 | 123 | 28.5 | 64 | 39.3 | 46 | 28.2 | ||||
| Other | 1 | 0.4 | 6 | 1.4 | 0 | 0.0 | 2 | 1.2 | ||||
| Squamous | 105 | 41.7 | 233 | 54.1 | 67 | 41.1 | 91 | 55.8 | ||||
| Hemoglobin, g/dL | 0.17 | .0346 | .3293 | |||||||||
| Mean | 12.9 | 12.6 | 12.9 | 12.7 | 0.11 | |||||||
| SD | 1.8 | 1.7 | 1.8 | 1.6 | ||||||||
| eGFR, mL/min | 0.13 | .1094 | .3779 | |||||||||
| Mean | 87.5 | 84.4 | 84.3 | 86.6 | 0.10 | |||||||
| SD | 23.9 | 24.4 | 23.8 | 24.3 | ||||||||
| Albumin, g/dL | 0.25 | .0032 | .0029 | |||||||||
| Mean | 3.7 | 3.5 | 3.8 | 3.6 | 0.33 | |||||||
| SD | 0.6 | 0.6 | 0.5 | 0.6 | ||||||||
| Weight loss, % | 0.12 | .1740 | .7829 | |||||||||
| Mean | 3.5 | 4.2 | 3.9 | 4.1 | 0.03 | |||||||
| SD | 6.5 | 6.7 | 6.2 | 5.7 | ||||||||
| Modified NCI score > 2 | 28 | 11.2 | 66 | 15.3 | 0.12 | .1259 | 21 | 12.9 | 19 | 11.7 | 0.04 | .7357 |
Abbreviations: CP, carboplatin-paclitaxel; eGFR, estimated glomerular filtration rate; EP, etoposide-cisplatin; NCI, National Cancer Institute; NOS, not otherwise specified; SD, standard deviation.
Table A2.
Histologies Included in the Study
| Histology/Behavior Code | Histology Description | Histology/Behavior Description |
|---|---|---|
| 8000/0 | Neoplasm | Neoplasm, benign |
| 8000/1 | Neoplasm | Neoplasm, uncertain whether benign or malignant |
| 8000/3 | Neoplasm | Neoplasm, malignant |
| 8001/3 | Neoplasm | Tumor cells, malignant |
| 8002/3 | Neoplasm | Malignant tumor, small-cell type |
| 8003/3 | Neoplasm | Malignant tumor, giant-cell type |
| 8004/3 | Neoplasm | Malignant tumor, spindle cell type |
| 8010/2 | Carcinoma, NOS | Carcinoma in situ, NOS |
| 8010/3 | Carcinoma, NOS | Carcinoma, NOS |
| 8011/3 | Carcinoma, NOS | Epithelioma, malignant |
| 8012/3 | Carcinoma, NOS | Large-cell carcinoma, NOS |
| 8013/3 | Carcinoma, NOS | Large-cell neuroendocrine carcinoma |
| 8014/3 | Carcinoma, NOS | Large-cell carcinoma with rhabdoid phenotype |
| 8020/3 | Carcinoma, undifferentiated NOS | Carcinoma, undifferentiated type, NOS |
| 8021/3 | Carcinoma, undifferentiated NOS | Carcinoma, anaplastic type, NOS |
| 8022/3 | Carcinoma, undifferentiated NOS | Pleomorphic carcinoma |
| 8030/3 | Giant- and spindle cell carcinoma | Giant-cell and spindle cell carcinoma |
| 8031/3 | Giant- and spindle cell carcinoma | Giant-cell carcinoma |
| 8032/3 | Giant- and spindle cell carcinoma | Spindle cell carcinoma |
| 8033/3 | Giant- and spindle cell carcinoma | Pseudosarcomatous carcinoma |
| 8041/3 | Small-cell carcinoma, NOS | Small-cell carcinoma, NOS |
| 8042/3 | Small-cell carcinoma, NOS | Oat cell carcinoma |
| 8043/3 | Small-cell carcinoma, NOS | Small-cell carcinoma, fusiform cell |
| 8044/3 | Small-cell carcinoma, NOS | Small-cell carcinoma, intermediate cell |
| 8045/3 | Small-cell carcinoma, NOS | Combined small-cell carcinoma |
| 8046/2 | Non–small-cell carcinoma, in situ | Non–small-cell carcinoma, in situ |
| 8046/3 | Non–small-cell carcinoma, NOS | Non–small-cell carcinoma |
| 8050/2 | Papillary carcinoma, NOS | Papillary carcinoma in situ |
| 8050/3 | Papillary carcinoma, NOS | Papillary carcinoma, NOS |
| 8052/2 | Papillary carcinoma, NOS | Papillary squamous cell carcinoma, noninvasive |
| 8052/3 | Papillary carcinoma, NOS | Papillary squamous cell carcinoma |
| 8070/2 | Squamous cell carcinoma, NOS | Squamous cell carcinoma in situ, NOS |
| 8070/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, NOS |
