Abstract
Background: The sporadic cases of radiation-activated multiple sclerosis (MS) has been previously described, with a few studies focused on the relationship between radiation and the risk of MS. The aim of our study was to evaluate the association between history of X-ray radiation and MS.
Methods: This case-control study was conducted on 150 individuals including 65 MS patients and 85 age- and sex-matched healthy controls enrolled using non-probability convenient sampling. Any history of previous Xray radiation consisted of job-related X-ray exposure, radiotherapy, radiographic evaluations including chest Xray, lumbosacral X-ray, skull X-ray, paranasal sinuses (PNS) X-ray, gastrointestinal (GI) series, foot X-ray and brain CT scanning were recorded and compared between two groups. Statistical analysis was performed using independent t test, Chi square and receiver operating characteristics (ROC) curve methods through SPSS software.
Results: History of both diagnostic [OR=3.06 (95% CI: 1.32-7.06)] and therapeutic [OR=7.54 (95% CI: 1.5935.76) X-ray radiations were significantly higher among MS group. Mean number of skull X-rays [0.4 (SD=0.6) vs. 0.1 (SD=0.3), p=0.004] and brain CT scanning [0.9 (SD=0.8) vs. 0.5 (SD=0.7), p=0.005] was higher in MS group as well as mean of the cumulative X-ray radiation dosage [1.84 (SD=1.70) mSv vs. 1.11 (SD=1.54) mSv; p=0.008].
Conclusion: Our study was one of the first to show higher history of X-ray radiation in patients with MS compared to healthy controls. A possible association was also found between the dose and the site exposed to X-ray radiation and risk of developing MS
Keywords: Multiple Sclerosis, Radiation, X-ray, Association, Risk factor
Introduction
Multiple sclerosis (MS) is a complex disease with no clear etiology. It occurs due to variable genetic susceptibility and environmental triggers. The increasing prevalence of MS in developing countries has drawn researchers’ attention towards the potential risk factors that might affect the incidence or severity of this disease (1 , 2).
Some of the probable etiological triggers that have been investigated are latitude, hours of daylight, carbon monoxide, ultraviolet light, temperature, viruses, pets, and toxic chemicals (3 , 4). Ionizing radiation is also proposed as another potential trigger (5-8). Some evidence support this hypothesis including central demyelination after radiotherapy (9 , 10), high prevalence of MS in northen and southern magnetic geographical areas that causes an increase in cosmic radiation (11), and exacerbation of experimental allergic encephalomyelitis (EAE) after exposure of spinal cord to X-ray in rats (12). Ionizing radiation could be emitted in different ways: natural sources (cosmic radiation), and human-made radiation (radionuclide fall-out, nuclear medicine, medical X-ray for diagnosis and therapy). The frequent usage of X-ray radiation in medicine has grown concerns about the potential consequence of X-ray as a risk exposure for various health conditions (13-17).
Until now, few studies have assessed the role of ionizing radiation as a risk factor in MS, and most of them have focused on radiation types other than the X-ray. Therefore, the aim of our study was to evaluate the relationship between history of X-ray radiation and MS using a case-control study design. In addition, we also determined whether the site, location and dose of X-ray radiation would make any significant risk difference for MS.
Methods
Subjects
The study was performed at Firoozgar University Hospital (affiliated to Iran University of Medical Sciences) in Tehran, Iran, during 2007-2008. This analytical case-control study was conducted on 150 individuals including 65 MS patients (case group) and 85 healthy controls (control group) enrolled using non-probability convenient sampling. At the time of enrollment, brain MRI, cerebrospinal fluid (CSF) analysis and visual evoked potential (VEP) tests were performed as part of the pre-study evaluation for patients. The results of the test were assessed using McDonald criteria (revision of 2005) (18) to determine MS diagnosis. Exclusion criteria were: other serious systemic illnesses or psychiatric disorders as well as any memory disorders or cognitive impairment.
The controls were consisted of 85 age- and sex-matched non-MS subjects who were selected from the healthy relatives of the referred patients during the same period of time.
