Abstract
Background: Dual-energy X-ray Absorptiometry (DXA) is considered the gold standard for non-invasive measurement of bone mass. T-scores and Z-scores are used to present the results of bone mass. The present study was designed to evaluate the discordance between T-scores and Z-scores calculated at a same level and its relation with age, gender and body mass index (BMI) in a representative sample of normal population.
Methods: This cross-sectional study was conducted as a part of a comprehensive survey, Iranian Multicenter Osteoporosis Study (IMOS), designed to assess bone health among healthy adults. Each individual underwent both L1–L4 antero-posterior lumbar spine and hip DXA scan. The difference between the T- and Z-scores measured at each of the four skeletal sites was then calculated.
Results: A -1.21 to 1.21 point difference was noted in the Z- and T- scores measured at each site. While the difference between the T-and Z-scores was less than 0.5 SD in most of the cases, the difference was higher than 1 SD in about 5% of the subjects.
Conclusion: Standardization of Z-score definition and calculation techniques as well as developing an ethnicity-matched reference population is needed to improve the reliability of DXA-generated Z-scores.
Keywords: Osteoporosis, Bone Mineral Density, T-scores, Z-scores, Iran
Introduction
Dual-energy X-ray Absorptiometry (DXA) is considered the gold standard for non-invasive measurement of bone mass(1-4); its use, therefore, is widely accepted because of its high safety and reliability(2, 5-7). While DXA measures bone density in g/cm2, T- and/or Z-scores are commonly used to present the results.
The normal aging process is associated with physiologic bone loss, characterized by reduced T-score values; Z-score, on the other hand, is believed to remain almost unchanged over time. In fact, this is because T-score reports the number of standard deviation difference away from a young adult reference population presumably at peak bone mass, whereas Z-score shows the divergence noted when comparing the individual with the an age-matched reference population (1, 8).
Considering the abovementioned definition previous publications have suggested that T- and Z-scores should be similar or identical in young adults as the relevant reference populations should be the same(6, 9). Although T- and Z-score values measured using DXA had long been used interchangeably in young adults, recent ISCD guidelines have not universally accepted the use of Z-scores instead of T-scores in premenopausal women and men aged 20-49 years (6, 9-12).
Some authors have also pointed out unexpectedly large differences between these values in the clinical practice, a fact which may lead to amendments in the diagnosis and treatment of osteoporosis (13, 14). Apart from the abovementioned studies, the magnitude of the discordance in the normal population and its relation with other variables remains unclear. The present study was therefore designed to assess this discordance and its relation with age, gender and body mass index (BMI) in a representative sample of normal population.
Methods
Study design and subjects
This cross-sectional study was conducted as a part of a comprehensive survey, Iranian Multicenter Osteoporosis Study (IMOS), designed to assess bone health among healthy adults between February and March 2001. Details on the survey design and methods have been reported previously (15). Briefly, the IMOS used a random cluster sampling design in five representative cities (Tehran, Tabriz, Mashhad, Shiraz and Booshehr); independent samples of healthy Iranian adults excluding those with metabolic bone disease, history of rheumatoid arthritis, type 1 diabetes mellitus, hypercortisilism, malabsorption, renal and hepatic diseases along with pregnant and lactating woman and those with a history of infertility, oligomenorrhea, malignancy, immobility for more than a week. Those with alcoholism, cigarette smokers and individuals taking drugs affecting bone metabolism were also excluded. A written informed consent was taken from each participant. The study was approved by the Ethical Board Committee of Tehran University of Medical Sciences (TUMS) and Ministry of Health and Medical Education.
Men and women aged between 20 to 49 years were drawn from this comprehensive survey and recruited in the present study.
Data collection and BMD measurements
The subjects were asked to complete a questionnaire on physical activity, duration of sun exposure, diet, drug history, and past medical history, before they underwent the bone mineral density analysis.
Anthropometric values, including weight and height, were measured with individuals wearing light clothing and no shoes by trained technicians based on the international guidelines (16, 17). The height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) measurements were performed using the same technique by a single wall-mounted stadiometer (Seca) and a mobile digital scale (Seca, Hamburg, Germany), respectively. The BMI was calculated by dividing the body weight by the height squared (kg/m2). Quality control for all measurements was regularly monitored.
Each individual underwent both L1–L4 antero-posterior lumbar spine and hip (total, femoral neck, and trochanter) with a dual energy x-ray absorptiometry (DXA) scan using a Lunar DPXMD densitometer (Lunar 7164, GE, Madison, WI) based on the manufacturer's guidelines. Adjusted Z-score in each subject was calculated for body weight and height in addition to age. Precision error for BMD measurements was calculated to be 1–1.5% at the lumbar and 2–3% at the femoral regions. Quality control procedures were carried out regularly based on the manufacturer’s recommendations and the instrument was weekly calibrated using appropriate phantoms supplied by the manufacturer.
