Abstract
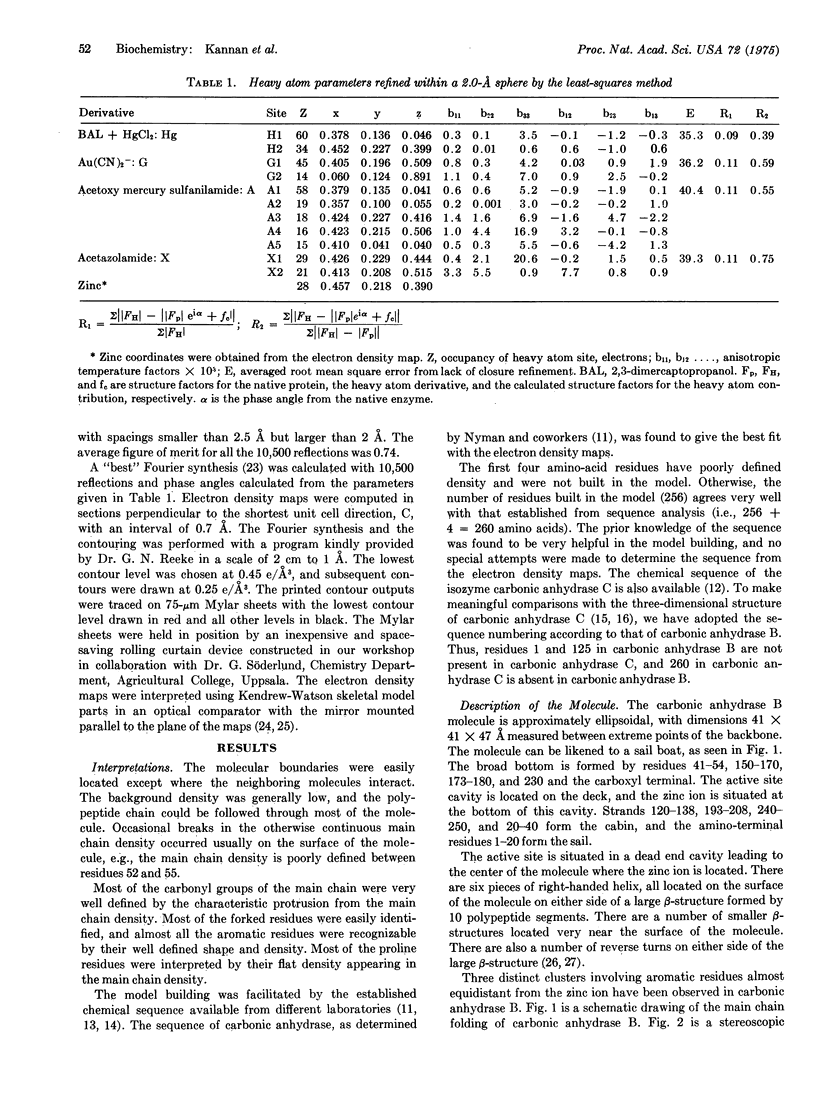
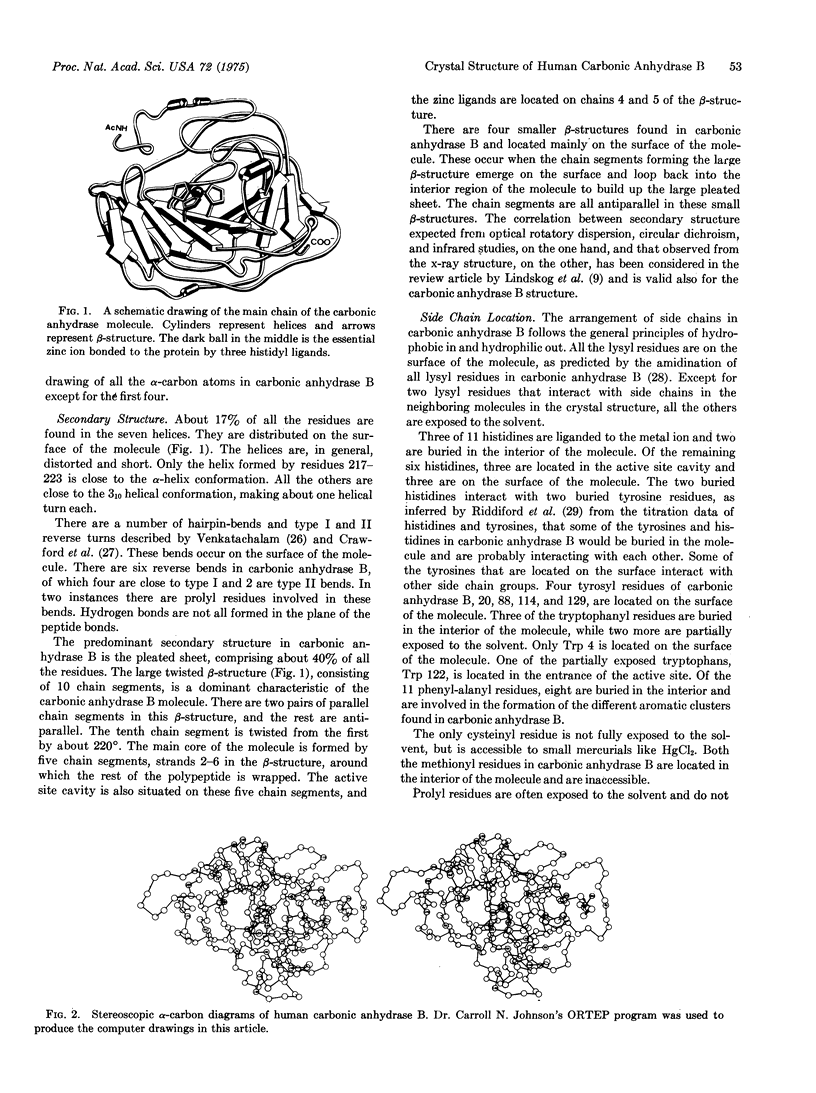
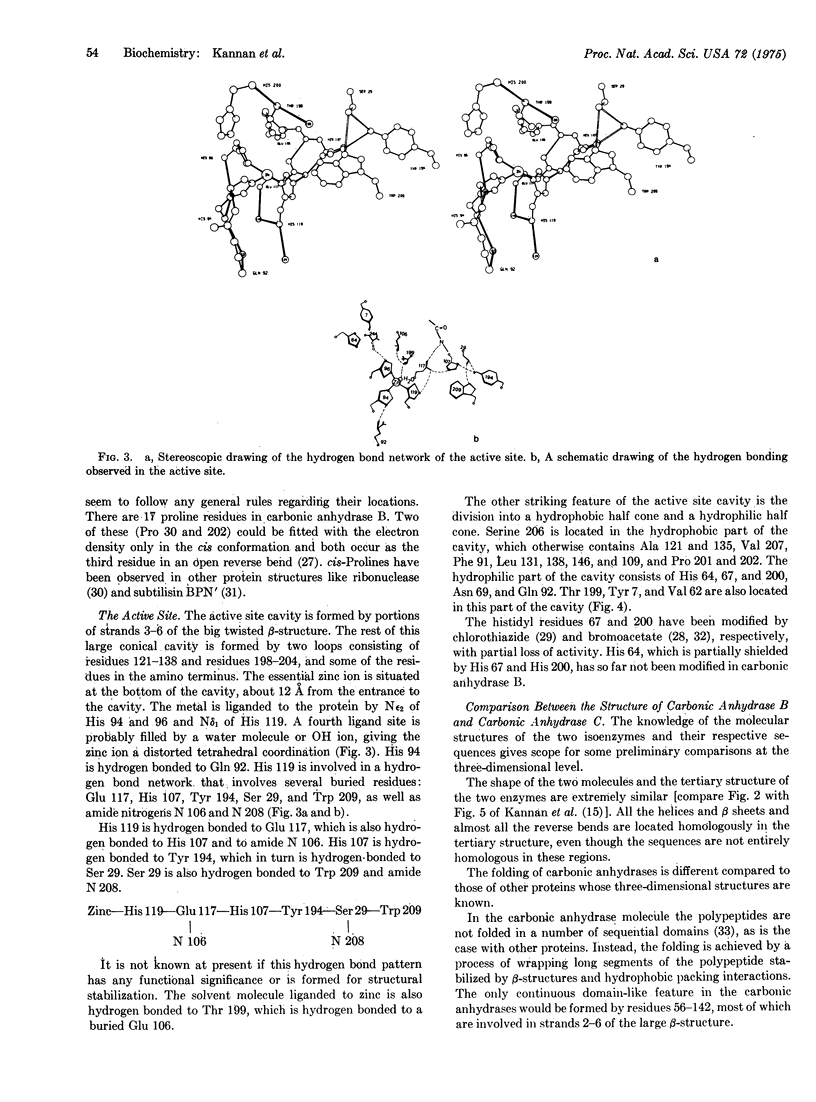
The three-dimensional structure of carbonic anhydrase B (EC 4,2,1,1; carbonate hydro-lyase) from human erythrocytes has been determined to high resolution. Parallel and antiparallel pleated sheet makes up the predominant secondary structure of the enzyme. The tertiary structure is unique for its folding and is very similar to the structure is unique for its folding and is very similar to the structure of the isoenzyme, human erythrocyte carbonic anhydrase C. The essential metal ion, zinc, is firmly bound to the enzyme through three histidyl ligands and located at the bottom of a 12-A deep conical cavity. The zinc ligands are involved in a number of hydrogen bond formations with residues in the immediate vicinity of the active site cavity. Some of the similarities and differences in the sidechain orientation and active site topography of the two isoenzymes are also discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L., Brundell J., Falkbring S. O., Nyman P. O. Carbonic anhydrase from Neisseria sicca, strain 6021. I. Bacterial growth and purification of the enzyme. Biochim Biophys Acta. 1972 Sep 19;284(1):298–310. doi: 10.1016/0005-2744(72)90068-x. [DOI] [PubMed] [Google Scholar]
- Andersson B., Nyman P. O., Strid L. Amino acid sequence of human erythrocyte carbonic anhydrase B. Biochem Biophys Res Commun. 1972 Aug 7;48(3):670–677. doi: 10.1016/0006-291x(72)90400-7. [DOI] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Henriksson D., Nyman P. O. Amino acid sequence of human erythrocyte carbonic anhydrase C. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1388–1394. doi: 10.1016/0006-291x(73)90655-4. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Fridborg K., Bergstén P. C., Liljas A., Lövgren S., Petef M., Strandberg B., Waara I., Adler L., Falkbring S. O. Structure of human carbonic anhydrase B. I. Crystallization and heavy atom modifications. J Mol Biol. 1972 Feb 14;63(3):601–604. doi: 10.1016/0022-2836(72)90452-4. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Liljas A., Waara I., Bergstén P. C., Lövgren S., Strandberg B., Bengtsson U., Carlbom U., Fridborg K., Järup L. Crystal structure of human erythrocyte carbonic anhydrase C. VI. The three-dimensional structure at high resolution in relation to other mammalian carbonic anhydrases. Cold Spring Harb Symp Quant Biol. 1972;36:221–231. doi: 10.1101/sqb.1972.036.01.030. [DOI] [PubMed] [Google Scholar]
- Liljas A., Kannan K. K., Bergstén P. C., Waara I., Fridborg K., Strandberg B., Carlbom U., Järup L., Lövgren S., Petef M. Crystal structure of human carbonic anhydrase C. Nat New Biol. 1972 Feb 2;235(57):131–137. doi: 10.1038/newbio235131a0. [DOI] [PubMed] [Google Scholar]
- Lin K. T., Deutsch H. F. Human carbonic anhydrases. XI. The complete primary structure of carbonic anhydrase B. J Biol Chem. 1973 Mar 25;248(6):1885–1893. [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Meldrum N. U., Roughton F. J. Carbonic anhydrase. Its preparation and properties. J Physiol. 1933 Dec 5;80(2):113–142. doi: 10.1113/jphysiol.1933.sp003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYMAN P. O. Purification and properties of carbonic anhydrase from human erythrocytes. Biochim Biophys Acta. 1961 Sep 2;52:1–12. doi: 10.1016/0006-3002(61)90898-8. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Meany J. E. The catalytic versatility of erythrocyte carbonic anhydrase. II. Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry. 1967 Jan;6(1):239–246. doi: 10.1021/bi00853a037. [DOI] [PubMed] [Google Scholar]