Assessments
In addition to the baseline and demographic data, any previous history of X-ray radiation consisted of job-related X-ray exposure, radiotherapy, radiographic evaluations and CT scanning were all recorded and compared between the two study groups. This information was collected using a structured questionnaire in a face-to-face interview. The recruited patients were asked about the history of exposures in the time period before the MS-related symptoms were started and the diagnosis was confirmed. Moreover, the type of modality, location of exposure and the number of each X-ray examination were also recorded to calculate the cumulative dosage of X-ray radiation for each organ. For this purpose, the standard typical dose of irradiation was considered as 0.02 mSv for chest X-ray, 0.7 mSv for lumbosacral X-ray, 0.06 mSv for PNS X-ray, 0.005 mSv for foot X-ray, 0.03 mSv for skull X-ray, 1.2 mSv for GI series (abdominal X-ray) and 2 mSv for the brain CT scanning (19).
The interview was performed and the questionnaire was completed by a single medical student after an appropriate training. All of the diagnostic work-ups and clinical examinations were performed by a single neurologist for the MS group.
Statistical Analysis
Data were analyzed using SPSS v.20 software (IBM, Chicago, IL, USA). To describe the quantitative measurements, mean and standard deviation (SD) were used. For nominal variables relative frequency and percentages were reported. In univariate statistical analysis, student t-test for independent samples was applied for between-group comparisons. In order to compare the prevalence of positive history of X-ray radiation (in overall and in different sites of exposure), Pearson Chi square or Fisher exact test were used wherever appropriate. The corresponding odds ratio (OR) and its 95% confidence interval (CI) were also calculated for each comparison.
Receiver operating characteristics (ROC) curve was performed to evaluate whether the cumulative dosage of X-ray radiation could statistically discriminate MS (case group) from normal condition (control group). For this purpose, the area under the curve (AUC) was calculated and the best cut-off point for the radiation dosage was selected based on the corresponding diagnostic values, sensitivity and specificity. In all analytical procedures, a two-tailed p-value of less than 0.05 was considered as statistically significant difference.
Results
Baseline Characteristics
A total number of 65 MS patients and 85 controls were recruited in this case-control study. The two groups were matched regarding the mean age and sex distribution. Majority of MS patients were female (66.2%, 43 out of 65) with mean age of 30.7 (SD=7.4) years. Correspondingly, the control group consisted of 63.5% (54 out of 85) females with the mean age of 28.8 (SD=6.8) years. Univariate comparisons showed no statistical significant difference in the age [Student t-test p=0.108) and sex (Chi square p=0.739] distribution between the two groups.
History of X-ray Radiation
In overall, 86.2% (n=56) of MS patients had a positive history for any exposure with X-ray radiation before they were diagnosed with MS. This feature was significantly higher than the control group where 67.1% (n=57) had positive history [OR=3.06 (95% CI: 1.32-7.06), Chi square p=0.007]. As listed in Table 1, the history of radiation therapy was also significantly higher in the MS group [15.4% vs. 2.4%, OR=7.54 (95% CI: 1.59-35.76), Chi square p=0.005].
Table 1 . Comparison of the frequency of different types of radiation between MS patientsand healthy controls [all values are presented as N.O (%) and the healthy controls are set as the reference group for all reported ORs.] .