Statistical analysis
All the gathered data were entered in Microsoft Access Databank, checked, and cleaned before analysis; participants with incomplete data were excluded from the study. The statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Chicago, IL). The difference between the T- and Z-scores measured at each of the four skeletal sites, the total hip, femoral neck, trochanter, and lumbar spine (antero-posterior spine L1-L4) were calculated for every individual and presented in mean and standard deviation (SD). The individuals were then classified into three main groups based on the difference rates (between 0- 0.5, 0.5- 1 & more than 1). The effect of age, sex, and BMI on the difference was thereafter assessed. Correlation coefficient (r) was used to display the univariate strength and direction of the relationship between the studied variables. The analysis was performed in the whole population and then in each gender and age group (20- 29, 30- 39 & 40- 49) separately. Finally, we performed multivariable regression analysis to assess the association of age, sex, and BMI with the discordance of T-scores and Z-scores for each skeletal site. As data were derived from 5 different cities, the multivariable model was adjusted for clustering effect (city of living). P values less than 0.05 were considered as statistically significant.
Results
The scans of 2947 individuals including 1200 male (40.7%) and 1747 female (59.3%) were analyzed. The mean age of the studied men and women was 34 and 35 years, correspondingly. The mean ± SD of BMI was 25.6 ± 4.73 kg/cm2, ranging from 14.10 to 44.51kg/cm2.
The measured Z-scores ranged from -7.25 to 4.33 in men and -6.09 to 4.48 in women. T-score values, on the other hand, ranged from -6.57 to 4.30 and -5.04 to 3.59 in the two genders, respectively. The highest T-score was reported in male total hip (-6.57 to 4.30) and the least was seen in female neck of femur (-2.64 to 3.16). As for Z-score, however, the highest amount was similarly found in male total hip (-7.25 to 4.27) but the least was seen in female lumbar spine (-3.02 to 3.75).
A -1.21 to 1.21 point difference was noted in the Z- and T- scores measured at each site. While the difference between the T- and Z-scores was less than 0.5 SD in most of the cases, the difference was higher than 1 SD in about 5% of the subjects (Fig. 1). Total hip, with the least discrepancy between the two values, was the most reliable site whereas the contrary was reported for the femoral neck. Individuals in their 40s (aged between 40 and 49 years) had the highest number of discrepancies in the measured values (Table 1).
Fig. 1 .
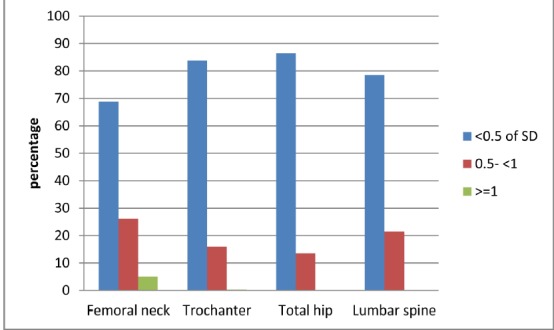
The magnitude of difference between Z- and T-scores in the whole study population stratified by skeletel sites.
Table 1 . The difference noted between the Z- and T-scores in units of SD stratified by age, sex, and skeletal sites.
| Male | Female | |||||||
| <0.5 | 0.5- <1 | >=1 | <0.5 | 0.5- <1 | >=1 | |||
| Age category(year) | Skeletal sites | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | |
| 20-29 | Femoral neck | 341(86) | 57(14) | 0(0) | 415(89) | 50(11) | 2(0) | |
| Trochanter | 208(71) | 86(29) | 1(0) | 303(96) | 11(4) | 0(0) | ||
| Total hip | 377(98) | 9(2) | 0(0) | 441(98) | 9(2) | 0(0) | ||
| Lumbar spine | 392(99) | 5(1) | 0(0) | 389(84) | 72(16) | 0(0) | ||
| 30-39 | Femoral neck | 318(100) | 0 | 0(0) | 337(63) | 187(35) | 13(2) | |
| Trochanter | 192(95) | 10(5) | 0(0) | 317(89) | 38(11) | 1(0) | ||
| Total hip | 302(100) | 1(0) | 0(0) | 477(94) | 32(6) | 0(0) | ||
| Lumbar spine | 306(97) | 11(3) | 0(0) | 521(100) | 0(0) | 0(0) | ||
| 40-49 | Femoral neck | 309(88) | 44(12) | 0(0) | 86(16) | 347(63) | 116(21) | |
| Trochanter | 216(100) | 0(0) | 0(0) | 218(62) | 132(37) | 4(1) | ||
| Total hip | 334(100) | 0(0) | 0(0) | 238(45) | 289(55) | 2(0) | ||
| Lumbar spine | 256(73) | 93(27) | 0(0) | 167(31) | 375(69) | 1(0) |
A significant correlation was found between the differences and the age of the subject. Figure 2 shows the correlation between age and the difference noted in Z- and T- score values at total hip. A similar pattern was noted in the other three skeletal sites. The strongest and weakest relation was seen in the lumbar spine (r= 0.87) and the trochanter (r= 0.6), respectively; the results were statistically significant (p<0.001).
