Abstract
Background: Currently, non-invasive methods for screening atrophic gastritis and gastric cancer are lacking. The purpose of this study was to evaluate the value of serological parameters including serum pepsinogen I (PGI), pepsinogen II (PGII) and pepsinogen I: II ratio for the screening atrophic gastritis and gastric cancer.
Methods: The study population consisted of 132 dyspeptic patients who had undergone upper endoscopy with biopsy. Blood samples for ELISA assays of serum PGI, PGII and IgG antibodies against Helicobacter pylori were drawn. Comparison between the two groups was done by Student’s t- test, and Mann Whitney test. Cut-off points were calculated using receiver operating curves (ROC).
Results: Mean (±SD) age of the study population was 51.4 (±15.5) years. Values of PGI and PG ratio decreased significantly in the atrophic gastritis as compared with the control group (p<0.05). Values of PG and PG ratio didn’t show any significant difference between the gastric cancer and control group (p>0.05). For patients with atrophic gastritis, the area under the ROC for PGI was 0.639 (95% CI:0.538-0.741, p=0.008) in which the best cut-off value was 40μg/L (sensitivity 90%, specificity 67%, accuracy 69%, negative predictive value 92%, YI : 0.429). The area under the ROC for PG ratio was 0.711 (95% CI: 0.617–0.806, p=0.0001) and the best cut-off value was 8 (sensitivity 71%, specificity 71%, accuracy 71%, negative predictive value 86%,YI : 0.431).
Conclusion: It seems that PGI, PGI: PGII ratio is potential biomarkers for screening atrophic gastritis with high sensitivity, specificity, accuracy and negative predictive value. Serology could be used as a screening method for the detection of precancerous states due to its convenience, relative low cost and safety.
Keywords: Pepsinogens, Atrophic gastritis, Gastric cancer, Biomarkers
Introduction
Incidence and mortality of gastric cancers (GC) have rapidly decreased in the past 50 years in many countries; however it remains a major cause of morbidity and mortality (1).
Mortality of GC is high in most parts of Asia, while it was reduced in Japan and Korea (2, 3). Atrophic gastritis (AG) is an important and prevalent condition that has no symptom, leading to GC without any alarming sign (4,5).
Atrophic gastritis often desires to progress to premalignant lesions, and even to gastric cancer (5). Endoscopy with gastric biopsies was suggested as the best and most effective diagnostic method for screening of upper GI malignancies (3). Endoscopic screening is an invasive procedure, needing experienced endoscopist. In addition, it is relatively costly, uncomfortable for patients, and is not appropriate in low risk areas; therefore non-invasive screening modalities are required in this population. The inflamed gastric mucosa shifts specific factors to the blood, leading to the presence of diagnostic serologic biomarkers. In previous investigations, a variety of serologic markers have been explained, including, pepsinogen I (PGI), pepsinogen II (PGII) and IgG antibodies against Helicobacter pylori (4, 6). Due to the variance in ethnic, dietary, environmental and disease factors, the application of serum pepsinogen (PG) screening requires local validation in different geographic regions (7). As the prognosis is a major concern to most patients with gastric lesions, the importance of a suitable and cost-benefit screening program for early detection of the disease which could result in reduction of mortality in gastric cancer is highlighted (8) and a proper screening test which reduces the endoscopy loading, affirms a simple, safe and effective method in the first stage for diagnosis of AG is required (6). Currently, few data is available on serologic PG levels in Iranian patients with AG and gastritis cancer. Herein, the authors used serum PG level, as the first choice screening test for AG and gastric cancer and confirmed the diagnosis by endoscopy and biopsy. In this study, we have investigated the characteristics of PG screening and the convenient cut off points for the identification of AG and GC using receiver operator characteristics (ROC) curves. In addition, we compared PG screening with screening by endoscopy.
Methods
Participants
A total of 132 consecutive patients with upper gastrointestinal symptoms entered the study. The patients were referred to the endoscopy ward of Imam Khomeini hospital, Tehran, Iran during 2011-2013. They were excluded if they had H. pylori treatment before the test, any type of gastrointestinal malignancies other than gastric cancer, or a drug history of anti-secretory medication. Patients with non-atrophic mild gastritis and without gastritis comprised the control group. All patients were informed about the study and signed a written informed consent. The project was approved by Ethical Committee of Tehran University of Medical Sciences.