| Variable | Study Group |
OR (95% CI) |
p | |
|
MS patients (n=65) |
Healthy controls (n=85) |
|||
| Radiation-exposed job | 4 (9.5%) | 2 (4.2%) | 2.42 (0.42-13.95) | 0.412 |
| Radiation therapy | 10 (15.4%) | 2 (2.4%) | 7.54 (1.59-35.76) | 0.005* |
| All kinds of X-rayes | 56 (86.2%) | 57 (67.1%) | 3.06 (1.32-7.06) | 0.007* |
| Chest X-ray | 51 (78.5%) | 48 (56.5%) | 2.81 (1.35-5.83) | 0.005* |
| Lumbosacral X-ray | 4 (9.5%) | 8 (9.4%) | 1.01 (0.29-3.58) | 0.984 |
| PNS X-ray | 20 (30.8%) | 17 (20.0%) | 1.78 (0.84-3.76) | 0.129 |
| Brain CT-scan | 40 (61.5%) | 31 (36.5%) | 2.79 (1.43-5.43) | 0.002* |
| Foot X-ray | 1 (2.4%) | 1 (2.1%) | 1.15 (0.07-18.91) | 0.924 |
| Skull X-ray | 20 (30.8%) | 10 (11.8%) | 3.33 (1.43-7.75) | 0.004* |
| GI series | 1 (2.4%) | 0 | 1.02 (0.98-1.07) | 0.467 |
* Statistical significant difference, OR: Odd’s ratio; CI: confidence interval; PNS: para nasal sinuses; GI: gastrointestinal
Prevalence of X-ray radiation is illustrated in Table 1 and Figure 1 with respect to different sites of exposure. Although the positive history of taking X-ray radiography was more likely among MS patients in all of the evaluated sites, the difference was statistically significant for the chest and skull X-rays and brain CT scanning. Chest X-ray was obtained in 78.5% of the MS patients while 56.5% of the controls had a positive history of being exposed to a chest X-ray [OR=2.81 (95% CI: 1.35-5.83), Chi square p=0.005]. Patients with MS were more likely to have skull X-ray compared to the healthy controls [30.8% vs. 11.8%, OR=3.33 (95% CI: 1.43-7.75), Chi square p=0.004]. Similarly, brain CT scanning was also more commonly performed in MS patients comparing to the controls [61.5% vs. 36.5%, OR=2.79 (95% CI: 1.43-5.43), Chi square p=0.002].
Fig. 1 .

Frequency distribution of different radiation types in MS patients and healthy controls
Table 2 summarizes the results for comparison of the mean number of different types of radiation between MS patients and healthy controls. In overall, the total number of times in which the MS patients were exposed to X-ray radiation was significantly higher than the controls [2.9 (SD=1.9) vs. 1.7 (SD=1.7), Student t-test p<0.001]. Moreover, the mean number of chest [1.3 (SD=1.0) vs. 0.8 (SD=0.8), Student t-test p=0.002] and skull X-rays [0.4 (SD=0.6) vs. 0.1 (SD=0.3), Student t-test p=0.004] and brain CT scanning [0.9 (SD=0.8) vs. 0.5 (SD=0.7), Student t-test p=0.005] was higher in the MS group. The results from independent Student t-test also showed that the mean of the cumulative radiation dosage was significantly higher in MS patients compared to the controls [1.84 (SD=1.70) mSv vs. 1.11 (SD=1.54) mSv; p=0.008].
Table 2 . Comparison of the mean number of different types of radiation and the cumulative radiation dosage between MS patients and healthy controls (all values are presented as mean±S.D.) .
| Variable | Study Group | p | |
|
MS patients (n=65) |
Healthy controls (n=85) |
||
| Total X-rayes | 2.88±1.87 | 1.74±1.71 | <0.001* |
| Chest X-ray | 1.25±0.97 | 0.79±0.83 | 0.002* |
| Lumbosacral X-ray | 0.10±0.30 | 0.13±0.43 | 0.645 |
| PNS X-ray | 0.32±0.50 | 0.20±0.40 | 0.109 |
| Brain CT-scan | 0.86±0.85 | 0.49±0.72 | 0.005* |
| Foot X-ray | 0.02±0.15 | 0.02±0.14 | 0.925 |
| Skull X-ray | 0.35±0.57 | 0.12±0.32 | 0.004* |
| GI series | 0.02±0.15 | 0 | 0.323 |
| Cumulative Radiation Dosage (mSv) | 1.84±1.70 | 1.11±1.54 | 0.008* |
Subgroup Analysis
Further analysis was performed within each sex subgroup. The higher probability of being exposed to any kind of X-ray radiation in MS patients remained significant only among the females [In females: OR=3.77 (95% CI: 1.48-9.62), Chi square p=0.004; in males: OR=2.25 (95% CI: 0.22-23.19), Chi square p=0.633]. Similar results were also found in other significant differences regarding the site of the X-ray exposure. The statistically significant higher exposure with X-ray radiation in chest was only observed when the comparison was performed between the female MS patients and female healthy controls [In females: OR=4.45 (95% CI: 1.83-10.83), Chi square p=0.001; in males: OR=1.08 (95% CI: 0.27-4.39), Chi square p=0.914]. Likely, the higher exposure with skull X-ray [In females: OR=5.42 (95% CI: 1.62-18.14), Chi square p=0.003; in males: OR=1.94 (95% CI: 0.55-6.88), Chi square p=0.299] and brain CT-scanning [In females: OR=3.30 (95% CI: 1.42-7.65), Chi square p=0.005; in males: OR=2.29 (95% CI: 0.73-7.15), Chi square p=0.152] was statistically significant only among the females.