Fig. 2 .
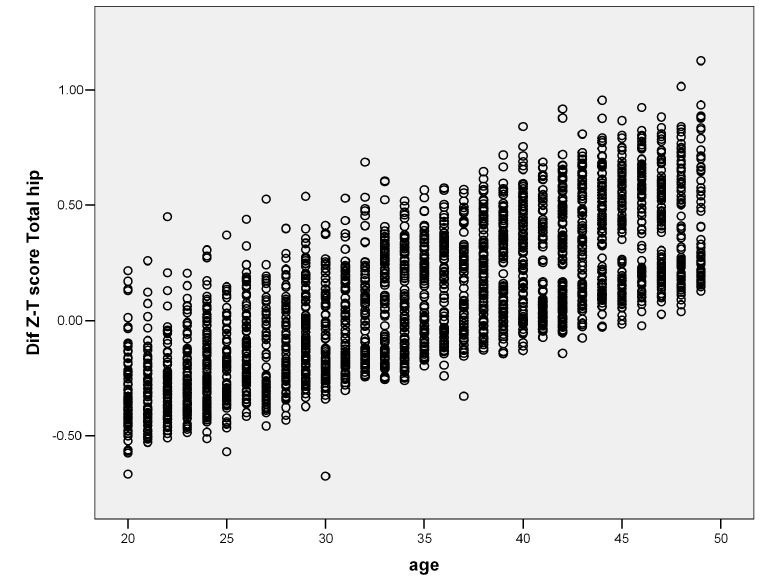
The correlation between age and the difference noted in Z- and T- score values at total hip
A moderate correlation was seen in the difference noted between Z- and T- score and the BMI values of the studied subjects (Fig. 3). The strongest and weakest correlation were seen in trochanter (r= 0.5) and lumbar spine (r= 0.24), correspondingly; all were statistically significant (p< 0.001).
Fig. 3 .
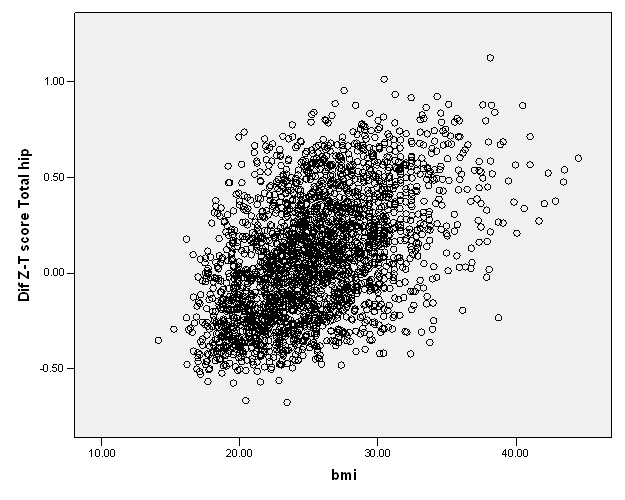
The correlation between BMI and the difference noted in Z- and T- score values at total hip
The discordance in all the three regions (trochanter, femoral neck, and total hip) was significantly greater in females (p<0.001). As for lumbar spine, however, the difference was slightly greater in males; the difference, however, was not statistically significant.
In the multivariate analysis, the β values were calculated for all the independent variables in all four skeletal sites (Table 2). While age and sex were positively correlated with the discordance in all four skeletal sites (p<0.001), the positive correlation between BMI and discordance was only recognized in total hip, femoral neck, and trochanter (p<0.001). The lowest β values referred to BMI and the greatest ones belonged to gender.