Endoscopy and histology
All patients underwent upper gastrointestinal endoscopy. At least four biopsy specimens were taken from the fundus of the stomach (two specimens from each site) and were sent to the Department of Pathology for histological examination by experienced pathologists. All specimens were reviewed as recommended in the last edition of Sydney System classification of gastritis (9).
Regards to Rugge M et al. study, we considered for Reliability, agreement between two observers with really having good Kappa =0.78 (10).
Blood samples
Blood samples for the measurement of PGI, PGII and IgG antibodies for H. pylori were drawn after endoscopy, immediately put into an ice bottle for 30 minutes, and then centrifuged at 200 g for 10 min to separate the serum for the assays. The serum samples were stored at −70°C until analysis.
Serological testing
PGI, PGII and antibodies to H. pylori were determined using specific commercial ELISA kits11 (Pepsinogen I, Pepsinogen II, H. pylori ELISA Kit, Shanghai Huitai Medical Technology Company, China). To decrease the bias, all calibrators and samples were assayed as duplicates. For determination of PGI and PGII, standard curves were used to extrapolate the concentrations of unknown samples. According to the given cut-off value of the kit, IgG ≥ 0.1 enzyme immunoassay units (EIU) were considered H. pylori positive.
Statistical Analysis
Data were analyzed using the SPSS 11.0 software. Serum concentrations of PGI, PGII and the ratio of PGI: PGII were compared between subjects with AG, non-AG, GC, non-GC and H. pylori positive, H. pylori negative were. We evaluated the difference between two groups with Student’s t- test, and Mann Whitney test. Cut-off points of PGI and the PGI: PGII ratios were calculated using receiver operating curves (ROC).
Results
The mean age (±SD) of the study patients was 51.4 (±15.5) years, ranging from 12 to 84 years. Of the overall 132 patients, 83 were men (62.9%) and 49 women (37.1%). According to the histologic examination of the fundic mucosa, 51 (39.2%) patients had atrophic gastritis, and 22 (16.7%) had gastric cancer. Values of PGI and PG ratio decreased significantly in the atrophic gastritis group as compared with the control group (86.4±77.7 vs. 113.7±75.7μg/L, 16.4 ±42.1 vs. 17.4±10.5, p=0.008, 0.01, respectively). Values of PG and PG ratio didn’t show any significant difference between the two groups in the gastric cancer and control (p> 0.05). Values of PG and PG ratio decreased significantly in the atrophic gastritis group without H. pylori infection as compared with control group (all p<0.05); in addition, PGI decreased significantly in those with atrophic gastritis with H. pylori infection as compared with control group (97.3±85.2 vs. 126.5±79.6μg/L; p=0.025, 0.012, respectively). While values of PGI and PG ratio changed significantly in the atrophic gastritis with H. pylori infection as compared with the control group, however, values of PG and PG ratio didn’t show any significant difference between two groups in the gastric cancer (Table 1).
Table1 . Comparison of the serum markers between different status of patients and controls.
| Serum Marker | PGI (μg/L) | PGII (μg/L) | PGI:PGII Ratio |
| AG (Mean±SD) | 86.4±77.7 | 12.3±16.7 | 16.4±42.1 |
| Non AG (Mean±SD) | 113.7±75.5 | 8.9±9.2 | 17.4±10.5 |
| p value | 0.008 | 0.360 | 0.010 |
| GC (Mean±SD) | 100.3±86.4 | 10.5±10.7 | 25.7±64.5 |
| Non GC (Mean±SD) | 103.3±75.8 | 10.2±13.3 | 15.3±10.5 |
| p value | 0.498 | 0.678 | 0.402 |
| AG H.pylori Negative (Mean±SD) | 54.6±36.07 | 15.8±25.3 | 7.2±5.9 |
| Non AG H.pylori Negative (Mean±SD) | 75.5±44.5 | 4.09±2.6 | 21.9±12.8 |
| p value | 0.015 | 0.005 | <0.001 |
| AG H.pylori Positive (Mean±SD) | 97.3±85.2 | 11.05±12.9 | 19.5±48.4 |
| Non AG H.pylori Positive (Mean±SD) | 126.5±79.6 | 10.5±10.06 | 15.9±9.2 |
| p value | 0.025 | 0.559 | 0.012 |
| GC H.pylori Negative (Mean±SD) | 33.5±22.8 | 5±4.07 | 9.5±6.8 |
| Non GC H.pylori Negative (Mean±SD) | 71.8±42.2 | 9.4±18 | 16.9±13.3 |
| p value | 0.053 | 0.550 | 0.333 |
| GC H.pylori Positive (Mean±SD) | 116±88.6 | 11.8±11.5 | 29.5±71.5 |
| Non GC H.pylori Positive (Mean±SD) | 114.5±81.9 | 10.5±11.2 | 14.7±9.4 |
| p value | 0.930 | 0.541 | 0.067 |
For patients with atrophic gastritis, the area under the ROC for PGI was 0.639 (95% CI: 0.538-0.741, p=0.008). The best cut-off value was 40μg/L (sensitivity 90%, specificity 67%, and accuracy 69%) (Fig.1 ).