Receiver Operating Characteristics (ROC) Curve
As illustrated in Figure 2, the results from ROC curve analysis showed that the cumulative dosage of X-ray radiation could significantly discriminate MS patients from healthy controls [AUC=0.661 (95% CI: 0.572-0.749), p=0.001]. As the best discriminant points, the cut-off value of 0.045 mSv had 78.5% sensitivity and 50.6% specificity, the cut-off value of 1.72 mSv showed 61.5% sensitivity and 63.5% specificity and the cut-off value of 2.01 mSv had 60% sensitivity and 67.1% specificity.
Fig. 2 .
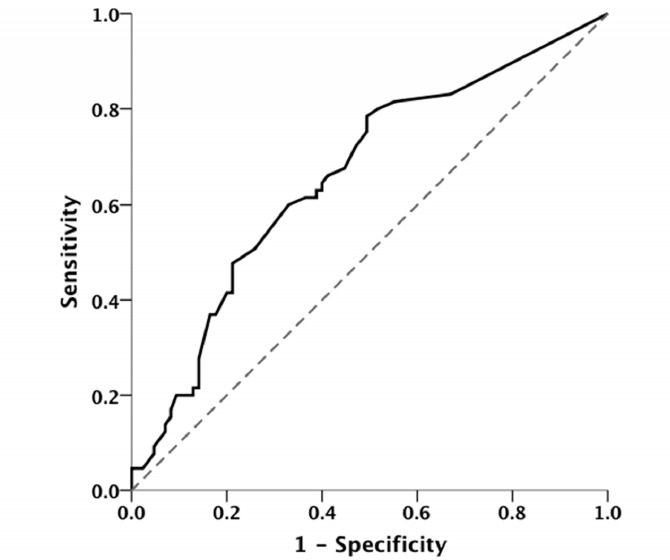
Receiver operating characteristics (ROC) curve to show the value of the cumulative dosage of X-ray radiation to discriminate MS patients and healthy controls [area under curve (AUC)=0.661 (95% CI: 0.572-0.749), p=0.001]
Discussion
We evaluated the relationship between history of diagnostic and/or therapeutic X-ray radiation and risk of MS. Our findings showed that positive history of exposure to X-ray radiation was more likely in patients with MS in comparison with the age- and sex-matched control group and this difference was statistically significant. Moreover, the mean number of chest X-ray, skull X-ray, and brain CT scanning was higher in MS group. The mean of the cumulative dosage of the radiation was also significantly higher in MS patients compared to the controls demonstrating the so-called “dose-response” association. Sex appeared to be an important factor, and the subgroup analysis showed a more prominent relationship between X-ray radiation and risk of MS in female patients.
Similar to our study, European researchers in Sweden reported that X-ray examination more frequently occurred among MS patients compared to the control group during a five-year period prior to MS diagnosis (5). In this study, therapeutic X–ray was reported only in five cases of the MS group, but not in the control group at all (6). This result was primary interpreted as simply reflecting the fact that some of these imaging could have been performed in the early stages of the disease when the diagnosis is still unclear and imaging was performed to help final diagnosis and identify the causes of the initial symptoms. In our study, mean number of chest X-ray episodes was also significantly higher among the MS patients, which may be due to sensory impairment, such as tightness around the torso or stomach (“the MS hug”) (20) as an initial symptom of MS where chest X- ray was performed to determine the final diagnosis. As a result, higher exposure to X-ray radiation in the MS group can be related to underlying medical problems including some of the early-onset signs and symptoms of the MS itself. Although this issue might lead to a confounding bias, the dose-response finding in our study suggests an independent risk-factor role for X-ray radiation.
