Abstract
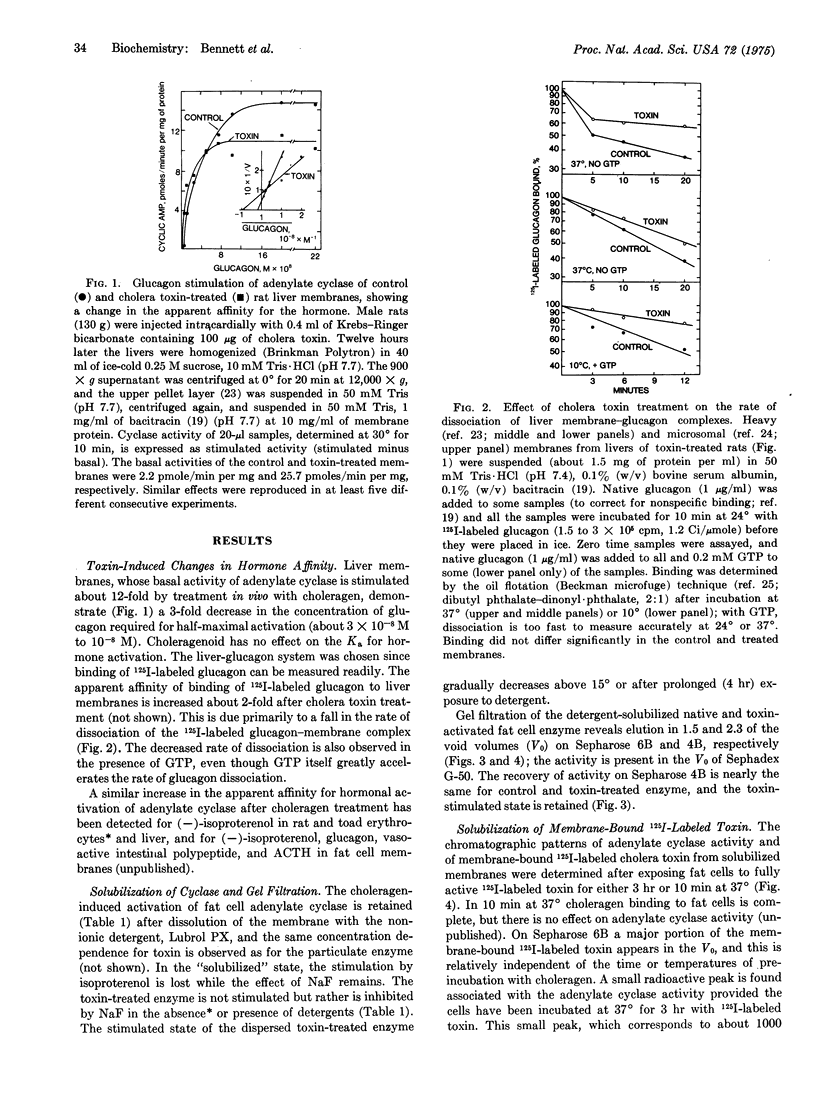
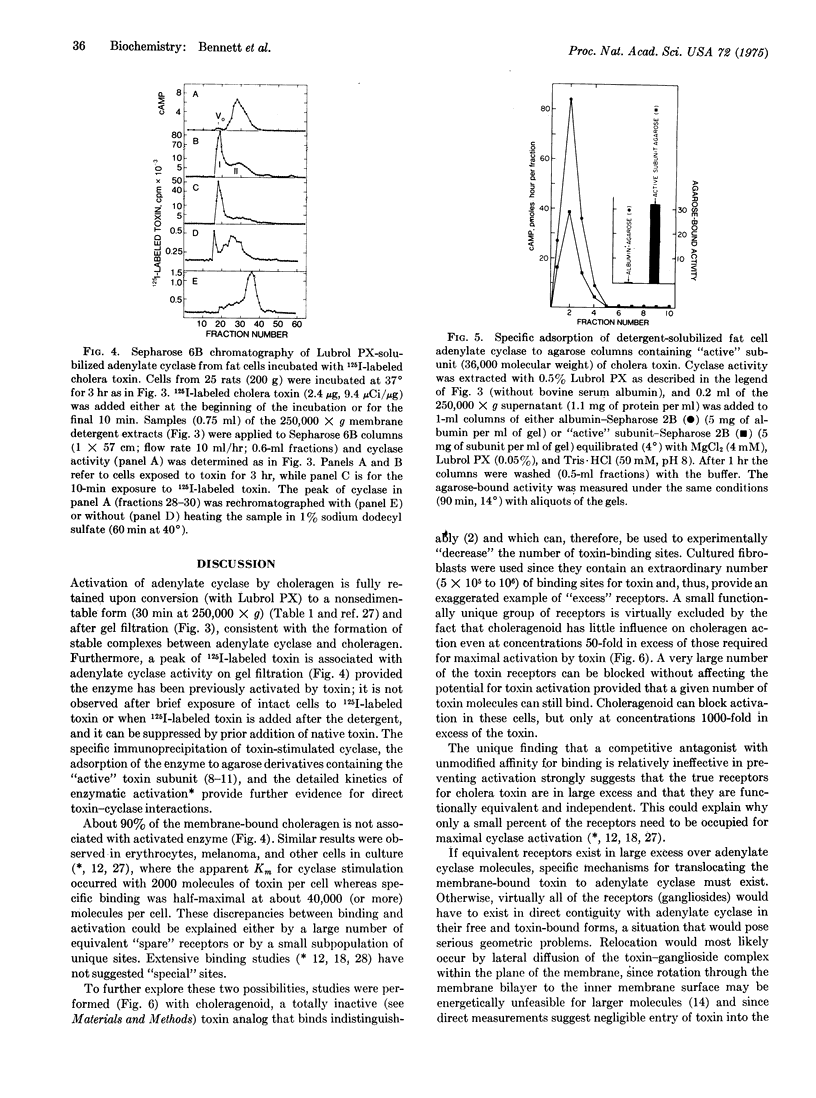
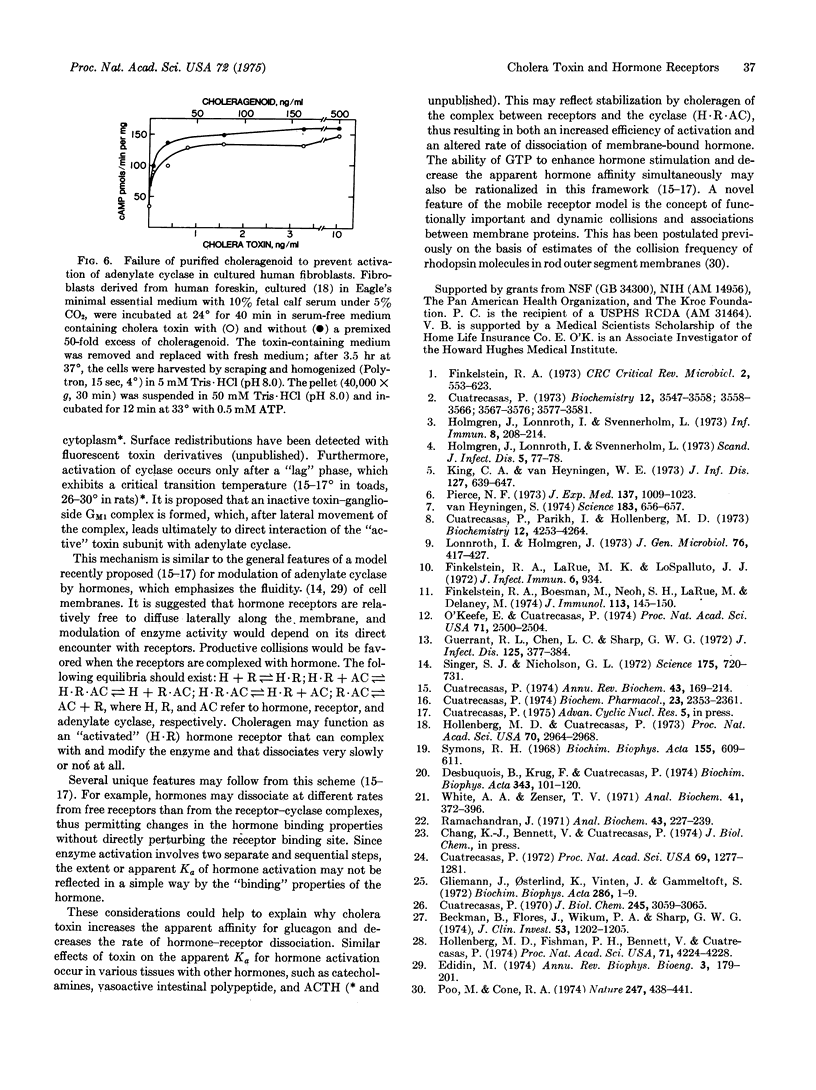
Rat liver membrane adenylate cyclase (EC 4.6.1.1) that has been stimulated more than 10-fold by cholera toxin (choleragen) has a 3-fold greater sensitivity to stimulation by glucagon. Choleragen similarly increases the sensitivity of cyclase to other peptide (ACTH, vasoactive intestinal polypeptide) and nonpeptide (catecholamines) hormones in this and other tissues. The rate of 125I-labeled glucagon-membrane dissociation is decreased about 2-fold in toxin-treated liver membranes. Toxin-activated cyclase activity of fat cell membranes is retained upon solubilization with Lubrol PX. Provided 125I-labeled choleragen is first incubated with cells under conditions resulting in enzyme activation, the solubilized cyclase activity migrates with a component of 125I-labeled choleragen on gel filtration chromatography. Agarose derivatives containing the "active" subunit (molecular weight 36,000) of the toxin can specifically adsorb solubilized adenylate cyclase. Toxin-stimulated cyclase can be immunoprecipitated with antitoxin or anti-"active" subunit antibodies. There is a large excess of membrane receptors (ganglioside GM1) which, with the use of choleragenoid, can be shown to be