Abstract
Purpose
Hip osteonecrosis frequently complicates treatment with glucocorticoids. When extensive (affecting ≥ 30% of the epiphyseal surface), 80% of joints collapse within 2 years, so interventions are needed to prevent this outcome.
Patients and Methods
This prospective cohort magnetic resonance imaging (MRI) screening study included all consecutive children treated for acute lymphoblastic leukemia on a single protocol. Hip MRI was performed at 6.5 and 9 months from diagnosis (early screening) and at completion of chemotherapy (final evaluation) to determine whether screening could identify extensive hip osteonecrosis before symptom development.
Results
Of 498 patients, 462 underwent screening MRI. Extensive asymptomatic osteonecrosis was identified by early screening in 26 patients (41 hips); another four patients (seven hips) were detected after the screening period, such that screening sensitivity was 84.1% and specificity was 99.4%. The number of joints screened to detect one lesion was 20.1 joints for all patients, 4.4 joints for patients older than 10 years, and 198 joints for patients ≤ 10 years old (P < .001). Of the 40 extensive lesions in patients older than 10 years, 19 required total hip arthroplasty and none improved. Of eight extensive lesions in younger patients, none required arthroplasty and four improved.
Conclusion
In patients age 10 years old or younger who require prolonged glucocorticoid therapy, screening for extensive hip osteonecrosis is unnecessary because their risk is low and lesions tend to heal. In children older than 10 years, early screening successfully identifies extensive asymptomatic lesions in patients who would be eligible for studies of interventions to prevent or delay joint collapse.
INTRODUCTION
Glucocorticoid-induced osteonecrosis occurs in up to 50% of children treated for acute lymphoblastic leukemia (ALL).1–7 Once symptoms develop, femoral head lesions are often extensive and joint collapse inevitable. Indeed, joints usually collapse within 2 years of osteonecrosis identification when affecting ≥ 30% of the epiphyseal surface, defined herein as extensive osteonecrosis.8 Early identification of osteonecrosis would allow for interventions that may prevent progression.9 However, the timing of onset and risk factors for development of extensive epiphyseal lesions are poorly understood.4
With modern therapy, up to 90% of children with ALL are cured of their disease, but femoral head osteonecrosis can cause lasting disability.8,10–12 Unlike other groups of patients at risk for osteonecrosis, children with ALL receive glucocorticoids at specified doses and schedules. They provide a uniformly treated patient cohort in whom the timing and risk factors for early and asymptomatic osteonecrosis can be evaluated to identify patients eligible for studies of early interventions to prevent progression.9
PATIENTS AND METHODS
Study Conduct
We prospectively performed hip MRI screening after completion of each of the two reinduction (delayed intensification) chemotherapy cycles at weeks 10 and 20 of continuation treatment (approximately 6.5 and 9 months from diagnosis, respectively) and at the completion of all therapy for patients age 1 to 18 years old with newly diagnosed ALL treated on the Total Therapy Study XV from June 2000 through October 2007 (Fig 1).10 Because patients experienced treatment delay for various reasons, the time from diagnosis to screening varied among patients, but initial screening was done within a year of diagnosis in all patients. The protocol was approved by the institutional review board and registered at ClinicalTrials.gov (identifier: NCT00137111). Parents or guardians provided signed informed consent, and patients provided assent, as appropriate. Race was reported by the parent, guardian, or patient. At the time of first screening, patients had received prednisone 40 mg/m2 per day for 28 days (all patients) and dexamethasone 200 mg/m2 (lower risk ALL) or 240 mg/m2 (higher risk ALL) and an additional 160 mg/m2 (lower risk) or 180 mg/m2 (higher risk) of dexamethasone before the second MRI. Details of the regimen have been published previously10,13 and are included in the Data Supplement. We have previously reported the cumulative incidence of osteonecrosis at all sites for 364 of these patients, but we did not evaluate extensive femoral head osteonecrosis in the prior study. Because extensive osteonecrosis at this site is the most common cause of morbidity in patients with osteonecrosis, specific study of the incidence of osteonecrosis, risk factors, and utility of MRI screening was warranted.13 As an indicator of skeletal maturity and to examine its potential association with development of extensive osteonecrosis lesions, we assessed proximal femoral physis patency (open v closed) at the time of MRI, because an open physis indicates immature bone that is still growing.
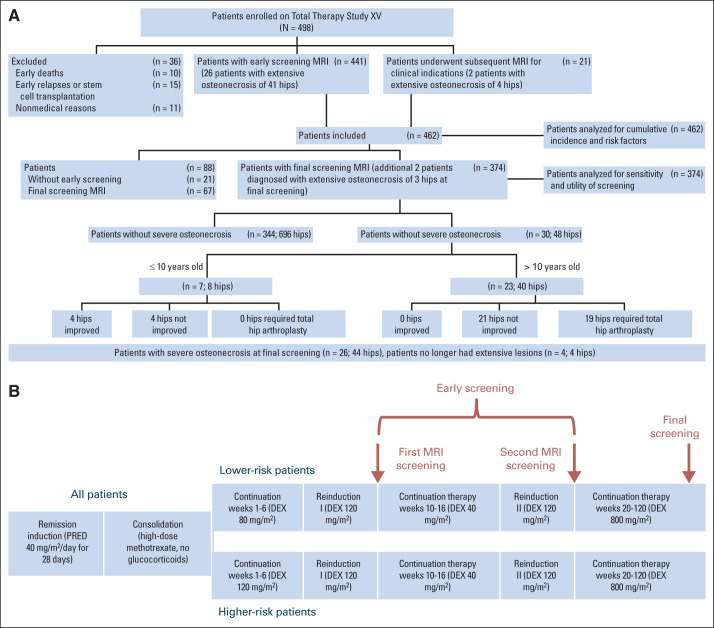
Fig 1.
(A) CONSORT diagram of patients undergoing hip magnetic resonance imaging (MRI) as part of the osteonecrosis screening portion of the Total Therapy Study XV. Extensive osteonecrosis is defined as that affecting 30% or more of the epiphyseal surface. (B) Protocol schema indicating time points of early screening magnetic resonance imaging and final assessment. DEX, dexamethasone; PRED, prednisone.