| 8071/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, keratinizing, NOS |
| 8072/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, large cell, nonkeratinizing |
| 8073/2 | Squamous cell carcinoma, CIS | Squamous cell carcinoma, CIS |
| 8073/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, small cell, nonkeratinizing |
| 8074/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, spindle cell |
| 8075/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, adenoid |
| 8076/2 | Squamous cell carcinoma, NOS | Squamous cell carcinoma in situ with questionable stromal invasion |
| 8076/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma, microinvasive |
| 8078/3 | Squamous cell carcinoma, NOS | Squamous cell carcinoma with horn formation |
| 8140/2 | Adenocarcinoma, NOS | Adenocarcinoma in situ |
| 8140/3 | Adenocarcinoma, NOS | Adenocarcinoma, NOS |
| 8141/3 | Adenocarcinoma, NOS | Scirrhous adenocarcinoma |
| 8144/3 | Adenocarcinoma, NOS | Adenocarcinoma, intestinal type |
| 8147/3 | Adenocarcinoma, NOS | Basal cell adenocarcinoma |
| 8200/3 | Adenoid cystic and cribriform carcinoma | Adenoid cystic carcinoma |
| 8230/3 | Solid carcinoma, NOS | Solid carcinoma, NOS |
| 8231/3 | Solid carcinoma, NOS | Carcinoma simplex |
| 8250/2 | Bronchioloalveolar adenocarcinoma in situ | Bronchioloalveolar adenocarcinoma in situ |
| 8250/3 | Bronchioloalveolar adenocarcinoma | Bronchioloalveolar adenocarcinoma |
| 8251/3 | Bronchioloalveolar adenocarcinoma | Alveolar adenocarcinoma |
| 8252/2 | Bronchioloalveolar adenocarcinoma in situ | Bronchioloalveolar adenocarcinoma in situ |
| 8252/3 | Bronchioloalveolar adenocarcinoma | Bronchioloalveolar carcinoma, nonmucinous |
| 8253/3 | Bronchioloalveolar adenocarcinoma | Bronchioloalveolar carcinoma, mucinous |
| 8254/3 | Bronchioloalveolar adenocarcinoma | Bronchioloalveolar carcinoma, mixed mucinous and nonmucinous |
| 8255/3 | Bronchioloalveolar adenocarcinoma | Adenocarcinoma with mixed subtypes |
| 8260/3 | Papillary adenocarcinoma, NOS | Papillary adenocarcinoma, NOS |
| 8263/3 | Papillary adenocarcinoma, NOS | Adenocarcinoma in tubulovillous adenoma |
| 8310/3 | Clear cell adenocarcinoma, NOS | Clear cell adenocarcinoma, NOS |
| 8320/3 | Granular cell carcinoma | Granular cell carcinoma |
| 8323/3 | Granular cell carcinoma | Mixed cell adenocarcinoma |
| 8430/3 | Mucoepidermoid carcinoma | Mucoepidermoid carcinoma |
| 8430/3 | Mucoepidermoid carcinoma | Mucoepidermoid carcinoma |
| 8480/3 | Mucinous adenocarcinoma | Mucinous adenocarcinoma |
| 8481/3 | Mucinous adenocarcinoma | Mucin-producing adenocarcinoma |
| 8490/3 | Signet ring cell carcinoma | Signet ring cell carcinoma |
| 8550/3 | Acinar cell carcinoma | Acinar cell carcinoma |
| 8560/3 | Adenosquamous carcinoma | Adenosquamous carcinoma |
| 8572/3 | Adenocarcinoma with metaplasia | Adenocarcinoma with spindle cell metaplasia |
| 8574/3 | Adenocarcinoma with metaplasia | Adenocarcinoma with neuroendocrine differentiation |
| 8575/3 | Adenocarcinoma with metaplasia | Metaplastic carcinoma, NOS |
| 8576/3 | Adenocarcinoma with metaplasia | Hepatoid adenocarcinoma |
| 8803/3 | Sarcoma, NOS | Small-cell sarcoma |
| 8940/3 | Mixed tumor, malignant, NOS | Mixed tumor, malignant, NOS |
| 8980/3 | Carcinosarcoma, NOS | Carcinosarcoma, NOS |
| 9015/3 | Adenocarcinofibroma | Mucinous adenocarcinofibroma |
Abbreviations: CIS, carcinoma in situ; NOS, not otherwise specified.