functionally equivalent with respect to cyclase activation. Choleragenoid, an inactive competitive antagonist of toxin binding, can occupy and block a large proportion of toxin receptors without affecting toxin activity. A scheme of toxin action is proposed that involves lateral membrane diffusion of the initially inactive toxin-receptor complex with subsequent direct interaction with and modulation of adenylate cyclase. The basic features of this scheme may be pertinent to the mechanisms by which hormone receptors normally modulate adenylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman B., Flores J., Witkum P. A., Sharp G. W. Studies on the mode of action of cholera toxin. Effects on solubilized adenylate cyclase. J Clin Invest. 1974 Apr;53(4):1202–1205. doi: 10.1172/JCI107660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Commentary. Insulin receptors, cell membranes and hormone action. Biochem Pharmacol. 1974 Sep 1;23(17):2353–2361. doi: 10.1016/0006-2952(74)90224-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Desbuquois B., Krug F., Cuatrecasas P. Inhibitors of glucagon inactivation. Effect on glucagon--receptor interactions and glucagon-stimulated adenylate cyclase activity in liver cell membranes. Biochim Biophys Acta. 1974 Mar 20;343(1):101–120. doi: 10.1016/0304-4165(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A., LaRue M. K., LoSpalluto J. J. Properties of the cholera exo-enterotoxin: effects of dispersing agents and reducing agents in gel filtration and electrophoresis. Infect Immun. 1972 Dec;6(6):934–944. doi: 10.1128/iai.6.6.934-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fishman P. H., Bennett V., Cuatrecasas P. Cholera toxin and cell growth: role of membrane gangliosides. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4224–4228. doi: 10.1073/pnas.71.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis. 1973;5(1):77–78. doi: 10.3109/inf.1973.5.issue-1.15. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Subunit structure of cholera toxin. J Gen Microbiol. 1973 Jun;76(2):417–427. doi: 10.1099/00221287-76-2-417. [DOI] [PubMed] [Google Scholar]
- O'Keefe E., Cuatrecasas P. Cholera toxin mimics melanocyte stimulating hormone in inducing differentiation in melanoma cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2500–2504. doi: 10.1073/pnas.71.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Modified procedure for the synthesis of 32P-labelled ribonucleoside 5'-monophosphates of high specific activity. Biochim Biophys Acta. 1968 Feb 26;155(2):609–610. doi: 10.1016/0005-2787(68)90205-0. [DOI] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]