MRI Screening
Studies included coronal T1-weighted, short tau inversion recovery, and sagittal fast low-angle shot two-dimensional sequences using sedation when necessary. Osteonecrosis was defined as a geographic area of decreased signal on T1-weighted images and increased signal on short tau inversion recovery images (Fig 2).14 All MRIs were interpreted by a single pediatric radiologist (S.C.K.). In contrast to our prior report, where all osteonecrotic lesions at all sites and of any size were considered as positive, in this report, we focus only on extensive femoral head osteonecrosis, because this leads to joint collapse in 80% of patients within 2 years of diagnosis, underscoring the need for trials of early intervention to prevent progression.13 Thus, we defined lesions affecting ≥ 30% of the epiphyseal surface as extensive hip osteonecrosis, because they are associated with a high risk of subsequent collapse.8 The first two MRIs performed were considered early screening evaluations. Some patients underwent additional MRI examinations between screening time points for clinical indications, which were used in the calculation of cumulative incidence. However, the final classification of each joint was based on the off-therapy MRI.
Fig 2.
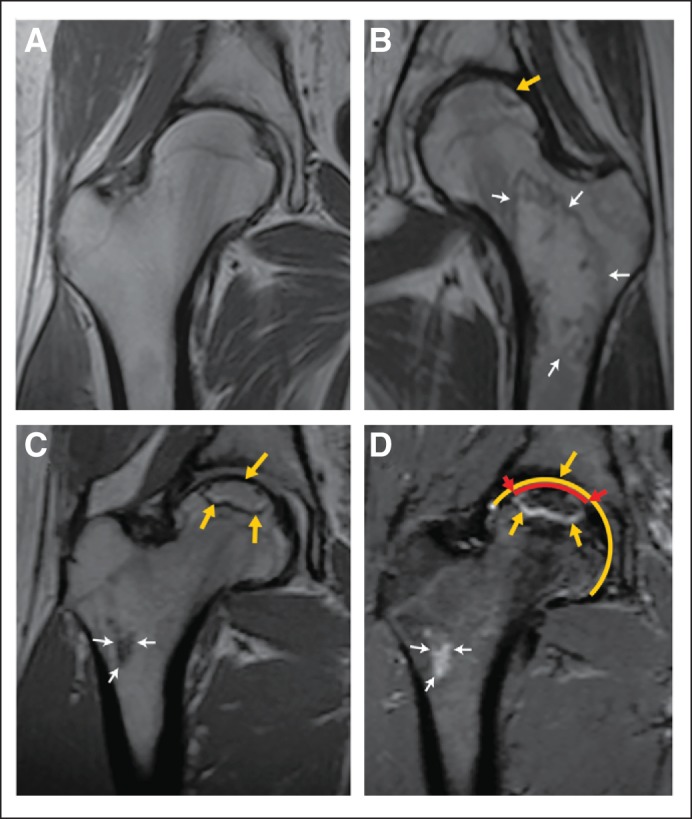
Magnetic resonance images (MRIs) showing a normal hip and hips with clinically significant osteonecrosis in an asymptomatic patient. Arrows indicate the border of the osteonecrotic lesions. Thick arrows mark epiphyseal osteonecrosis, and thin arrows mark metaphyseal lesions. These (A to C) coronal noncontrast T1-weighted and (D) short tau inversion recovery–weighted MRIs show (A) a normal hip, (B) a hip with a small (< 30%) epiphyseal osteonecrotic lesion and involvement of the femoral neck and proximal diaphysis, and (C and D) a more than 30% lesion of the femoral head along with involvement of the femoral neck. Panel D shows how the percentage involvement of the epiphyseal surface is measured. The yellow circumference shows the entire epiphyseal surface, and the red line shows the length of the epiphyseal surface affected by the lesion (delimited by red arrows).
Sensitivity, specificity, the number of joints needed to screen (NJNTS) to identify each lesion, and the number of patients needed to screen to identify patients with at least one hip affected were calculated for the entire cohort and by age group (≤ v > 10 years old). In the sensitivity and specificity calculations, only patients with both early screening and final screening MRIs were evaluated; we considered osteonecrosis of the metaphysis or less than 30% of the epiphysis as negative screening tests. Hips without extensive osteonecrosis identified by screening MRI that subsequently developed extensive lesions were considered to have had false-negative screening results (Fig 1). Patients with extensive lesions at screening that had improved to involve less than 30% of the epiphyseal surface or resolved at the time of final screening were considered false-positive screening results (Tables 1 and 2).
Table 1.
Utility of Early MRI Screening for Extensive Osteonecrosis Affecting ≥ 30% of the Epiphyseal Surface in Pediatric Patients Treated With Fixed Doses and Schedules of Glucocorticoids for Acute Lymphoblastic Leukemia
| Patient Group | No. of Hips |
Sensitivity (%) |
Specificity (%) |
No. of Joints Needed to Screen |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total* | True-Positive Screening | False-Positive Screening | False-Negative Screening | True-Negative Screening | Positive Final Outcome | Rate | 95% CI | Rate | 95% CI | No. | 95% CI | |
| All patients* | 744 | 37 | 4 | 7 | 696 | 44 | 84.1 | 70.6 to 99.8 | 99.4 | 98.5 to 99.8 | 20.1 | 14.8 to 27.6 |
| Age at diagnosis, years | ||||||||||||
| 1-10 | 595 | 3 | 4 | 1 | 587 | 4 | 75.0 | 30.1 to 95.4 | 99.3 | 98.3 to 99.7 | 198 | 67.9 to 583 |
| 11-20 | 149 | 34 | 0 | 6 | 109 | 40 | 85.0 | 70.2 to 94.3 | 100 | 96.7 to 100 | 4.4 | 3.3 to 5.9 |
Abbreviation: MRI, magnetic resonance imaging.
Although 462 patients had at least one screening MRI and are included in calculations of cumulative incidence and risk factors for osteonecrosis, only 374 patients (744 joints) had both an early screening MRI and a final screening MRI and are included in calculations of the sensitivity, specificity, and the number of joints and patients needed to screen to identify each case of extensive osteonecrosis. In all patients, MRI was performed on both hips when it was performed. To understand the apparent discrepancy between 374 patients and 744 joints, see Screening Outcomes under Results.
Table 2.
