Abstract
Background:
Glossopharyngeal neuralgia (GN) is a rare condition characterized by severe, paroxysmal episodes of pain mainly localized to the external ear canal, pharynx, and tongue, usually caused by a neurovascular conflict between postero-inferior cerebellar artery (PICA) and IX cranial nerve. Sometimes there is also a compression of X c.n.
Case Description:
We present a case of a 71-year-old female with a 3-year history of intense pain localized in the pharynx and posterior portion of the tongue. Preoperative magnetic resonance imaging (MRI) documented a neurovascular conflict between a loop of PICA and IX left c.n. Surgery was performed through a retrosigmoid craniectomy. The intraoperative findings documented a loop of PICA compressing IX, X, and XI c.n. Microvascular decompression (MVD) of IX c.n. was performed using the interposing technique. No rhizotomy and MVD of the X c.n. was performed. Postoperative course showed the regression of all symptoms.
Conclusions:
The surgical treatment of patients with GN caused by complex neurovascular conflicts can be safely performed with the classical MVD of IX c.n. A double MVD of both IX and X c.n. has a role only in patients presenting symptoms from both nerves. Rhizotomy, in our opinion, has to be avoided in all cases. The authors review the literature concerning GN caused by complex neurovascular conflicts.
Keywords: Glossopharyngeal neuralgia, interposing technique, microvascular decompression, neurovascular conflict
INTRODUCTION
Glossopharyngeal neuralgia (GN) is an uncommon disease characterized by pain and paroxysms along the branches of the ninth cranial nerve (auricular and pharyngeal).[7] This condition can cause throat, ear, and neck pain. Classically, the pain is described as severe, transient, and stabbing and is localized in the ear, base of the tongue, tonsilar fossa, or beneath the angle of the jaw. Unusual presentations are cardiac arrhythmias associated with pain episodes and syncope (related to the compression of X c.n.).[17]
In clinical practice, GN is often misdiagnosed as trigeminal neuralgia, because of similar clinical features. Clusters of unilateral attacks of sharp, stabbing, and shooting pain localized in the throat radiating to the ear or vice versa are characteristic of GN. The distribution of pain is diagnostic: The pain usually starts from the pharynx, tonsil, and posterior tongue base and then rapidly involves the Eustachian tube and inner ear or spreads to the mandibular angle.[17] Swallowing is the most common trigger factor and cold liquids mainly induce the pain.
GN is usually idiopathic, but it can be rarely associated to cerebellopontine angle masses, oropharyngeal tumors, arachnoiditis, stylohyoid ligament ossification, multiple sclerosis and vascular malformations. GN can be isolated or associated to trigeminal neuralgia, or be part of a combined hyperactive dysfunction syndrome.[23]
The idiopathic type of GN is, usually, caused by a compression of the postero-inferior cerebellar artery (PICA) on the glossopharyngeal nerve as it exits or enters the brainstem.
It is important to make a “clinical” differential diagnosis between idiopathic GN and secondary forms due to inflammation and tumors. In the first case, the neuralgic pain is severe, episodic, lancinating, and of short duration, whereas inflammatory or neoplastic pain is more constant, long-lasting, and of deep-seated quality. The “mapping” of the distribution of the pain has to be performed in order to evaluate if other cranial nerves are involved.
Brain Magnetic Resonance Imaging Angiography (angio-MRI) is the diagnostic procedure in idiopathic GN. Three radiological findings are important to make diagnosis of GN due to vascular compression syndrome: High-origin of PICA, the PICA making upward loop, the PICA coursing and compressing the supraolivary fossette.[10]
The treatment of choice of GN caused by a neurovascular conflict is microvascular decompression (MVD). Some authors advocate the possibility to perform a rhizotomy of the IX c.n., although this procedure carries the risk of causing sensorial deficits and remains a second-line therapy. The use of rhizotomy for X c.n., even if still under debate, is not considered as a valid option by many authors because of the high risk of dysphagia and vocal cord paralysis.