Fig. 1 .
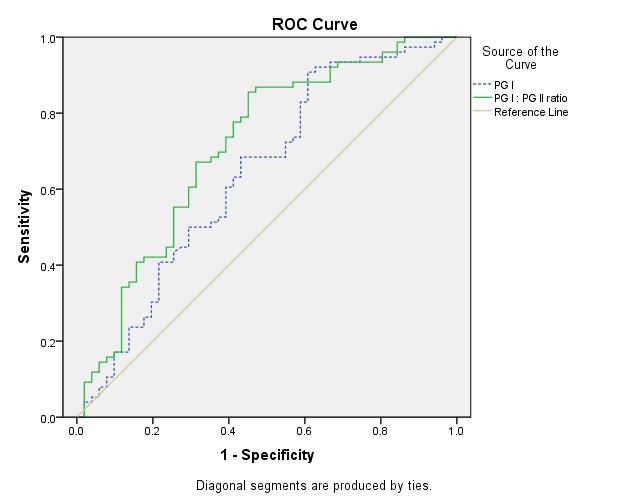
Receiver operating curve of pepsinogen I (PGI) (dashed line), PGI: PGII ratio (line) for the diagnosis of atrophic gastritis
The area under the ROC for PG ratio was 0.711 (95% CI: 0.617–0.806, p=0.0001) and the best cut-off value was 8 (sensitivity 71%, specificity 71%, accuracy 71%) (Fig.1).
For patients with gastric cancer, the area under the ROC for PGI was 0.547 (95% CI: 0.400-0.694, p=0.498), and for PGI: PGII the ratio was 0.558 (95% CI: 0.427-0.689, p=0.402) which showed no value for the diagnosis of gastric cancer (Fig. 2). The results of ROC analysis and the corresponding diagnostic indices are summarized in Table 2.
Fig. 2 .
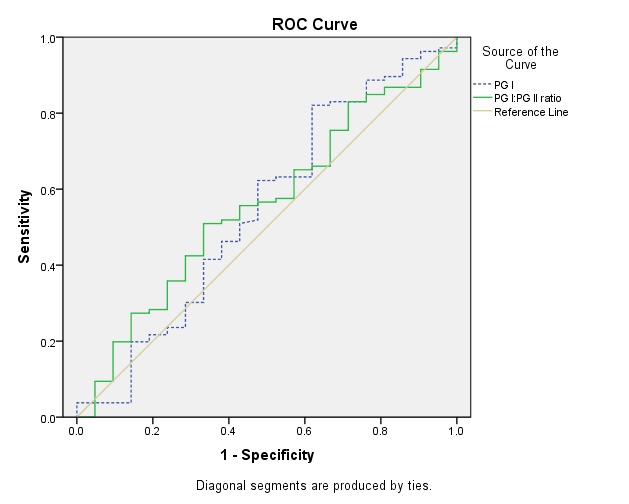
Receiver operating curve of pepsinogen I (PGI) (dashed line), PGI: PGII ratio (line) for the diagnosis of gastric cancer
Table 2 . Summary of the receiver operating characteristic (ROC) curve analysis for atrophic gastritis detection by the serum biomarkers .
| Serum marker | PGI (μg/L) | PGI:PGII ratio |
| AUC% (95% CI) | 0.639 | 0.711 |
| P value | 0.008 | 0.0001 |
| Cut-off | 40 | 8 |
| Sensitivity (95% CI) | 90% (95% CI=76%-95%) | 71% (95% CI=53%-85%) |
| Specificity (95% CI) | 67% (95% CI=58%-76%) | 71% (95% CI=61%-80%) |
| Accuracy (%) | 69% | 71% |
| PPV | 35% (95% CI=22%-49%) | 49% (95% CI=34%-63%) |
| NPV | 92% (95% CI=83%-97%) | 86% (95% CI=77%-93%) |
| Youden index | 0.429 (95% CI=0.234-0.624) | 0.431 (95% CI=0.256-0.607) |
Discussion
Despite the decline in gastric cancer in western countries, it remains a leading cause of cancer-related deaths in Asian countries such as Japan and Iran (11, 12).