On the other hand, there are some clues implicating the potential role of X-ray radiation on MS pathogenesis. Investigations show that oxidative stress (OS) (21) and immune system play an important role in the MS pathogenesis (22). It seems that consequences of X-ray radiation on these pathways might be the cause of relationship between MS and X-ray radiation. The immune system responds to radiation, which is dependent on the dose (23), radiation quality, immune cell types and patient sensitivity (24). Some studies explain that low dose radiation leads to up-regulated T cell activity especially TH1, increases the level of IFN-γ, TNF-α, IL-2, decreases the level of IL-10, and causes free radical formation and oxidative damage (25). All of these changes lead to demyelination and axonal damage similar to MS pathogenesis (21). Furthermore, some other studies mentioned that low dose radiation (once per week for 4 weeks) could suppress pro-inflammatory cytokines and induction of Tregs, the regulatory T cells (25). Ionizing radiation can also induce viral synthesis as another etiological trigger (6). In addition, experimental studies showed that X-ray radiation increases vascular permeability, which itself may play a key role in development of demyelination plaques (9 , 10). Similar to other immune regulation disorders (26), it has been suggested that MS patients are more sensitive to ionizing (27) and X-ray radiation, which might trigger demyelination process in susceptible patients.
In our study, obtaining skull X-ray and brain CT scanning were also found to be significantly more common in patients with MS. Regarding the potential reverse causation, this finding may be due to non-specific early neurologic dysfunction in MS patients such as vertigo. From another point of view, some studies show the relationship between cranial trauma and MS onset (28), which could also lead to more prevalent imaging assessment among this group. Besides the probability for reverse causation bias and confounding effects of other risk factors, there are some case-reports of disseminated plaque of demyelination that directly developed after central nervous system radiotherapy (29 , 30). However, it is not clear which of the abovementioned causal directions, interpretations or their combination is the realy beneath all of these findings. On the other hand and in line with the dose-response findings, our results showed that cumulative dosage of X-ray radiation is statistically related to the risk of MS. For this purpose, the best discriminant points were 0.045 mSv, 1.72 mSv and 2.01 mSv. However, due to the rather low sensitivity and specificity, this variable failed to show a clinically relevant predictive value for MS.
Subgroup analysis showed that higher probability of being exposed to any kind of X-ray radiation, chest X-ray, skull X-ray and brain CT-scan in MS patients remained significant only in females. It must be noted that the male subgroup had smaller sample size, which leads to a lower statistical power. This inequity in sex distribution could be expected where MS is approximately three-fold more common in females than males (20). However, genome-wide studies have failed to provide any convincing support for any MS-related genes on the X chromosome, thus the increased prevalence of MS in females might be related to female-specific physiology and hormone related (31). This higher prevalence of MS in females over time proposes that sex effect makes females more vulnerable to potential environmental risk factors such as X-ray radiation.
Similar to other case–control studies on neurodegenerative disorders, the most important technical problem in this study is the risk for misclassification bias. It is not clear when exactly the pathological changes of the MS has been started in the case group or how sure are the researchers about the absence of these changes in the non-symptomatic period among the controls, which could lead to reverse causation bias, too. Although not completely preventing, we considered a lag time between the history of exposure and MS diagnosis to look for the X-ray radiation when any MS-related symptom had not been started yet in order to decrease the risk for this misclassification bias. On the other hand, like many other retrospective research using self-reported data, recall bias represents a major threat in our study. The tendency of MS patients to report past medical events including their exposure to X-ray radiation might have been higher than the controls. Nevertheless, since the exposure of interest (X-ray radiation) is usually considered as a rather memorizable clinical event in the medical history of every person, the risk for recall bias was expected not to be high in our study. Furthermore, we also excluded any individual with memory and/or cognition dysfunction. While up to now, X-ray radiation is not considered as a well-known risk factor for MS, the risk for ascertainment bias is also ignorable in our study. In other words, the individuals with any history of X-ray radiation are not more likely to be evaluated for MS according to current work-ups.