CASE REPORT
A 71-year-old female with a 3-year history of intense pain in ingesting solids and liquids was admitted to our Department on October 2013. The patient complained of sudden and violent pain accesses, especially localized at the left side of the pharynx, following the ingestion of solid foods and liquids. These episodes were getting worse in recent months, thus preventing the patient to lead a normal life. Preoperative neurological examination documented a mild left hypoacusia and odynophagia. No symptoms or signs related to compression of the X and XI c.n. were found.
Presurgical MRI examination was performed using a 1.5 T superconductive scanner (Magnetom Siemens Medical Solutions, Erlangen, Germany), gradient strength 26 mT/m, slew rate 200 T/m/ms and head coil. The baseline MRI protocol for neurovascular conflicts included:
Constructive interference in steady-state (CISS-3D) sequence; slice thickness 0.70 mm; two acquisitions;
Magnetic resonance angiography performed by a time-of-flight (TOF-3D); slice thickness 0.88 mm; one acquisition.
The CISS-3D and TOF-3D data were transferred to a commercially available independent workstation (Leonardo Workstation, VD 30B, Siemens, Erlangen, Germany). The data sets were processed by the “3D fusion” function of the Leonardo software. In the CISS-3D/TOF-3D fusion images, the nervous structures were automatically displayed in blue and the arterial vessels in deep red.
Presurgical MRI examination showed a significant anomaly of the PICA course. The vessel from its origin moved cranially [Figure 1] describing a long intracisternal route. A double anomalous contact between PICA and IX, X, and XI nerves was present: At root entry or exit zone (REZ) and along the intracisternal tract of the nerve itself [Figure 2]. This complex neurovascular assessment was well depicted on fusion images [Figure 3].
Figure 1.
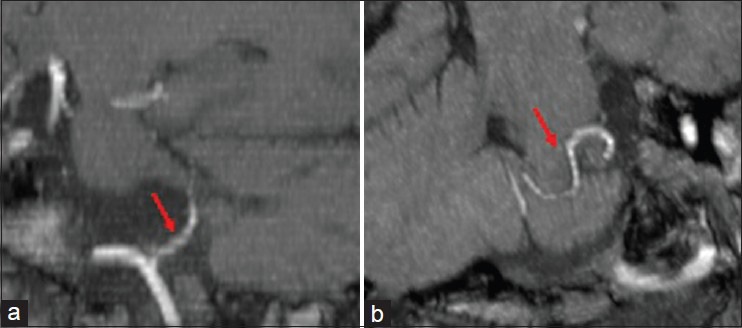
Time-of-flight (TOF-3D) magnetic resonance angiography processed by maximum intensity projection (MIP) on sagittal (a) and oblique (b) reconstruction. Left postero-inferior cerebellar artery (PICA) from its origin moves cranially describing a long intracisternal route (red arrows)
Figure 2.
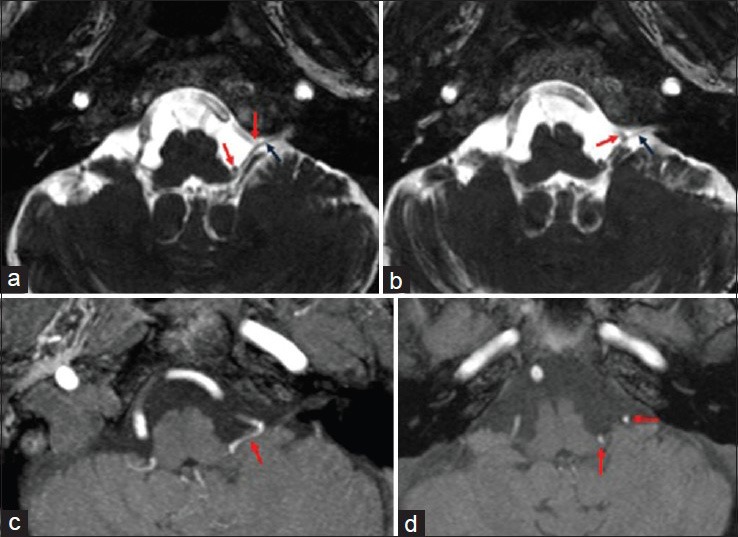
Axial constructive interference in steady-state (CISS-3D) image (a, b). Axial TOF-3D source image (c, d). (a, b) Left PICA (red arrows) impacts IX, X, and XI cranial nerves at root entry or exit zone (REZ) and along the nerves intracisternal tract (black arrows). Vascular and nervous structures are all hypointense. (c, d) Only the tortuous left postero-inferior cerebellar artery (PICA) is visible (red arrows)
Figure 3.