Table 2 . Multivariable regression analysis for each skeletal site considering the discordance between Z- score and T-scores as dependent variable.
|
Skeletal sites Independent variables |
Total Hip | Femoral Neck | Trochanter | Lumbar Spine |
| Age (yr) | 0.023 | 0.032 | 0.016 | 0.036 |
| BMI (kg/m2) | 0.012 | 0.015 | 0.017 | -0.005 |
| Sex (female/male) | 0.33 | 0.35 | 0.47 | 0.21 |
The presented data are β values of each independent variable in any skeletal site. All β values were statistically significant (P value<0.001). The analyses are adjusted for city of living.
Discussion
Osteoporosis, defined as having low bone density, is characterized by having T score values equal to or lower than 2.5 standard deviations (SD) below the normal peak values for young adults in the reference population(2, 10, 18). In 1994, the WHO recommended the use of T-score in diagnosing osteoporosis in postmenopausal women(18). The International Society for Clinical Densitometry (ISCD), however, recently urged physicians to use Z-score, rather than T-score, for diagnosing low bone mass in children, premenopausal women, and men younger than 50 years(9, 18, 19).
The definite prevalence of low bone mass and osteoporosis in this population, however, remains unknown for several reasons. Lack of validated DXA or any other universally accepted diagnostic criteria for premenopausal women or men mainly due to the low number of published scientific studies on this age group is the main factor hampering the accurate diagnosis of the condition in these populations(18, 20-22).
More recent studies have revealed a substantial and significant difference between these two values. This, mainly caused due to various definitions and calculation methods used for Z-score) and perhaps reference population, may alter the diagnostic and management process if Z-scores are used instead of T-scores (13, 14).
In the other words, any individual’s Z-score is dependent not only on specific subject factors such as BMD, age, ethnicity, gender and weight but also on the DXA manufacturer and the actual skeletal site being measured(18, 23). These findings have important implications for interpretation of Z-scores in clinical practice, medical guidelines and scientific research studies, as they may result in significant ascertainment bias and alter the diagnosis, evaluation and treatment an individual may receive(13, 14).
In our study, T-score was lower than or equal to their corresponding Z-score in most of the cases; in younger subjects, though, T-score values were higher than Z-scores in their age-matched peers. In other words, this study revealed a significant discordance between T- and Z-scores for any given BMD measurement in a young adult population. Although the difference was less than 0.5 SD in most of the cases, about 5% of them had a difference greater than 1 SD. The studies performed by Carey et al are the only other similar studies conducted in this regard (13, 14).
In the first study, at least one SD difference was noted between the Z- and T-scores at the lumbar spine, total hip, femoral neck, and trochanter measured using Lunar and Hologic technologies in more than 12% of the studied cases, which is much higher than that of our study (14). The fact that they have studied a population generally older than ours (59% of them aged between 40 and 49 years), mainly consisted of women (77%), and with the majority of the subjects suffering an underlying condition affecting the bone metabolism may explain this dissimilarity (14). It should be noted that Carey et al stressed that the different characteristics used for calculating T- and Z-score in each DXA technology (Lunar technology adjusts the data for ethnicity and weight as well as age resulting in a greater magnitude of difference is responsible for the mentioned discrepancy (14).
Collaborating with our results, the second study revealed that using Z-score, rather than T-score, lowers the number of individuals diagnosed with osteopenia and osteoporosis as well as low bone mass for age (13). The absence of a unique definition for standard Z-score and different methods used for calculating it are believed to be the main reasons contributing to the above mentioned discordance(8, 14). They also showed that while T-scores measured using Hologic technology were equal to or lower than their corresponding Z-scores, T-scores measured using Lunar were often greater than Z-scores (13).
They concluded that DXA-generated Z-scores can be unreliable measures in these populations, stressing that the diagnosis of osteoporosis in premenopausal women and men aged less than 50 should not be based on DXA alone and Z-score thresholds should only be used for the diagnosis of low bone mass for age (9, 14).
Previous studies have reported that weight, BMI and weight are important determinants of BMD, stressing that they may explain the differences noted in BMD values seen in population with different ethnicities (24, 25). Corroborating with these studies, the present research revealed age, sex and BMI as the three main variables affecting the discordance noted between the two values. While ISCD currently does not adjust Z-scores for body weight, doing so as well as using an ethnicity-matched reference population instead of a White reference may improve the calculations (14).
Conclusion
It could be concluded that standardization of Z-score definition and calculation techniques as well as developing an ethnicity-matched reference population is needed to improve the reliability of DXA-generated Z-scores. Additional studies highlighting the magnitude and the importance of these differences, its impact on the diagnosis of the disease and calculating the fracture risk are also needed. Until such data are available, however, DXA-generated Z-scores should be interpreted and used with caution in premenopausal women and men aged less than 50 years.