Conclusion
To the best of our knowledge, our study is one of the first attempts not only to show a potential risk factor role for X-ray radiation in MS, but also to propose cut-off values. Conclusively, our findings showed a higher prevalence of X-ray radiation in individuals who further developed MS. Regarding the site of radiation, the difference was more prominent for brain and skull exposure and both the cumulative number and dose of radiation relate to the risk of MS. This study could be considered as an initial attempt to show the relationship between X-ray radiation and risk of MS development. Yet, this study could not demonstrate any causal association and for a better understanding of the underlying relationship, there is a need for longitudinal community-based cohort studies with larger number of participants taking into account the exact reason for the administration of X-ray radiation.
Conflict of Interest
We declare that we have neither financial disclosure nor conflict of interest in this manuscript.
Cite this article as: Motamed M.R, Fereshtehnejad S.M, Abbasi M, Sanei M, Abbaslou M, Meysami S. X-ray radiation and the risk of multiple sclerosis: Do the site and dose of exposure matter? . Med J Islam Repub Iran 2014 (9 December). Vol. 28:145.
References
- 1.Ebers GC, Kukay K, Bulman DE, Sadovnick AD, Rice G, Anderson C. et al. A full genome search in multiple sclerosis. Nat Genet. 1996;13:472–476. doi: 10.1038/ng0896-472. [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker BG. Epidemiology of multiple sclerosis. Neurol Clin. 1996;14:291–308. doi: 10.1016/s0733-8619(05)70257-7. [DOI] [PubMed] [Google Scholar]
- 3. Matthews WB, Compton A, Allen IV, Marten C, eds. McAlpine's multiple sclerosis. 2nd S. New York, NY: Churchill Livingstone; 1991: 18-27.
- 4.Powell JJ, Van de Water J, Gershwin ME. Evidence for the role of environmental agents in the initiation or progression of autoimmune conditions. Environ Health Perspect. 1999;107(suppl 5):667–672. doi: 10.1289/ehp.99107s5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flodin U, Söderfeldt B, Noorlind-Brage H, Fredriksson M, Axelson O. Multiple Sclerosis, Solvents, and PetsA Case-Referent Study. Arch Neurol. 1988;45(6):620–623. doi: 10.1001/archneur.1988.00520300038015. [DOI] [PubMed] [Google Scholar]
- 6.Landtblom AM, Flodin U, Karlsson M, Pålhagen S, Axelson O, Söderfeldt B. Multiple sclerosis and exposure to solvents, ionizing radiation and animals. Scand J Work Environ Health. 1993;19(6):399–404. doi: 10.5271/sjweh.1455. [DOI] [PubMed] [Google Scholar]
- 7.Axelson O, Landtblom AM, Flodin U. Multiple sclerosis and ionizing radiation. Neuroepidemiology. 2001;20:175–178. doi: 10.1159/000054784. [DOI] [PubMed] [Google Scholar]
- 8. Bölviken O. Ecological associations: nasopharyngeal carcinoma and multiple sclerosis versus radioactive elements. In: Bölvikin O, ed. Proceedings from a Symposium Held at the Norwegian Academy of Science and Letters; 2002 June 6-7; Oslo, Norway.