Definitive diagnosis of AG through endoscopy and histological examination is invasive. While histological examination is considered the gold standard diagnostic test, it may be subject to error due to the patchy nature of atrophy and the limitation in the number of biopsies. PGI and PGII can be used to indicate the function of the gastric mucosa (13-15).PGI and PGI: PGII ratios are decreased in atrophic chronic gastritis which is acknowledged as a major risk factor for the development of gastric cancer. Variable cut-off-values for PGI and the PG-ratio were applied in former studies (16),but levels below 70 μg/ ml and 3.0, respectively, were considered the most efficient resulting in a sensitivity of 66.7-84.6% and a specificity of 73.5-87.1% for the detection of atrophic gastritis (17-19).
In our study, the best cut off value for PGI was found to be 40 μg/L with an accuracy of 69%, 90% sensitivity, 67% specificity and Youden index (YI) of 0.429. Additionally, the best cut off value for PGI: PGII ratio was 8, with 71% accuracy, 71% sensitivity, 71% specificity and YI 0.431, in discriminating atrophic from non atrophic specimens. Other studies showed a broad range of cut off values for the diagnosis of gastritis atrophy, which can be attributed to the use of different methods for patients selection, atrophic intensity, risk factors and different type of studies.
With respect to studies from Iran, Hosseini et al. reported that the PGI: PGII ratio is diagnostically significant in detecting gastric atrophy, while serum biomarkers of atrophic gastritis (PGI and PGII) are not useful screening tests due to their low sensitivity (50%) (20). Haj Sheykholeslami et al. demonstrated that by setting a cutoff value of 7.5 microg/mL for PGII, all types of gastritis were diagnosed with 80% sensitivity and 80%. While PGII is a suitable marker for screening any gastritis from normal mucosa, PGI, PGI: PGII ratio or their combination failed to select those with precancerous conditions and corpus-predominant gastritis among first-degree relatives of gastric cancer patients (21).
In the study conducted by Nasrollahzadeh et al. for defining an efficient biomarker for gastric atrophy, the best cut off value for PGI was 56 mg/l (sensitivity: 61.9%, specificity: 94.8%), while a PGI: PGII ratio=5 presented as the most efficient cut off value (sensitivity: 75.0%, specificity: 91.0%) (22).
Irvani et al. described PGI: PGII ratio as an efficient biomarker with 96.1% sensitivity and 97.7% negative predictive value; PGI has the highest specificity (94.6%), and PGII also had a high negative predictive value (90.7%). Thus, Pepsinogen I/II ratio appears to be the most suitable single measurement for screening purposes in atrophic gastritis (23).
Storskrubb et al. reported a sensitivity and specificity of 71% and 98%, respectively. Serological biomarkers show a high degree of accuracy as a non-invasive method to diagnose corpus atrophy (24).
Most studies have indicated a superior ability of the combined PGI and PGI: PGII ratio (24) or the PGI: PGII ratio alone (25-27) in the diagnosis of atrophic gastritis, which was confirmed by our findings.
In the present study, serum PGI levels and PGI: PGII ratio was significantly decreased in patients with atrophic gastritis, whereas there was no significant association between these biomarkers and gastric cancer.
Conclusion
This study demonstrates that PGI and PGI: PGII ratios are potential serologic biomarkers for screening of atrophic gastritis in fundus region, possessing high sensitivity, specificity, accuracy and negative predictive value. Serologic biomarker could be used as a screening method for the detection of precancerous states due to its well-established advantages, such as its convenience, relative cheapness and safety.
Acknowledgments
We would like to thank Mostofi Laboratory colleagues, who helped, our in many ways. In particular Hesami MD, Divanbeigi MD for her assistance.