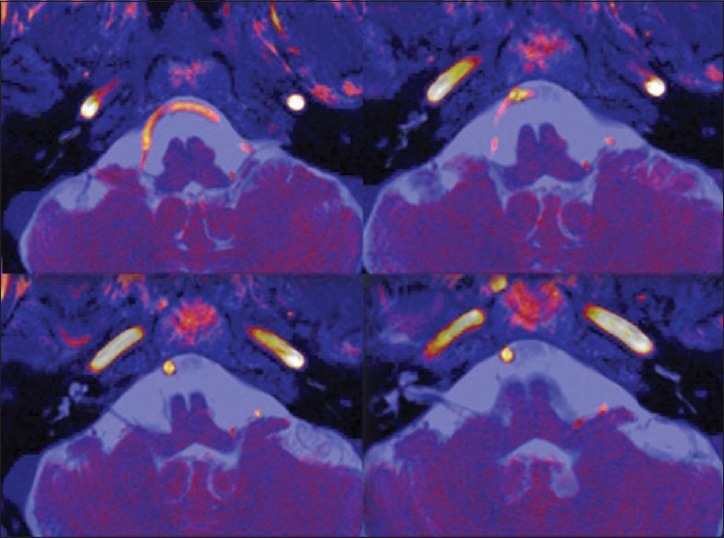
Axial CISS-3D/TOF-3D bidimensional image fusion. Simultaneous display of the offending vessel (red color) and nerves (blue color)
MVD was performed via a left suboccipital retrosigmoid approach. The intraoperative findings showed a loop of the PICA compressing the inferior surface of the IX c.n. and the superior surface of the X and XI c.n. [Figure 4]. This artery had a very twisty course interposed between the ninth and tenth nerve and compressing both nervous structures. After dissection of the arachnoidal adhesions, a fragment of a previously prepared autologous muscle was interposed, thus obtaining an optimal decompression of the IX c.n. Neither MVD nor additional rhizotomy was performed for X c.n. because the patient did not present any related symptoms. For the same reason there was no need to perform an additional rhizotomy. The postoperative course was uneventful. The patient left the hospital on postoperative day 7, with a complete regression of her symptoms. At 7-month follow-up, the patient is still pain-free.
Figure 4.
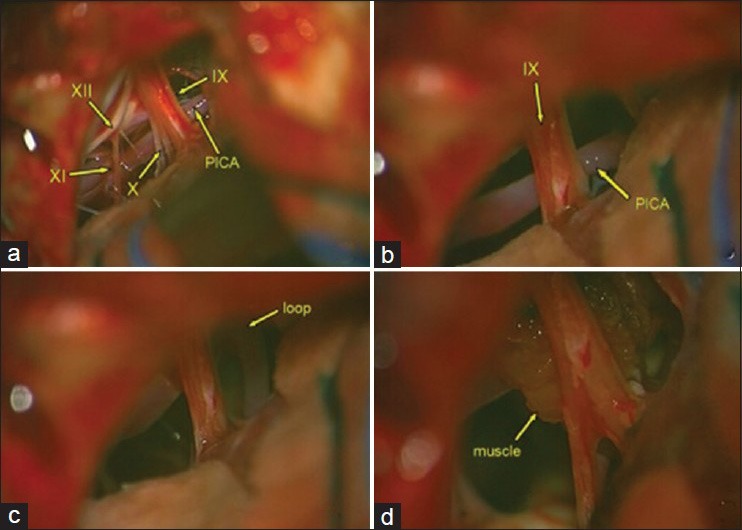
Intraoperative view: (a) Left PICA offending IX, X, and XI nerves in the intracisternal tract (b, c) The PICA and its loop impacting the IX nerve (d) Insertion of autologous muscle
DISCUSSION
GN is an uncommon painful disease, often caused by a neurovascular conflict. In such cases, the medical treatment is generally less effective in controlling pain while surgery is the gold standard procedure. However, old patients with some age-related diseases or carrying a high-risk medical status are not candidates for surgery. In such situations, many authors suggest to perform a peripheral glycerol injection, in order to control pain.[24] Glycerol injection, in the presence of a neurovascular conflict, has to be considered a second-line therapy.