Utility of Early MRI Screening for Extensive Osteonecrosis Affecting ≥ 30% of the Epiphyseal Surface in Pediatric Patients Treated With Fixed Doses and Schedules of Glucocorticoids for Acute Lymphoblastic Leukemia
| Patient Group | No. of Patients |
Sensitivity (%) |
Specificity (%) |
No. of Joints Needed to Screen |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total* | True-Positive Screening | False-Positive Screening | False-Negative Screening | True-Negative Screening | Positive Final Outcome | Rate | 95% CI | Rate | 95% CI | No. | 95% CI | |
| All patients* | 374 | 22 | 4 | 4 | 344 | 26 | 84.6 | 66.5 to 93.8 | 98.8 | 97.1 to 99.6 | 17.0 | 11.4 to 25.5 |
| Age at diagnosis, years | ||||||||||||
| 1-10 | 298 | 2 | 4 | 1 | 291 | 3 | 66.7 | 20.8 to 93.8 | 98.6 | 96.6 to 99.5 | 149.0 | 41.4 to 543 |
| 11-20 | 76 | 20 | 0 | 3 | 53 | 23 | 86.9 | 66.4 to 97.2 | 100 | 93.2 to 100 | 3.8 | 2.7 to 5.6 |
Abbreviation: MRI, magnetic resonance imaging.
Although 462 patients had at least one screening MRI and are included in calculations of cumulative incidence and risk factors for osteonecrosis, only 374 patients (744 joints) had both an early screening MRI and a final screening MRI and are included in calculations of the sensitivity, specificity, and the number of joints and patients needed to screen to identify each case of extensive osteonecrosis. In all patients, MRI was performed on both hips when it was performed. To understand the apparent discrepancy between 374 patients and 744 joints, see Screening Outcomes under Results.
Symptom Assessment
At weekly clinic visits for chemotherapy administration, patients were directly asked about symptoms that could be attributable to osteonecrosis, including pain or disability and the joint involved, as part of a general review of systems performed by treating clinicians. Patients reporting joint pain only after being informed of the presence of osteonecrosis by screening MRI were considered to have been asymptomatic at the time MRI was performed. Patients with femoral head osteonecrosis who reported lower extremity pain were considered to have symptomatic osteonecrosis, even if the pain may have resulted from osteonecrosis lesions in the distal femur or tibia. Those reporting transient lower extremity pain that was temporally associated with vincristine administration were considered to have asymptomatic osteonecrosis.
Management of Extensive Osteonecrosis Detected by Screening
Patients with hip osteonecrosis were referred to orthopedics; management was individualized based on the size and location of lesions, the degree of symptoms, timing of lesion development, patient age, and ALL risk group. Interventions used included reduction or subsequent omission of steroids, use of crutches to prevent weight-bearing, nutritional intervention for obese patients to encourage weight loss, recommendations for non–weight-bearing exercise, and combinations of these. No patient received statins, bisphosphonates, or other pharmacologic interventions for osteonecrosis. Surgical intervention was performed at the discretion of the orthopedist to relieve symptoms in patients who became symptomatic before joint collapse.
Statistical Analyses
Potential risk factors analyzed for osteonecrosis included age at diagnosis, sex, body mass index (BMI) at diagnosis, self-reported race, physeal status at the time of MRI, and leukemia risk group. Age was categorized as older than 10 years versus 10 years or younger, a cutoff typically used in studies of children with ALL, but age was also categorized in 5-year increments to determine whether very young children differed from those age 10 to 15 years old (Appendix Tables A1 and A2, online only). BMI was defined using criteria from the Centers for Disease Control and Prevention.15 The Fine and Gray model was used to examine the relationship between potential risk factors and timing of osteonecrosis.16 Death, relapse, and progression of leukemia were considered competing risks for osteonecrosis. Risk factors significant at the level of P = .1 in univariable analysis were included in multivariable analysis, except for age categories, which were analyzed as a binary variable (≤ v > 10 years) and also in categories of 5 years each. The cumulative incidence of osteonecrosis was calculated according to the method of Kalbfleisch and Prentice and compared between groups using Gray's test. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Results were analyzed by patient to determine risk factors for osteonecrosis and by joint to determine the utility of screening and the progression to collapse and total hip arthroplasty. There were no adjustments for multiple comparisons.
RESULTS
Patient Demographics
Of 498 patients enrolled, 462 underwent at least one MRI of the hips and compose the study cohort (Appendix Table A1, Fig 1). Thirty-six patients did not undergo MRI screening as a result of early death (n = 10), early relapse or hematopoietic stem-cell transplantation (n = 15), or nonmedical reasons (n = 11) including refusal and logistical conflicts. Twenty-seven patients underwent a single MRI of the hips, 89 underwent two MRIs, and 346 completed all three protocol-specified MRI studies, for a total of 1,243 protocol-specified MRIs. The 88 patients missing early screening MRI or the final screening MRI were excluded from analyses of sensitivity and specificity of screening (Fig 1). We included 281 additional MRIs performed for clinical indications in the calculations of cumulative incidence of osteonecrosis, but not in the sensitivity and specificity calculations, which evaluated only MRIs done as early screening or final screening (Fig 1). No patients were diagnosed with hip osteonecrosis before the first screening study, consistent with previously published studies of symptomatic osteonecrosis, in which lesions were rarely identified within 6 months of starting glucocorticoids.6,7,17 There were no statistically significant differences in age at diagnosis, race, or sex between the 462 patients with at least one MRI and the 36 patients who did not undergo any MRI screening. However, patients with higher risk ALL were more likely than lower risk patients to have no MRI because of their higher rates of early death and stem-cell transplantation before undergoing the first protocol-specified MRI (12% v 2%, respectively; P < .001).
Hip Osteonecrosis by Patient
The cumulative incidence of osteonecrosis involving the epiphysis or metaphysis of at least one hip was 17.1% ± 1.8% after early screening (1 year) and 21.7% ± 1.9% after completion of therapy (4 years; Fig 3; Appendix Table A1; and Appendix Fig A1, online only). Independent risk factors included age more than 10 years at diagnosis (P < .001), treatment on the higher risk arm of the protocol (P < .006), and race other than black, Hispanic, or white (P = .034), but not sex, BMI at diagnosis, or physeal patency. Among patients 11 to 15 years old at the time of ALL diagnosis (11.5 to 15.5 years old at the time of MRI screening), the risk of osteonecrosis was not associated with physeal patency (Appendix Fig A2, online only).