Cite this article as: Zoalfaghari A, Aletaha N, Roushan N, Taslimi R, Foroutan H, Faridnia B. Accuracy of pepsinogens for early diagnosis of atrophic gastritis and gastric cancer in Iranian population. Med J Islam Repub Iran 2014 (16 December). Vol. 28: 150.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;1(118)):2315–2321. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;14(12):4873–4874. doi: 10.3748/wjg.v12.i30.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sipponen P, Härkönen M. et al. Diagnosis of atrophic gastritis from a serum sample. Clin Lab. 2002;48:505–15. [PubMed] [Google Scholar]
- 5.Suovaniemi O, Härkönen M. et al. Diagnosis of atrophic gastritis from a serum sample. Jordan Med J. 2002;36:117–21. [Google Scholar]
- 6.Mardh E, Mardh S, Mardh B. et al. Diagnosis of gastritis by means of a combination of serological analyses. Clin Chim Acta. 2002;320:17–27. doi: 10.1016/s0009-8981(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 7.So JB-Y, Yeoh K-G, Moochala S. et al. Serum pepsinogen levels in gastric cancer patients and their relationship with Helicobacter pylori infection: a prospective study. Gastric Cancer. 2002;5:228–32. doi: 10.1007/s101200200039. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara M, Hiyama T, Yoshida S, Ito M, Tanaka S, Watanabe Y. et al. Reduction in gastric cancer mortality by screening based on serum pepsinogen concentration: a case-control study. Scand J Gastroenterol. 2007;42:760–764. doi: 10.1080/00365520601097351. [DOI] [PubMed] [Google Scholar]
- 9.Dixon FM, Genta RM. et al. Classification and grading of gastritis: The updated Sydney System. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J. et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16(7):1249–59. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- 11.Correa P. The epidemiology of gastric cancer. World J Surg. 1991;15:228–234. doi: 10.1007/BF01659057. [DOI] [PubMed] [Google Scholar]
- 12.Islami F, Kamangar F, Aghcheli K. et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–1406. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oksanen A, Sipponen P, Miettinen A, Sarna S, Rautelin H. Evaluation of blood tests to predict normal gastric mucosa. Scand J Gastroenterol. 2000;35:791–5. doi: 10.1080/003655200750023138. [DOI] [PubMed] [Google Scholar]
- 14.Schlemper RJ, van der Werf SD, Biemond I, Lamers CB. Seroepidemiology of gastritis in Japanese and Dutch male employees with and without ulcer disease. Eur J Gastroenterol Hepatol. 1996;8:33–9. doi: 10.1097/00042737-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sipponen P, Ranta P, Helske T. et al. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002;37:785–91. [PubMed] [Google Scholar]
- 16.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;15(121):2782–2786. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]
- 17.Hattori Y, Tashiro H, Kawamoto T, Kodama Y. Sensitivity and specificity of mass screening for gastric cancer using the measurment of serum pepsinogens. Jpn J Cancer Res. 1995;86:1210–1215. doi: 10.1111/j.1349-7006.1995.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi S, Kato M, Katsuyama T, Tominaga S, Asaka M. Design and planned analyses of an ongoing randomized trial assessing the preventive effect of Helicobacter pylori eradication on occurrence of new gastric carcinomas after endoscopic resection. Helicobacter. 2006;11:147–151. doi: 10.1111/j.1523-5378.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini M, Amoueian S, Attaranzadeh A, Montazer M, Soltani GH, Asadollahi KH, Abangah GH. Serum gastrin 17, pepsinogen I and pepsinogen II in atrophic gastritis patients living in North-East of Iran. Journal of Research in Medical Sciences. 2013:225–229. [PMC free article] [PubMed] [Google Scholar]
- 21.Haj-Sheykholeslami A, Rakhshani N, Amirzargar A, Rafiee R, Shahidi SM, Nikbin B. et al. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: Comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol. 2008;6:174–9. doi: 10.1016/j.cgh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Shakeri R, Persson EC, Islami F. et al. Accuracy and cut-off values of pepsinogens I, II and gastrin 17 for diagnosis of gastric fundic atrophy: Influence of gastritis. PLoS One. 2011;6:e26957. doi: 10.1371/journal.pone.0026957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvani S, Hashemi MR, Moghadam KG, Saeidee S, Khavaran K, Najari O. et al. Accuracy of serum pepsinogens I and II, gastrin-17 and anti-helicobacter pylori antibodies in histological diagnoses of atrophic gastritis. Minerva Gastroenterol Dietol. 2010 Mar;56(1):13–7. [PubMed] [Google Scholar]
- 24.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ. et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43(12):1448–55. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Nurgalieva ZZ, El-Zimaity HM, Opekun AR, Campos A. et al. Noninvasive versus histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol. 2006;4:306–314. doi: 10.1016/j.cgh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Broutet N, Plebani M, Sakarovitch C, Sipponen P, Megraud F. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JM, Kim N, Yoo JY, Park YS, Lee DH. et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter. 2008;13:146–156. doi: 10.1111/j.1523-5378.2008.00592.x. [DOI] [PubMed] [Google Scholar]