In few cases, GN can be associated to other neurovascular compression syndromes.[9,21] The combination of GN with trigeminal neuralgia and hemifacial spasm is often associated with a looped VBA and a smaller posterior fossa. MVD is still a good choice for the treatment of both syndromes; to achieve a safe and effective outcome, many authors strongly recommend dissection of the caudal cranial nerves and proximal transposition of the vertebral artery, before decompression of the affected nerve roots.[21]
Neurovascular conflict at the level of the root exit zone of cranial nerves IX and X is believed to be the cause of this pain syndrome in most cases. Many authors suggest to perform a vagus nerve rhizotomy for cases in which vascular conflict is not evident.[13] A review of the literature reveals that although the addition of cranial nerve X rhizotomy may improve the chances of long-term pain control, this maneuver also increases the risk of permanent dysphagia and vocal cord paralysis.[13]
For a better evaluation of neurovascular conflicts, the endoscope is a useful adjunctive imaging tool in verifying the adequate nerve decompression.[2,15]
Most authors consider suboccipital retromastoid craniectomy as the best approach to surgically treat those syndromes. However, some authors prefer a midline suboccipital subtonsillar approach.[15] In fact the root entry zone of caudal cranial nerves at the brainstem are rarely visible via the retrosigmoid approach. The use of a midline suboccipital osteoplastic craniotomy together with a continuous electrophysiological monitoring of the glossopharyngeal nerve, can be a useful therapeutic option. Intradurally, an endoscopic-assisted subtonsillar exposure of the lateral recess and the nerve root exit zone of the glossopharyngeal nerve can also be performed.[15]
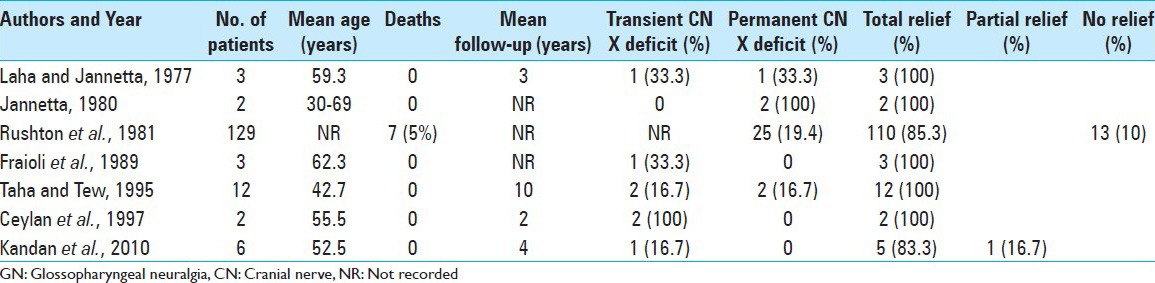
In cases in which an exploratory surgery does not show any conflict, the rhizotomy can be considered as a curative option. In a large series by Rushton et al.[16] and in a smaller series by Taha et al.,[19] there were no pain recurrences after preganglionic section of the IX and upper X c.n. Both studies showed that rhizotomy can be curative in almost all patients with GN. Sectioning of IX and X cranial nerves is followed, in many cases, by severe complications as dysphonia and dysphagia that usually remain persistent.[1,4,5] This is because all neural ablative procedures, including rhizotomy, carry the risk of neuritis and deafferentation pain.[14] In addition, sectioning the upper rootlets of the vagus nerve increases the pharyngeal sensory loss already caused by cranial nerve IX rhizotomy, potentially resulting in paralysis of the ipsilateral vocal cord and motor arc of the gag reflex.[13]
Rushton has reported a series of 217 cases of GN treated with sectioning of the IX c.n. alone or combined with X c.n. (in 129 patients). Pain relief was obtained in 100 of these patients. The most common reported postoperative complication was difficulty in swallowing, which occurred in 25 of the 129 patients.[16]
In a recent review, Rey-Dios and Cohen-Gadol[13] reported a total of 454 patients, from 10 different series, affected by idiopathic GPN who underwent MVD and 157 patients, from 7 different series, who underwent rhizotomy of the upper rootlets of cranial nerve X [Tables 1 and 2]. The rate of long-term pain control for patients who underwent MVD was 84.7%, with recurrence in 7% of patients. Pain control for patients who underwent rhizotomy was 87.3% with pain recurrence in 8.2%. Transient X c.n. dysfunction (dysphagia and/or hoarseness) occurred in 13.2% of MVD cases and in 25% of rhizotomy cases. Permanent X c.n. deficits occurred in 5.5% of MVD operations on average and 19.1% of rhizotomy cohorts.[13] Definitively it appears that rhizotomy leads to slightly better pain control at the expense of higher postoperative permanent cranial nerve dysfunction.