Footnotes
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Kiran Devisetty, Abbott Laboratories, AbbVie Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Rafael Santana-Davila, Rodney Sparapani, Jeffrey Whittle
Administrative support: Jeffrey Whittle
Collection and assembly of data: Rafael Santana-Davila, Amy Moran, Jeffrey Whittle
Data analysis and interpretation: Rafael Santana-Davila, Kiran Devisetty, Aniko Szabo, Carlos Arce-Lara, Elizabeth M. Gore, Amy Moran, Christina D. Williams, Michael J. Kelley, Jeffrey Whittle
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Price A. Emerging developments of chemoradiotherapy in stage III NSCLC. Nat Rev Clin Oncol. 2012;9:591–598. doi: 10.1038/nrclinonc.2012.135. [DOI] [PubMed] [Google Scholar]
- 2.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 4.Hoang T, Dahlberg SE, Schiller JH, et al. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: The ECOG 3598 study. J Clin Oncol. 2012;30:616–622. doi: 10.1200/JCO.2011.36.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol. 2013;31:1029–1038. doi: 10.1200/JCO.2012.44.5064. [DOI] [PubMed] [Google Scholar]
- 6.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 7.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Crowley JJ, Turrisi AT, 3rd, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–3460. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: Phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 10.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 11.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EW, Wei LJ, Amato DA, et al. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Columbus, OH: Springer; 1992. pp. 237–247. [Google Scholar]
- 16.Gayat E, Resche-Rigon M, Mary JY, et al. Propensity score applied to survival data analysis through proportional hazards models: A Monte Carlo study. Pharm Stat. 2012;12:222–229. doi: 10.1002/pst.537. [DOI] [PubMed] [Google Scholar]
- 17.Lin DY, Wei L-J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 18.Baiocchi M, Small DS, Lorch S, et al. Building a stronger instrument in an observational study of perinatal care for premature infants. J Am Stat Assoc. 2010;105:1285–1296. [Google Scholar]
- 19.Baiocchi M, Small DS, Yang L, et al. Near/far matching: A study design approach to instrumental variables. Health Serv Outcomes Res Method. 2012;12:237–253. doi: 10.1007/s10742-012-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therneau TM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 21.Akerley W, Herndon JE, Jr, Lyss AP, et al. Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small-cell lung cancer: A limited-access study—CALGB 9534. Clin Lung Cancer. 2005;7:47–53. doi: 10.3816/CLC.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 22.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Wu S, Ou G, et al. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2012;77:89–96. doi: 10.1016/j.lungcan.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Maitland ML, Hudoba C, Snider KL, et al. Analysis of the yield of phase II combination therapy trials in medical oncology. Clin Cancer Res. 2010;16:5296–5302. doi: 10.1158/1078-0432.CCR-10-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber RM, Engel-Riedel W, Kollmeier J, et al. GILT study: Oral vinorelbine (NVBo) and cisplatin (P) with concomitant radiotherapy (RT) followed by either consolidation (C) with NVBo plus P plus best supportive care (BSC) or BSC alone in stage (st) III non-small cell lung cancer (NSCLC): Final results of a phase (ph) III study. J Clin Oncol. 2012;(suppl):30. abstr 7001. [Google Scholar]
- 26.Tsujino K, Kurata T, Yamamoto S, et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol. 2013;8:1181–1189. doi: 10.1097/JTO.0b013e3182988348. [DOI] [PubMed] [Google Scholar]
- 27.Park K, Ahn YC, Ahn JS. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN) J Clin Oncol. 2014;(suppl):32. doi: 10.1200/JCO.2014.60.0130. abstr 7500. [DOI] [PubMed] [Google Scholar]
- 28.Santana-Davila R, Szabo A, Arce-Lara C, et al. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer: An analysis of Veterans Health Administration data. J Thorac Oncol. 2014;9:702–709. doi: 10.1097/JTO.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30:1072–1079. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]