- 9.Lampert P, Tom MI, Rider WD. Disseminated demyeli- nation of the brain following Co60 (gamma) radiation. Arch Pathol. 1959;68:322–330. [PubMed] [Google Scholar]
- 10.Lampert PW, Davis RL. Delayed effects of radiation on the human central nervous system: 'early' and 'late' delayed reactions. Neurology. 1964;14:912–917. doi: 10.1212/wnl.14.10.912. [DOI] [PubMed] [Google Scholar]
- 11.Barlow J. Correlation of the geographic distribution of multiple sclerosis with cosmic-ray intensities. Acta Neurol Scand. 1960;35(Suppl 147):108–130. doi: 10.1111/j.1600-0447.1960.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 12.Oldendorf WH, Cornford EM. A comparison of total body and local spinal cord irradiation in experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1977;36(1):50–61. doi: 10.1097/00005072-197701000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81(965):362–78. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 14.Hammer GP, Seidenbusch MC, Schneider K, Regulla DF, Zeeb H. et al. A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res. 2009;171(4):504–512. doi: 10.1667/RR1575.1. [DOI] [PubMed] [Google Scholar]
- 15.Grufferman S, Ruymann F, Ognjanovic S, Erhardt EB, Maurer HM. Prenatal X-ray exposure and rhabdomyosarcoma in children: a report from the children's oncology group. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1271–1276. doi: 10.1158/1055-9965.EPI-08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Sun Z, Wang J, Nan G, Ma Y. et al. X-ray exposure induces apoptosis of some proliferative epidermal cells following traumatic spinal cord injury in adult rats. Int J Neurosci. 2009;119(1):141–154. doi: 10.1080/00207450802540599. [DOI] [PubMed] [Google Scholar]
- 17.Bailey HD, Armstrong BK, de Klerk NH, Fritschi L, Attia J, Lockwood L, Milne E. Exposure to diagnostic radiological procedures and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2010;19:2897–2909. doi: 10.1158/1055-9965.EPI-10-0542. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L. et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 19.Wall BF, Hart D. Revised radiation doses for typical x-ray examinations. The British Journal of Radiology. 1997;70:437–439. doi: 10.1259/bjr.70.833.9227222. [DOI] [PubMed] [Google Scholar]
- 20. Hauser SL, Goodkin DE. Multiple sclerosis and other demyelinating diseases. In Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine, 15th ed. New York: McGraw- Hill, 2001:2452–2461.
- 21.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004 Mar;251(3):261–8. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 22.Lassmann H, Brьck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukimoto M, Nakatsukasa H, Sugawara K, Yamashita K, Kojima S. Repeated 05-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat Res. 2008;170(4):429–36. doi: 10.1667/rr1352.1. [DOI] [PubMed] [Google Scholar]
- 24.Amundson SA. Functional Genomics and a New Era in Radiation Biology and Oncology. Bio Science. 2008;58(6):491–500. doi: 10.1641/B580606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu SZ. Nonlinear Dose-Response Relationship in the Immune System Following Exposure to Ionizing Radiation: Mechanisms and Implications. Nonlinearity BiolToxicol Med. 2003;1(1):71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor AM, Harnden DG, Arlett CF, Harcourt SA, Lehmann AR, Stevens S. et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivityNature. 1975. 4;258(5534):427–9. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- 27.Gipps E, Kidson C. Ionising radiation sensitivity in multiple sclerosis. Lancet. 1981 Apr 25;1(8226):947. doi: 10.1016/s0140-6736(81)91644-5. [DOI] [PubMed] [Google Scholar]
- 28.Goodin DS, Ebers GC, Johnson KP, Rodriguez M, Sibley WA, Wolinsky JS. The relationship of MS to physical trauma and psychological stressReport of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1999;52:1737–45. doi: 10.1212/wnl.52.9.1737. [DOI] [PubMed] [Google Scholar]
- 29.Peterson K, Rosenblum MK, Powers JM, Alvord E, Walker RW, Posner JB. Effect of brain irradiation on demyelinating lesions. Neurology. 1993;43:2105–2112. doi: 10.1212/wnl.43.10.2105. [DOI] [PubMed] [Google Scholar]
- 30.McMeekin RR, Hardman JM, Kempe LG. Multiple sclerosis after x-radiationActivation by treatment of metastatic glomus tumor. Arch Otolaryngol. 1969;90(5):617–21. doi: 10.1001/archotol.1969.00770030619017. [DOI] [PubMed] [Google Scholar]
- 31.Orton SM, Herrera BM, Yee IM. et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–36. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]