Table 1.
Summary of studies involving MVD of the upper rootlets of cranial nerve X for GN (Rey-Dios and Cohen-Gadol, 2013)
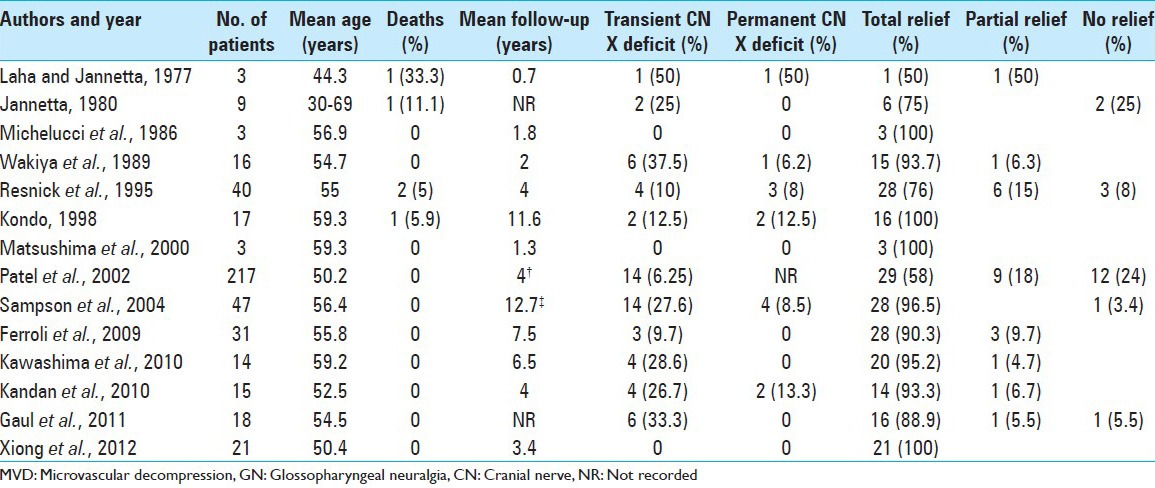
Table 2.
Summary of studies involving rhizotomy of the upper rootlets of cranial nerve X for GN (Rey-Dios and Cohen-Gadol, 2013)
With the improvement of microsurgical, anesthesiological and neurophysiological techniques (brainstem-evoked potentials), MVD has proven to be an effective and safe available treatment and should be considered the first-line treatment in idiopathic GN.[8] In a study by Resnic et al.,[12] MVD provided complete pain relief in 76% of the cases and substantial improvement in a further 16%. Stevens and Onofrio[18] found pain relief of more than 10 years by MVD, hence indicating its efficacy and safety even on long-term follow-up.
Stylectomy done for elongated styloid process has been promising, once the central causes of GPN have been ruled out[6] and an associated styloid enlargement is diagnosed.