Fig 3.
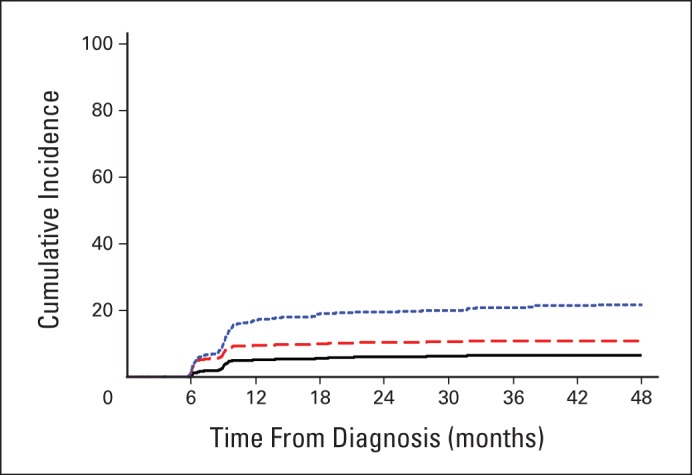
Cumulative incidence of hip osteonecrosis by femur site in pediatric patients with acute lymphoblastic leukemia. The blue dotted line represents patients with any hip osteonecrosis, including epiphysis (to any extent) or metaphysis. The red dashed line represents patients with osteonecrosis of the epiphysis (regardless of the extent of epiphyseal involvement, but excluding patients whose only sites of hip osteonecrosis were in the metaphysis). The black line shows patients with epiphyseal osteonecrosis affecting ≥ 30% of the articular surface, which represents extensive osteonecrosis with high risk of subsequent joint collapse and need for arthroplasty.
Extensive Hip Osteonecrosis Affecting ≥ 30% of the Epiphyseal Surface
By the end of therapy, extensive femoral head osteonecrosis affecting ≥ 30% of the epiphyseal surface had developed in 6.5% ± 1.1% of all patients and 24% ± 4.4% of those older than 10 years (Fig 2; Appendix Table A2). Of 48 involved joints in 30 patients, 41 joints in 26 asymptomatic patients were identified by early screening, and seven joints in four patients developed extensive osteonecrosis at later times and were classified as false-negative screening results (Fig 1, Tables 1 and 2). The hazard ratio for extensive osteonecrosis in patients age 11 to 20 years old was 15.6 (95% CI, 6.7 to 36.2; P < .001), and multivariable analysis found that age was the only independent predictor of extensive osteonecrosis (Appendix Table A2). Patients 6 to 10 years old had 1.7 times more extensive osteonecrosis than those 0 to 5 years old, but this difference was not statistically significant (P = .50). Furthermore, patients 11 to 15 years old had the same incidence of extensive osteonecrosis as those older than 15 years, supporting the age of 10 years chosen for dichotomy (Appendix Table A2).
Screening Outcomes
A total of 48 joints (30 patients) were identified with extensive lesions (Fig 1, Tables 1 and 2). Of the 40 extensive lesions in children older than 10 years, 19 progressed to joint collapse and required total hip arthroplasty, and none of the remaining 21 hips had improved at the time of final evaluation. Of the eight extensive lesions in children age 10 years and younger, none required arthroplasty; four had improved significantly at the time of final evaluation. The NJNTS for all patients was 20.1 joints (95% CI, 14.8 to 27.6 joints) to identify each extensive epiphyseal lesion; the NJNTS was 4.4 joints (95% CI, 3.3 to 5.9 joints) among children older than 10 years and 198 joints (95% CI, 67.9 to 583 joints) among those age 10 years and younger (Tables 1 and 2). The number of patients needed to screen for all patients was 17.0 patients; for those older than 10 years, the number of patients needed to screen was 3.8 patients, and for those age 10 years or younger, it was 149 patients. The sensitivity and specificity of MRI screening for extensive epiphyseal lesions are listed in Tables 1 and 2.
Once an extensive osteonecrosis lesion was identified, the affected hip was considered positive for analysis of final screening, whether a final screening MRI was done or not, because in many cases, the patient would have had hip arthroplasty that makes final screening unnecessary, and in no case did the lesions resolve by the end of the study period (although in four hips of younger children, improvement was noted; Fig 1). In four patients, one hip was positive at early screening and the other negative, but no final screening was performed to determine the final status of the hip without osteonecrosis. Therefore, these four patients were classified as having extensive osteonecrosis (based on one affected hip), but the contralateral joint was excluded from analysis of sensitivity of screening in the joint-by-joint analysis, because a new lesion may have developed in that hip that was not detected as a result of lack of final screening.
Symptomatic Osteonecrosis
No patient was diagnosed with symptomatic hip osteonecrosis before early screening, but 19 of 30 patients reported hip symptoms within 0 to 12 months of diagnosis of extensive femoral head osteonecrosis, and 12 patients required arthroplasty of 19 hips (Fig 1).
DISCUSSION
Screening MRI identified a high incidence (22%) of early extensive hip osteonecrosis in patients treated for ALL, especially among those older than 10 years. Importantly, 24% of patients older than 10 years had extensive osteonecrosis, placing them at high risk for subsequent joint collapse.8 Even patients 6 to 10 years old had 1.7 times more hip osteonecrosis than those younger than 6 years (Appendix Table A1). Although the risk of symptomatic osteonecrosis has been reported to be higher during puberty, we found no difference in osteonecrosis rates between patients 11 to 15 years old and those 16 to 20 years old. Among patients 11 to 15 years old, physeal patency, a marker of skeletal immaturity, did not alter osteonecrosis risk (Appendix Fig A2).6,7,13,18,19 However, whether or not physeal patency influences the rate of progression and collapse is unknown. Because, to our knowledge, this is the first large-scale screening study conducted in children, the findings may differ from studies of symptomatic osteonecrosis, in which lesions that do not progress or cause pain would not be detected. It is not surprising that more osteonecrosis occurred in children treated on the standard-/high-risk arm of the protocol, because this arm included more dexamethasone and more intensive asparaginase therapy, which in turn increased systemic exposure to dexamethasone.13 Neither sex nor BMI at diagnosis affected the incidence of osteonecrosis.