Cite this article as: Heidari B, Khashayar P, Rezai Homami M, Pajouhi A, Soltani A, Larijani B. Dual-energy X-ray absorptiometry diagnostic discordance be-tween Z-scores and T-scores in a young Iranian population. Med J Islam Repub Iran 2014 (22 December. Vol. 28:151.
References
- 1.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. American journal of obstetrics and gynecology. 2006;194:S3–S11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Nanes MS, Kallen CB. Osteoporosis. Seminars in nuclear medicine. 2014;44:439–50. doi: 10.1053/j.semnuclmed.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Kendler DL, Borges JL, Fielding RA. et al. The Official Positions of the International Society for Clinical Densitometry: Indications of Use and Reporting of DXA for Body Composition. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2013;16:496–507. doi: 10.1016/j.jocd.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Andreopoulou P, Bockman RS. Management of Postmenopausal Osteoporosis. Annual review of medicine. 2014 doi: 10.1146/annurev-med-070313-022841. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporosis international. 2005;16:S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 6.Bates DW, Black DM, Cummings SR. Clinical use of bone densitometry: clinical applications. Jama. 2002;288:1898–900. doi: 10.1001/jama.288.15.1898. [DOI] [PubMed] [Google Scholar]
- 7.Cauley JA, Lui L-Y, Ensrud KE. et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. Jama. 2005;293:2102–8. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Melton LJ, Christiansen C. et al. The diagnosis of osteoporosis. Journal of Bone and Mineral Research. 1994;9:1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Adler RA, El-Hajj Fuleihan G. et al. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. Journal of Clinical Densitometry. 2006;9:22–30. doi: 10.1016/j.jocd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10. Bonnick S. Bone Densitometry in Clinical Practice Totowa. NJ: Humana Press; 2004.
- 11.Kanis JA, Seeman E, Johnell O. et al. The perspective of the International Osteoporosis Foundation on the official positions of the International Society for Clinical Densitometry. Journal of Clinical Densitometry. 2005;8:145–7. doi: 10.1385/jcd:8:2:145. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Miller PD, Leib ES. et al. Response to “The perspective of the International Osteoporosis Foundation on the official positions of the International Society for Clinical Densitometry” by John A Kanis et al. Osteoporosis international. 2005;16:579–80. doi: 10.1007/s00198-005-1861-0. [DOI] [PubMed] [Google Scholar]
- 13.Carey JJ, Delaney MF, Love TE. et al. Dual-energy X-ray absorptiometry diagnostic discordance between Z-scores and T-scores in young adults. Journal of Clinical Densitometry. 2009;12:11–6. doi: 10.1016/j.jocd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Carey JJ, Delaney MF, Love TE. et al. DXA-generated Z-scores and T-scores may differ substantially and significantly in young adults. Journal of Clinical Densitometry. 2007;10:351–8. doi: 10.1016/j.jocd.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Meybodi HA, Heshmat R, Maasoumi Z. et al. Iranian Osteoporosis Research Network: Background, mission and its role in osteoporosis management. Iranian Journal of Public Health. 2008;37:1–6. [Google Scholar]
- 16.Lohman T, Roache A, Martorell R. Anthropometric standardization reference manual. Medicine & Science in Sports & Exercise. 1992;24:952. [Google Scholar]
- 17.Lau D, Douketis JD, Morrison KM. et al. Obesity Canada Clinical Practice Guidelines Expert Panel 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;176:1–117. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Organization WH. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992] 1994;. [PubMed]
- 19.Densitometry ISfC. Updated 2005 official positions for the international society for clinical densitometry. 2006 Nov 1 [Google Scholar]
- 20.Wainwright SA, Marshall LM, Ensrud KE. et al. Hip fracture in women without osteoporosis. The Journal of Clinical Endocrinology & Metabolism. 2005;90:2787–93. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- 21.Burge R, Dawson‐Hughes B, Solomon DH. et al. Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005–2025. Journal of Bone and Mineral Research. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ, Chrischilles EA, Cooper C. et al. How many women have osteoporosis? Journal of Bone and Mineral Research. 2005;20:886–92. doi: 10.1359/jbmr.2005.20.5.886. [DOI] [PubMed] [Google Scholar]
- 23.Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult US population: ACPM position statement on preventive practice. American journal of preventive medicine. 2009;36:366–75. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein JS, Lee M-LT, Sowers M. et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. The Journal of Clinical Endocrinology & Metabolism. 2002;87:3057–67. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 25.De Laet C, Kanis J, Odén A. et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis international. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]