Recently, various case reports have been published, which have shown beneficial effects of pulsed radiofrequency neurolysis (PRN) and gamma-knife surgery (GKS). PRN is a neuromodulatory, nonablative method to treat both idiopathic and secondary GN in which short pulses of radiofrequency energy, delivered at a constant temperature, produce central and peripheral neuromodulatory effects.[3,20,22] It might serve as a potential alternative to other percutaneous techniques and surgical options for patients with secondary GN. Also stereotactic radiosurgery (SRS) offers a less-invasive option for patients with GN. Pollock and Boes have reported the largest series (5 patients), with suspected GN being treated with SRS directed at the glossopharyngeal and vagus nerves, within the jugular foramen, with a failure rate of 40%.[11] These new techniques offer a promising direction that might successfully treat patients who are not candidates to surgery. Larger series need to be carried out together with a detailed long-term follow-up in order to compare these new therapeutic procedures to MVD. At the moment, MVD represents the first-line treatment in patients with idiopathic GN, allowing pain control with a low rate of pain recurrence and cranial nerve deficits.
CONCLUSIONS
GN is an uncommon facial pain syndrome often misdiagnosed as trigeminal neuralgia. These aspects often lead to a significant delay in diagnosis. The most important diagnostic examination is brain angio-MRI with specific sequences (CISS and TOF). The use of a software allowing a “fusion” of CISS-3D/TOF-3D images is a useful tool which well depicts the relation between cranial nerves and vascular structures in the cerebello-pontine angle. Microvascular decompression is currently the most effective strategy to treat idiopathic GN. If exploratory surgery does not identify an offending vessel, sectioning cranial nerve IX and the upper rootlets of cranial nerve X is an option. However, this maneuver can lead to dysphagia and vocal cord paralysis and has to be performed only in exceptional cases. In cases where surgery is not possible because of poor conditions of the patients, a peripheral injection of glycerol or SRS are good therapeutic options. We believe that in the typical form of GN caused by a neurovascular conflict offending both IX and X c.n., it is sufficient to perform a MVD in order to decompress the IX c.n., without decompressing also the X c.n. A careful patients selection and a safe operation allows the identification of the site of vascular compression, avoiding cranial nerve X rhizotomy, and, as a consequence, a higher rate of vagus dysfunction. This minimizes the rate of “negative” exploratory operations requiring cranial nerve IX and X rhizotomy.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/19/150810
Contributor Information
Concetta Alafaci, Email: calafaci@unime.it.
Francesca Granata, Email: effegranata@alice.it.
Mariano Cutugno, Email: cutugnomariano@libero.it.
Daniele Marino, Email: marinodott.daniele@gmail.com.
Alfredo Conti, Email: alfredo.conti@unime.it.
Francesco Tomasello, Email: ftomasel@unime.it.
REFERENCES
- 1.Bernard EJ, Nashold BS, Caputi F, Moossy JJ. Nucleus caudalis DREZ lesions for facial pain. Br J Neurosurg. 1987;1:81–92. doi: 10.3109/02688698709034343. [DOI] [PubMed] [Google Scholar]
- 2.Broggi M, Acerbi F, Ferroli P, Tringali G, Schiariti M, Broggi G. Microvascular decompression for neurovascular conflicts in the cerebello-pontine angle: Which role for endoscopy? Acta Neurochir (Wien) 2013;155:1709–16. doi: 10.1007/s00701-013-1824-8. [DOI] [PubMed] [Google Scholar]
- 3.Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in pain management: Mechanisms and potential indications-a review. Acta Neurochir (Wien) 2010;153:763–71. doi: 10.1007/s00701-010-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferroli P, Fioravanti A, Schiariti M, Tringali G, Franzini A, Calbucci F, et al. Microvascular decompression for glossopharyngeal neuralgia: A long-term retrospectic review of the Milan-Bologna experience in 31 consecutive cases. Acta Neurochir (Wien) 2009;151:1245–50. doi: 10.1007/s00701-009-0330-5. [DOI] [PubMed] [Google Scholar]