On the basis of prospectively acquired protocol-driven MRI of the hips obtained after standardized exposure to glucocorticoids, we found osteonecrosis in 17.1% of patients within 1 year of starting glucocorticoids and in 21.7% of patients by the end of ALL therapy, such that 79% of patients who would ultimately develop osteonecrosis did so within 1 year of starting glucocorticoids (Appendix Table A1). Screening of asymptomatic patients who have not developed hip osteonecrosis by 1 year has a low yield, because only 4.6% of patients develop new osteonecrosis beyond this time point. Even if subsequent screening is limited to patients older than 10 years, only an additional 6.3% of patients had lesions detected between 1 year (46.9% cumulative incidence) and 4 years (53.2% cumulative incidence) from ALL diagnosis.
Optimizing interventions to prevent or mitigate osteonecrosis requires identification of high-risk patient cohorts (eg, children > 10 years old), identification of the timing of lesion development relative to glucocorticoid exposure (eg, within 1 year of starting glucocorticoids), and identification of MRI abnormalities that may presage osteonecrosis.20 At the first screening MRI, 16 (4.3%) of 374 patients with early and final screening MRIs already had extensive hip osteonecrosis, including 12 patients older than 10 years and four patients age ≤ 10 years, indicating that osteonecrosis may occur in some patients even after short durations of glucocorticoid therapy. Thus, future studies should include even earlier screening to determine when the earliest lesions develop. Novel MRI techniques to identify prelesions may provide further opportunities for early intervention.20,21 Finally, detailed studies of the natural history of asymptomatic and symptomatic osteonecrosis are needed to document the frequency, risk factors, and time course for progression or resolution. In this study, we identified spontaneous resolution of extensive osteonecrosis in four of eight hips of children ≤ 10 years old but in none of 34 hips among those older than 10 years.
This large cohort of patients treated uniformly with defined doses and schedules of glucocorticoids identified patients who could potentially benefit from interventions aimed at limiting and ameliorating osteonecrosis-related joint damage. We have demonstrated that most osteonecrosis begins during the first year of glucocorticoid therapy, although symptomatic osteonecrosis may occur months or years later.7,22–24 Furthermore, early hip lesions can be extensive, with high risk of subsequent joint collapse.8 However, additional follow-up is needed to quantify the ultimate need for surgery, determine functional outcomes, and develop effective interventions.
The only strategy proven in a randomized controlled trial to reduce the development of symptomatic osteonecrosis is avoidance of prolonged, continuous exposure to dexamethasone.7 In the Children's Cancer Group 1961 trial in high-risk ALL, 823 patients age 10 to 21 years at diagnosis who had a rapid early response to induction chemotherapy were randomly assigned to receive dexamethasone 10 mg/m2 per day on days 0 through 20 of delayed intensification versus 10 mg/m2 per day on days 0 through 6 and 14 through 20. The cumulative incidence of symptomatic osteonecrosis at any site was 17% in patients treated with 21 days of continuous dexamethasone but only 8.7% in those treated with the interrupted schedule (hazard ratio, 2.1; P < .001). Physical therapy, avoidance of weight bearing, and other interventions have been proposed to reduce the risk of osteonecrosis or mitigate its effects but require prospective evaluation in high-risk cohorts.25,26 Detection of lesions at an early asymptomatic stage affords opportunities for prompt enrollment onto clinical trials of interventions to prevent progressive joint damage. Screening of patients older than 10 years frequently identifies extensive epiphyseal lesions (NJNTS, 4.4 joints), with high sensitivity and specificity. Patients without osteonecrosis at 1 year do not need to undergo further screening unless symptoms develop.
Supplementary Material
Appendix
Table A1.
Cumulative Incidence and Risk Factors for Hip Osteonecrosis Diagnosed by Early Screening MRI (1 year) and After Completion of Therapy (4 years)
| Demographic or Clinical Characteristic | No. of Patients | 1 Year* |
4 Years |
Univariable Analysis |
Multivariable Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Incidence | SE | Cumulative Incidence | SE | Hazard Ratio | 95% CI | P† | Hazard Ratio | 95% CI | P‡ | ||
| All patients | 462 | 17.1 | 1.8 | 21.7 | 1.9 | ||||||
| Age at diagnosis (5-year increments), years | |||||||||||
| 1-5 | 262 | 8.0 | 1.7 | 11.5 | 2.0 | Reference | Reference | ||||
| 6-10 | 104 | 12.5 | 3.3 | 18.3 | 3.8 | 1.708 | 1.0 to 3.0 | .060 | 1.474 | 0.8 to 2.6 | .187 |
| 11-15 | 70 | 47.1 | 6.0 | 52.9 | 6.1 | 6.580 | 4.1 to 10.6 | < .001 | 4.361 | 2.4 to 7.8 | < .001 |
| 16-20 | 26 | 46.2 | 10.1 | 53.8 | 10.1 | 6.585 | 3.5 to 12.4 | < .001 | 3.412 | 1.3 to 9.1 | .014 |
| Age at diagnosis, years | |||||||||||
| 1-10 | 366 | 9.3 | 1.5 | 13.4 | 1.8 | Reference | |||||
| 11-20 | 96 | 46.9 | 5.1 | 53.2 | 5.1 | 5.511 | 3.7 to 8.2 | < .001 | |||
| Race (self-reported) | |||||||||||
| White, non-Hispanic | 319 | 17.6 | 2.1 | 22.3 | 2.3 | Reference | Reference | ||||
| Black | 71 | 18.3 | 4.6 | 22.6 | 5.0 | 1.007 | 0.6 to 1.7 | .979 | 0.798 | 0.5 to 1.4 | .419 |
