Abstract
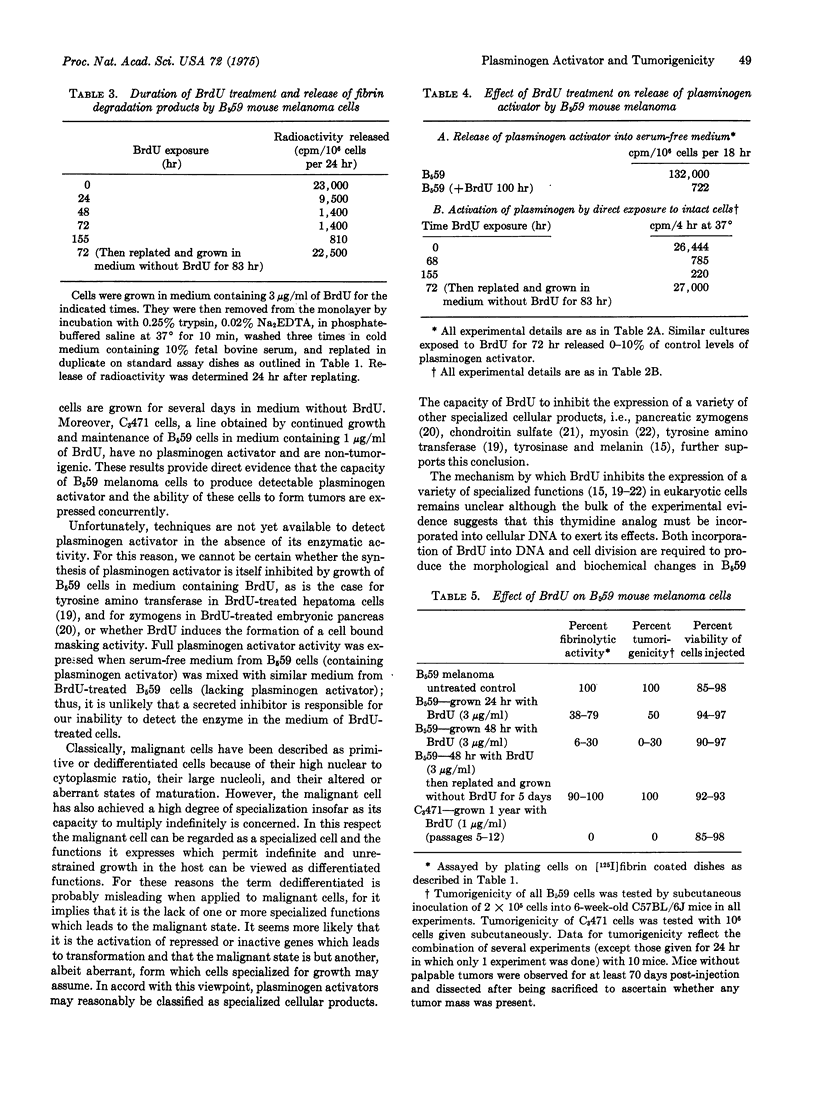
The decrease of tumorigenicity by mouse melanoma clone B559 after growth in the presence of 5-bromodeoxyuridine (BrdU) has been correlated with a decrease in detectable cellular plasminogen activator. Reduction of both activities occurs after one to two cell divisions in the presence of this thymidine analog and is virtually complete within three to four cell cycles. These changes are fully reversible; four to five cell divisions in the absence of BrdU are sufficient to allow both tumorigenicity and plasminogen activator levels to return to normal. These results support the hypotheses that (a) the expression of a cellular plasminogen activator is closely associated with the transformation of normal to malignant cells and that (b) the suppression of tumorigenicity by BrdU reflects the capacity of this base analog to inhibit the expression of specialized functions which accompany the malignant state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Elevated glycosidases and proteolytic enzymes in cells transformed by RNA tumor virus. Biochim Biophys Acta. 1972 Apr 21;264(2):339–343. doi: 10.1016/0304-4165(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. K., Acs G. Purification and characterization of a cellular fibrinolytic factor associated with oncogenic transformation: the plasminogen activator from SV-40-transformed hamster cells. Biochim Biophys Acta. 1974 Mar 27;340(3):339–347. doi: 10.1016/0005-2787(74)90279-2. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Glynn R. D., Thrash C. R., Cunningham D. D. Maximal concanavalin A-specific agglutinability without loss of density-dependent growth control. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2676–2677. doi: 10.1073/pnas.70.9.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Kazakova O. V., Orekhovich V. N. Neitral'naia proteinaza iz tkanei perevivaemoi sarkomy krys. Biokhimiia. 1969 Jan-Feb;34(1):73–77. [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKDALE F., OKAZAKI K., NAMEROFF M., HOLTZER H. 5-BROMODEOXYURIDINE: EFFECT ON MYOGENESIS IN VITRO. Science. 1964 Oct 23;146(3643):533–535. doi: 10.1126/science.146.3643.533. [DOI] [PubMed] [Google Scholar]
- Silagi S., Beju D., Wrathall J., Deharven E. Tumorigenicity, immunogenicity, and virus production in mouse melanoma cells treated with 5-bromodeoxyuridine. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3443–3447. doi: 10.1073/pnas.69.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagi S., Bruce S. A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc Natl Acad Sci U S A. 1970 May;66(1):72–78. doi: 10.1073/pnas.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagi S. Modification of malignancy by 5-bromodeoxyuridine. Studies of reversibility and immunological effects. In Vitro. 1971 Sep-Oct;7(2):105–114. doi: 10.1007/BF02628269. [DOI] [PubMed] [Google Scholar]
- Silagi S., Newcomb E. W., Weksler M. E. Relationship of antigenicity of melanoma cells grown in 5-bromodeoxyuridine to reduced tumorigenicity. Cancer Res. 1974 Jan;34(1):100–104. [PubMed] [Google Scholar]
- Stellwagen R. H., Tomkins G. M. Preferential inhibition by 5-bromodeoxyuridine of the synthesis of tyrosine aminotransferase in hepatoma cell cultures. J Mol Biol. 1971 Feb 28;56(1):167–182. doi: 10.1016/0022-2836(71)90092-1. [DOI] [PubMed] [Google Scholar]
- Taylor J. C., Hill D. W., Rogolsky M. Detection of caseinolytic and fibrinolytic activities of BHK-21 cell strains. Exp Cell Res. 1972 Aug;73(2):422–428. doi: 10.1016/0014-4827(72)90067-5. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Walther B. T., Pictet R. L., David J. D., Rutter W. J. On the mechanism of 5-bromodeoxyuridine inhibition of exocrine pancreas differentiation. J Biol Chem. 1974 Mar 25;249(6):1953–1964. [PubMed] [Google Scholar]
- Wrathall J. R., Oliver C., Silagi S., Essner E. Suppression of pigmentation in mouse melanoma cells by 5-bromodeoxyuridine: effects on tyrosinase activity and melanosome formation. J Cell Biol. 1973 May;57(2):406–423. doi: 10.1083/jcb.57.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]