- 5.Giorgi C, Broggi G. Surgical treatment of glossopharyngeal neuralgia and pain from cancer of the nasopharynx. J Neurosurg. 1984;61:952–5. doi: 10.3171/jns.1984.61.5.0952. [DOI] [PubMed] [Google Scholar]
- 6.Graff-Radford SB, Newman A, Ananda A. Treatment options for glossopharyngeal neuralgia. Therapy. 2005;2:733–7. [Google Scholar]
- 7.International Association for the Study of Pain. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- 8.Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: Long-term outcome following surgical treatment and literature review. Br J Neurosurg. 2010;24:441–6. doi: 10.3109/02688697.2010.487131. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Aida T, Moriwaki T, Yoshino M, Aoki T, Abumiya T, et al. A case of combined glossopharyngeal and trigeminal nevralgia. No Shinkei Geka. 2012;40:533–7. [PubMed] [Google Scholar]
- 10.Kawashima M, Matsushima T, Inoue T, Mineta T, Masuoka J, Hirakawa N. Microvascular decompression for glossopharyngeal neuralgia through the transcondylar fossa (supracondylar transjugular tubercle) approach. Neurosurgery. 2010;66:275–80. doi: 10.1227/01.NEU.0000369662.36524.CF. [DOI] [PubMed] [Google Scholar]
- 11.Pollock BE, Boes CJ. Stereotactic radiosurgery for glossopharyngeal neuralgia: Preliminary report of 5 cases. J Neurosurg. 2011;11:936–9. doi: 10.3171/2011.5.JNS1133. [DOI] [PubMed] [Google Scholar]
- 12.Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–9. doi: 10.1227/00006123-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rey-Dios R, Cohen-Gadol AA. Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery. Neurosurg Focus. 2013;34:E8. doi: 10.3171/2012.12.FOCUS12391. [DOI] [PubMed] [Google Scholar]
- 14.Rohof OJ. Radiofrequency treatment of peripheral nerves. Pain Pract. 2002;2:257–60. doi: 10.1046/j.1533-2500.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- 15.Roser F, Ebner FH, Schuhmann MU, Tatagiba M. Glossopharyngeal neuralgia treated with an endoscopic assisted midline suboccipital subtonsillar approach: Technical note. J Neurol Surg A Cent Eur Neurosurg. 2013;74:318–20. doi: 10.1055/s-0032-1327447. [DOI] [PubMed] [Google Scholar]
- 16.Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (Vagoglossopharyngeal) Neuralgia-A Study of 217 Cases. Arch Neurol. 1981;38:201–5. doi: 10.1001/archneur.1981.00510040027002. [DOI] [PubMed] [Google Scholar]
- 17.Singh PM, Kaur M, Trikha A. An uncommonly common: Glossopharyngeal neuralgia. Ann Indian Acad Neurol. 2013;18:1–8. doi: 10.4103/0972-2327.107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens MK, Onofrio BM. Glossopharyngeal rhizotomy for glossopharyngeal neuralgia. In: Barbaro NM, Larson PS, Starr PA, editors. Neurosurgical Operative Atlas: Functional neurosurgery. Stuttgart, Germany: Thieme Medical Publishers; 2008. pp. 127–30. [Google Scholar]
- 19.Taha JM, Tew JM., Jr Long-term results of treatment of idiopathic neuralgias of the glossopharyngeal and vagal nerves. Neurosurgery. 1995;36:926–31. doi: 10.1227/00006123-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira MJ, de Siqueira SR, Bor-Seng-Shu E. Glossopharyngeal neuralgia: Neurosurgical treatment and differential diagnosis. Acta Neurochir (Wien) 2008;150:471–5. doi: 10.1007/s00701-007-1493-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang YN, Zhong J, Zhu J, Dou NN, Xia L, Visocchi M, et al. Microvascular decompression in patients with coexistent trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Acta Neurochir (Wien) 2014;156:1167–71. doi: 10.1007/s00701-014-2034-8. [DOI] [PubMed] [Google Scholar]
- 22.Williams BJ, Schlesinger D, Sheehan J. Glossopharyngeal neuralgia treated with gamma knife radiosurgery. World Neurosurg. 2010;73:413–7. doi: 10.1016/j.wneu.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Yang KH, Na JH, Kong DS, Park K. Combined hyperactive dysfunction syndrome of the cranial nerves. J Korean Neurosurg Soc. 2009;46:351–4. doi: 10.3340/jkns.2009.46.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue WL, Zhang Y. Treatment of glossopharyngeal neuralgia with peripheral glycerol injection: Our experience in twenty one older patients. Clin Otolaryngol. 2013 doi: 10.1111/coa.12181. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