| Hispanic | 55 | 9.1 | 3.9 | 12.7 | 4.5 | 0.509 | 0.2 to 1.1 | .088 | 0.589 | 0.3 to 1.3 | .185 |
| Asian or other | 17 | 35.3 | 12.1 | 41.2 | 12.5 | 1.702 | 0.7 to 3.9 | .211 | 2.510 | 1.1 to 5.9 | .034 |
| Sex | |||||||||||
| Male | 258 | 19.8 | 2.5 | 24.1 | 2.7 | 1.391 | 0.9 to 2.1 | .107 | |||
| Female | 204 | 13.7 | 2.4 | 18.6 | 2.7 | Reference | |||||
| Body mass index category§ | |||||||||||
| < 2 years old‖ | 32 | 3.1 | 3.1 | 6.3 | 4.4 | 0.261 | 0.1 to 1.1 | .062 | |||
| Underweight | 29 | 17.2 | 7.2 | 24.1 | 8.1 | 1.132 | 0.5 to 2.5 | .757 | |||
| Normal | 281 | 17.1 | 2.2 | 21.0 | 2.4 | Reference | |||||
| Overweight | 65 | 12.3 | 4.1 | 18.5 | 4.9 | 0.862 | 0.5 to 1.6 | .627 | |||
| Obese | 55 | 30.9 | 6.3 | 36.4 | 6.6 | 1.897 | 1.1 to 3.2 | .013 | |||
| Risk classification | |||||||||||
| LR | 235 | 9.4 | 1.9 | 11.9 | 2.1 | Reference | Reference | ||||
| HR | 227 | 25.1 | 2.9 | 31.8 | 3.1 | 3.055 | 2.0 to 4.7 | < .001 | 1.979 | 1.2 to 3.2 | .006 |
| Combination of risk classification and age category | |||||||||||
| LR, 1-10 years | 220 | 7.3 | 1.8 | 9.5 | 2.0 | Reference | |||||
| HR, 1-10 years | 146 | 12.3 | 2.7 | 19.2 | 3.3 | 2.080 | 1.2 to 3.6 | .009 | |||
| LR, 11-20 years | 15 | 40.0 | 13.2 | 46.7 | 13.5 | 5.837 | 2.5 to 13.6 | < .001 | |||
| HR, 11-20 years | 81 | 48.1 | 5.6 | 54.4 | 5.6 | 8.142 | 4.9 to 13.5 | < .001 | |||
| Physis status at time of first MRI | |||||||||||
| Open | 412 | 13.8 | 1.7 | 18.0 | 1.9 | Reference | Reference | ||||
| Closed | 50 | 44.0 | 7.1 | 52.0 | 7.2 | 4.260 | 2.7 to 6.7 | < .001 | 1.442 | 0.7 to 2.9 | .301 |
Abbreviations: HR, higher risk (includes both standard- and high-risk groups); LR, lower risk; MRI, magnetic resonance imaging.
Although screening MRIs were scheduled at 6.5 and 9 months of treatment; they were performed up to 2 months late in some patients, so that the cumulative incidence of osteonecrosis at 1 year reflects those with osteonecrosis identified at early screening.
P values for comparisons of each group with the reference group in univariable analysis.
P values for comparisons of each group with the reference group in multivariable analysis.
Body mass index was defined as underweight if < 5th percentile, normal if 5th to less than 85th percentile, overweight if 85th to 95th percentile, and obese if > 95th percentile for age and sex.
Body mass index is not used for children younger than 2 years old.
Table A2.
Cumulative Incidence and Risk Factors for Extensive Hip Osteonecrosis Involving ≥ 30% of the Epiphyseal Surface Diagnosed by Early Screening MRI (1 year) and After Completion of Therapy (4 years)
| Demographic or Clinical Characteristic | No. of Patients | 1 Year* |
4 Years |
Univariable Analysis |
Multivariable Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Incidence | SE | Cumulative Incidence | SE | Hazard Ratio | 95% CI | P† | Hazard Ratio | 95% CI | P‡ | ||
| All patients | 462 | 5.2 | 1.0 | 6.5 | 1.1 | ||||||
| Age at diagnosis (5-year increments), years | |||||||||||
| 1-5 | 262 | 1.5 | 0.8 | 1.5 | 0.8 | Reference | Reference | ||||
| 6-10 | 104 | 1.9 | 1.4 | 2.9 | 1.7 | 1.950 | 0.4 to 8.7 | .382 | 1.694 | 0.4 to 7.7 | .496 |
| 11-15 | 70 | 20.0 | 4.8 | 24.3 | 5.2 | 19.745 | 6.6 to 59 | < .001 | 13.887 | 4.0 to 48 | < .001 |
| 16-20 | 26 | 15.4 | 7.2 | 23.1 | 8.5 | 19.600 | 5.7 to 67 | < .001 | 12.275 | 2.3 to 66 | .004 |
| Age at diagnosis, years | |||||||||||
| 1-10 | 366 | 1.6 | 0.7 | 1.9 | 0.7 | Reference | |||||
| 11-20 | 96 | 18.8 | 4.0 | 24.0 | 4.4 | 15.587 | 6.7 to 36 | < .001 | |||
| Race (self-reported) | |||||||||||
| White, non-Hispanic | 319 | 5.6 | 1.3 | 7.2 | 1.5 | Reference | Reference | ||||
| Black | 71 | 5.6 | 2.8 | 5.6 | 2.8 | 1.001 | 0.4 to 2.6 | .998 | 0.772 | 0.3 to 2.1 | .605 |
| Hispanic | 55 | 1.8 | 1.8 | 3.6 | 2.5 | 0.478 | 0.1 to 2.0 | .316 | 0.637 | 0.1 to 2.7 | .546 |
| Asian or other | 17 | 5.9 | 5.9 | 5.9 | 5.9 | 0.838 | 0.1 to 6.2 | .862 | 1.151 | 0.2 to 8.8 | .892 |
| Sex | |||||||||||
| Male | 258 | 4.7 | 1.3 | 5.8 | 1.5 | 0.748 | 0.4 to 1.5 | .419 | |||
| Female | 204 | 5.9 | 1.7 | 7.4 | 1.8 | Reference | |||||
| Body mass index category§ | |||||||||||
| < 2 years old‖ | 32 | 0 | 0 | — | .984 | ||||||
| Underweight | 29 | 3.4 | 3.5 | 6.9 | 4.8 | 0.960 | 0.2 to 4.1 | .956 | |||
| Normal | 281 | 5.7 | 1.4 | 6.8 | 1.5 | 0.410 | 0.1 to 1.8 | .229 | |||
| Overweight | 65 | 1.5 | 1.5 | 3.1 | 2.2 | 1.866 | 0.8 to 4.4 | .156 | |||
| Obese | 55 | 10.9 | 4.2 | 12.7 | 4.5 | 1.705 | 0.7 to 4.0 | .219 | |||
| Risk classification | |||||||||||
| LR | 235 | 2.6 | 1.0 | 2.6 | 1.0 | Reference | Reference | ||||
| HR | 227 | 7.9 | 1.8 | 10.6 | 2.0 | 4.912 | 2.0 to 12 | < .001 | 1.786 | 0.7 to 4.8 | .251 |
| Combination of risk classification and age category | |||||||||||
| LR, 1-10 years | 220 | 1.4 | 0.8 | 1.4 | 0.8 | Reference | |||||
| HR, 1-10 years | 146 | 2.1 | 1.2 | 2.7 | 1.4 | 2.211 | 0.5 to 9.9 | .299 | |||
| LR, 11-20 years | 15 | 20.0 | 10.7 | 20.0 | 10.7 | 16.708 | 3.4 to 83 | < .001 | |||
| HR, 11-20 years | 81 | 18.5 | 4.3 | 24.7 | 4.8 | 23.921 | 7.1 to 80 | < .001 | |||
| Physis status at time of first MRI | |||||||||||
| Open | 412 | 3.9 | 1.0 | 4.6 | 1.0 | Reference | Reference | ||||
| Closed | 50 | 16.0 | 5.2 | 22.0 | 5.9 | 6.407 | 3.1 to 13 | < .001 | 1.221 | 0.4 to 3.5 | .706 |
Abbreviations: HR, higher risk (includes both standard- and high-risk groups); LR, lower risk; MRI, magnetic resonance imaging.