- RIDDIFORD L. M. HYDROGEN ION EQUILIBRIA OF HUMAN CARBONIC ANHYDRASE B. J Biol Chem. 1964 Apr;239:1079–1086. [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Rossman M. G., Liljas A. Letter: Recognition of structural domains in globular proteins. J Mol Biol. 1974 May 5;85(1):177–181. doi: 10.1016/0022-2836(74)90136-3. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER F., LIEFLAENDER M. UBER DIE REAKTION VON CARBONAT-HYDRO-LYASE MIT P-NITROPHENYLACETAT. Hoppe Seylers Z Physiol Chem. 1963;334:279–282. doi: 10.1515/bchm2.1963.334.1.279. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Douglas D. P., Yu Y. S. Esterase and hydrase activity of carbonic anhydrase. I. From primate erythrocytes. Biochem Biophys Res Commun. 1964;14:256–261. doi: 10.1016/0006-291x(64)90445-0. [DOI] [PubMed] [Google Scholar]
- Tilander B., Strandeberg B., Fridborg K. Crystal structure studies on human erythrocyte carbonic anhydrase C. (II). J Mol Biol. 1965 Jul;12(3):740–760. doi: 10.1016/s0022-2836(65)80324-2. [DOI] [PubMed] [Google Scholar]
- Tobin A. J. Carbonic anhydrase from parsley leaves. J Biol Chem. 1970 May 25;245(10):2656–2666. [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Fölsch G., Nyman P. O., Malmström B. G. Inhibition of human erythrocyte carbonic anhydrase B by chloroacetyl sulfonamides with labeling of the active site. J Biol Chem. 1967 Sep 25;242(18):4206–4211. [PubMed] [Google Scholar]
- Whitney P. L., Nyman P. O., Malmström B. G. Inhibition and chemical modifications of human erythrocyte carbonic anhydrase B. J Biol Chem. 1967 Sep 25;242(18):4212–4220. [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Tsernoglou D., Hanson A. W., Knox J. R., Lee B., Richards F. M. The three-dimensional structure of ribonuclease-S. Interpretation of an electron density map at a nominal resolution of 2 A. J Biol Chem. 1970 Jan 25;245(2):305–328. [PubMed] [Google Scholar]