Although screening MRIs were scheduled at 6.5 and 9 months of treatment; they were performed up to 2 months late in some patients, so that the cumulative incidence of osteonecrosis at 1 year reflects those with osteonecrosis identified at early screening.
P values for comparisons of each group with the reference group in univariable analysis.
P values for comparisons of each group with the reference group in multivariable analysis.
Body mass index was defined as underweight if < 5th percentile, normal if 5th to less than 85th percentile, overweight if 85th to 95th percentile, and obese if > 95th percentile for age and sex.
Body mass index is not used for children younger than 2 years old.
Fig A1.
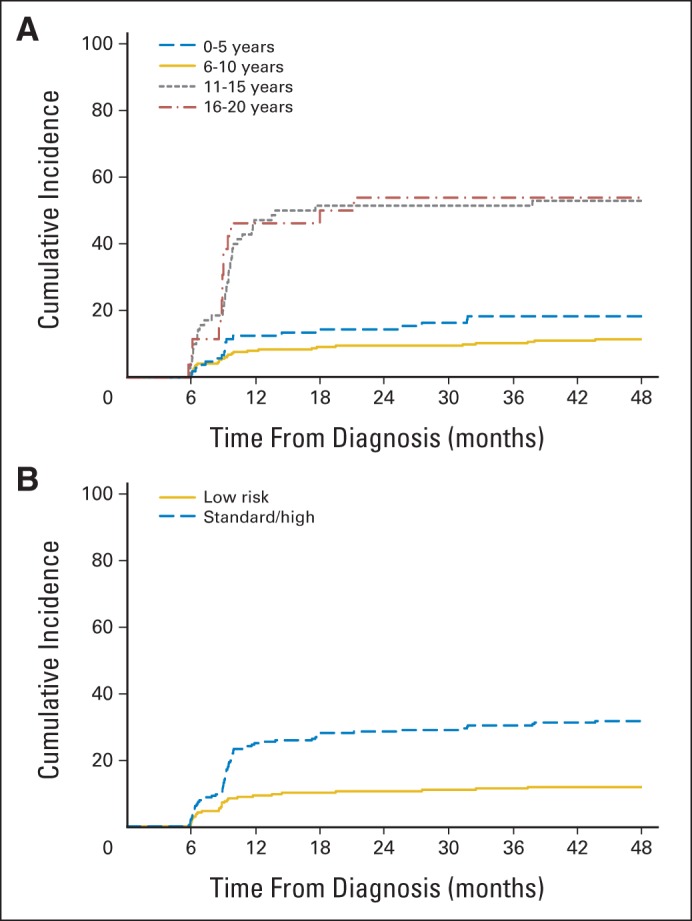
(A) Cumulative incidence of osteonecrosis in the epiphysis or metaphysis of either hip in pediatric patients with acute lymphoblastic leukemia by age group. (B) Cumulative incidence of osteonecrosis in the epiphysis or metaphysis of either hip in pediatric patients with acute lymphoblastic leukemia by leukemia risk group.
Fig A2.
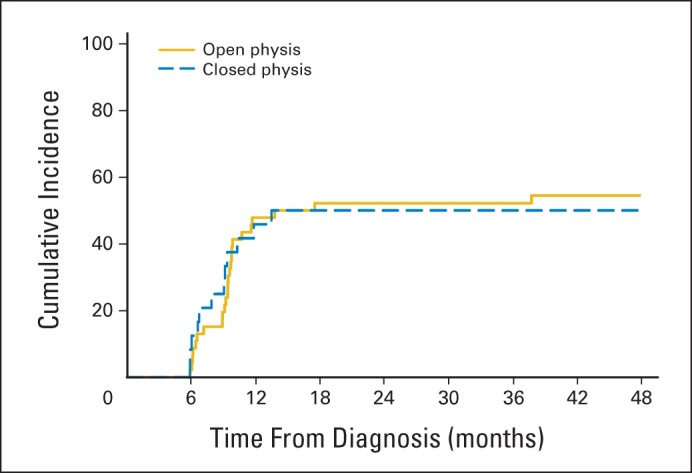
Cumulative incidence of osteonecrosis of either hip by physeal patency status among patients 11 to 15 years old.
Footnotes
Supported in part by Grant No. CA21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities.
The funding organizations had no role in the study design or conduct; collection, management, analysis, or interpretation of the data; or preparation, review approval, or decision to submit the article for publication.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00137111.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Sue C. Kaste, Cheng Cheng, Michael D. Neel, Monika L. Metzger, James R. Downing, Ching-Hon Pui, Scott C. Howard
Administrative support: James R. Downing, Ching-Hon Pui
Provision of study materials or patients: W. Paul Bowman, Raul C. Ribeiro, Monika L. Metzger, Ching-Hon Pui
Collection and assembly of data: Sue C. Kaste, Michael D. Neel, W. Paul Bowman, Monika L. Metzger, John T. Sandlund, Scott C. Howard
Data analysis and interpretation: Sue C. Kaste, Deqing Pei, Cheng Cheng, Raul C. Ribeiro, Deepa Bhojwani, Hiroto Inaba, Patrick Campbell, Jeffrey E. Rubnitz, Sima Jeha, John T. Sandlund, Mary V. Relling, Ching-Hon Pui, Scott C. Howard
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Utility of Early Screening Magnetic Resonance Imaging for Extensive Hip Osteonecrosis in Pediatric Patients Treated With Glucocorticoids
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Sue C. Kaste
Research Funding: National Institute of Child Health and Human Development
Deqing Pei
No relationship to disclose
Cheng Cheng
No relationship to disclose
Michael D. Neel
Consulting or Advisory Role: Wright Medical Technology
Patents, Royalties, Other Intellectual Property: Wright Medical Technology (Inst)
Travel, Accommodations, Expenses: Wright Medical Technology
W. Paul Bowman
No relationship to disclose
Raul C. Ribeiro
No relationship to disclose
Monika L. Metzger
No relationship to disclose
Deepa Bhojwani
Research Funding: Medimmune (Inst), Teva Pharmaceutical Industries (Inst)
Travel, Accommodations, Expenses: Inlyte Corporation
Hiroto Inaba
Research Funding: Bayer/Onyx (Inst)
Patrick Campbell
No relationship to disclose
Jeffrey E. Rubnitz
No relationship to disclose
Sima Jeha
Research Funding: Sigma Tau Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: JAZZ
John T. Sandlund
No relationship to disclose
James R. Downing
No relationship to disclose
Mary V. Relling
Research Funding: Investigator-initiated research grant from Sigma Tau Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Prometheus Labs (royalties from licensing TPMT genotyping)
Ching-Hon Pui
No relationship to disclose
Scott C. Howard
Consulting or Advisory Role: Sigma Tau Pharmaceuticals
Research Funding: Jazz Pharmaceuticals
Speaking Engagements: Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals
REFERENCES
- 1.Okazaki S, Nagoya S, Yamamoto M, et al. High risk of osteonecrosis of the femoral head in autoimmune disease patients showing no immediate increase in hepatic enzyme under steroid therapy. Rheumatol Int. 2013;33:51–55. doi: 10.1007/s00296-011-2295-y. [DOI] [PubMed] [Google Scholar]
- 2.Rahman WA, Garbuz DS, Masri BA. Total hip arthroplasty in steroid-induced osteonecrosis: Early functional and radiological outcomes. Can J Surg. 2013;56:41–46. doi: 10.1503/cjs.032510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroer WC. Current concepts on the pathogenesis of osteonecrosis of the femoral head. Orthop Rev. 1994;23:487–497. [PubMed] [Google Scholar]
- 5.Bürger B, Beier R, Zimmermann M, et al. Osteonecrosis: A treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)—Experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–225. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 6.Mattano LA, Jr, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: A report from the Children's Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 7.Mattano LA, Jr, Devidas M, Nachman JB, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012;13:906–915. doi: 10.1016/S1470-2045(12)70274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimova EJ, Rai SN, Howard SC, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 9.Fessel J. There are many potential medical therapies for atraumatic osteonecrosis. Rheumatology. 2013;52:235–241. doi: 10.1093/rheumatology/kes241. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: A systematic literature review. J Bone Joint Surg Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 12.Kaste SC, Karimova EJ, Neel MD. Osteonecrosis in children after therapy for malignancy. AJR Am J Roentgenol. 2011;196:1011–1018. doi: 10.2214/AJR.10.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karimova EJ, Rai SN, Deng X, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma: Part 1, observer agreement. AJR Am J Roentgenol. 2006;186:470–476. doi: 10.2214/AJR.04.1598. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. A SAS program for the 2000 CDC growth charts. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Ojala AE, Pääkkö E, Lanning FP, et al. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: A prospective MRI study. Med Pediatr Oncol. 1999;32:11–17. doi: 10.1002/(sici)1096-911x(199901)32:1<11::aid-mpo4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Lackner H, Benesch M, Moser A, et al. Aseptic osteonecrosis in children and adolescents treated for hemato-oncologic diseases: A 13-year longitudinal observational study. J Pediatr Hematol Oncol. 2005;27:259–263. doi: 10.1097/01.mph.0000163215.37147.13. [DOI] [PubMed] [Google Scholar]
- 20.Sansgiri RK, Neel MD, Soto-Fourier M, et al. Unique MRI findings as an early predictor of osteonecrosis in pediatric acute lymphoblastic leukemia. AJR Am J Roentgenol. 2012;198:W432–W439. doi: 10.2214/AJR.11.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miettunen PM, Lafay-Cousin L, Guilcher GM, et al. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clin Orthop Relat Res. 2012;470:3587–3595. doi: 10.1007/s11999-012-2579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblicq C, Laverdière C, Décarie JC, et al. Effectiveness of pamidronate as treatment of symptomatic osteonecrosis occurring in children treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:741–747. doi: 10.1002/pbc.24313. [DOI] [PubMed] [Google Scholar]
- 23.Patel B, Richards SM, Rowe JM, et al. High incidence of avascular necrosis in adolescents with acute lymphoblastic leukaemia: A UKALL XII analysis. Leukemia. 2008;22:308–312. doi: 10.1038/sj.leu.2405032. [DOI] [PubMed] [Google Scholar]
- 24.te Winkel ML, Pieters R, Hop WC, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2011;29:4143–4150. doi: 10.1200/JCO.2011.37.3217. [DOI] [PubMed] [Google Scholar]
- 25.Marti-Carvajal AJ, Sola I, Agreda-Perez LH. Treatment for avascular necrosis of bone in people with sickle cell disease. Cochrane Database Syst Rev. 2012;5:CD004344. doi: 10.1002/14651858.CD004344.pub4. [DOI] [PubMed] [Google Scholar]
- 26.Marchese VG, Connolly BH, Able C, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88:341–350. doi: 10.2522/ptj.20070108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.