Abstract
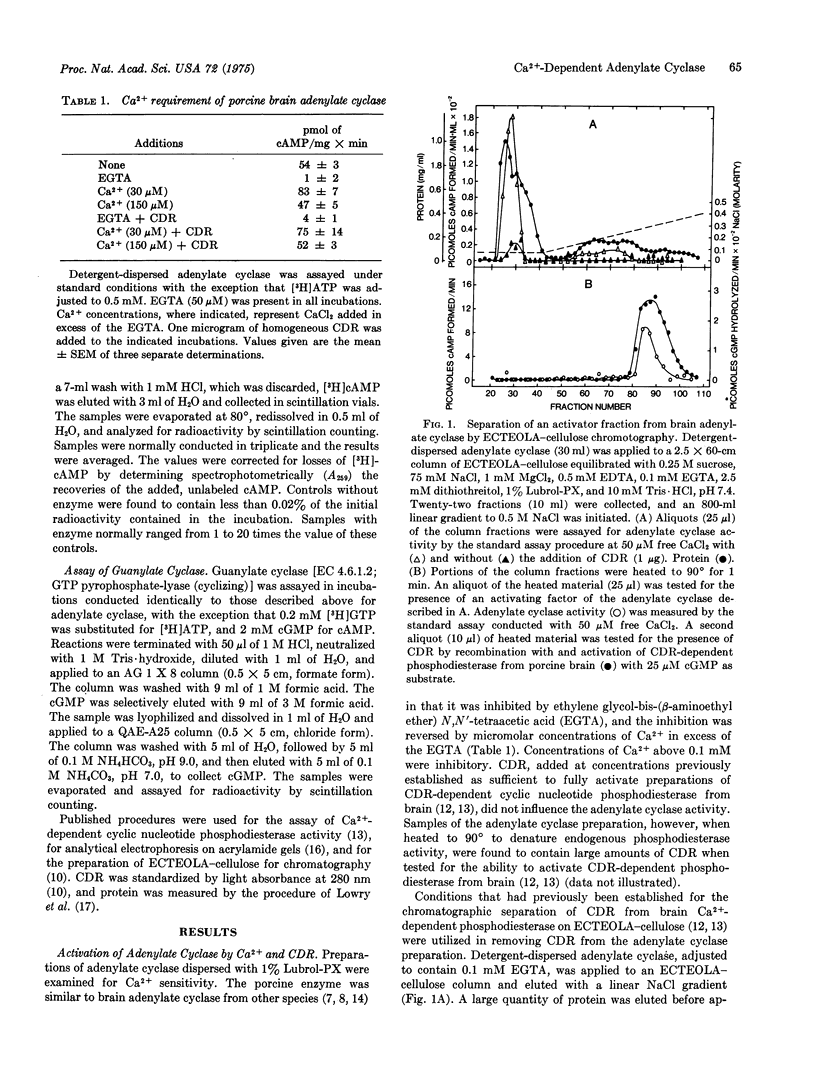
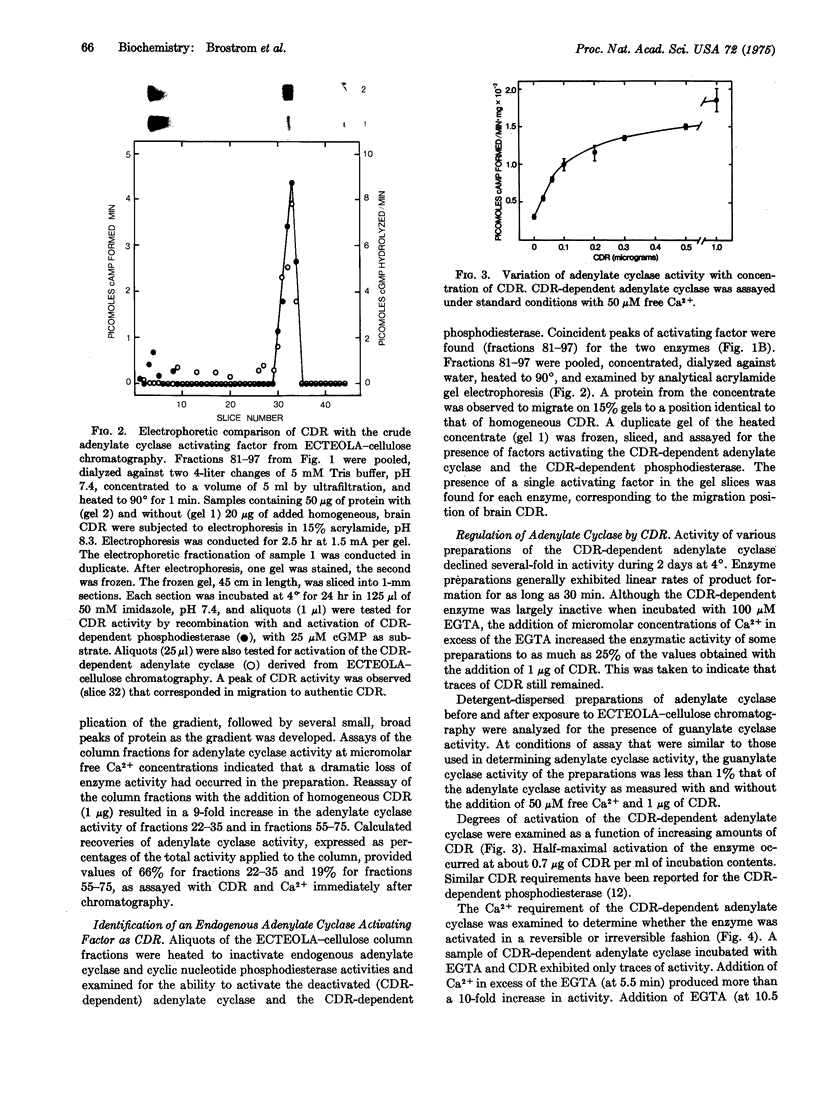
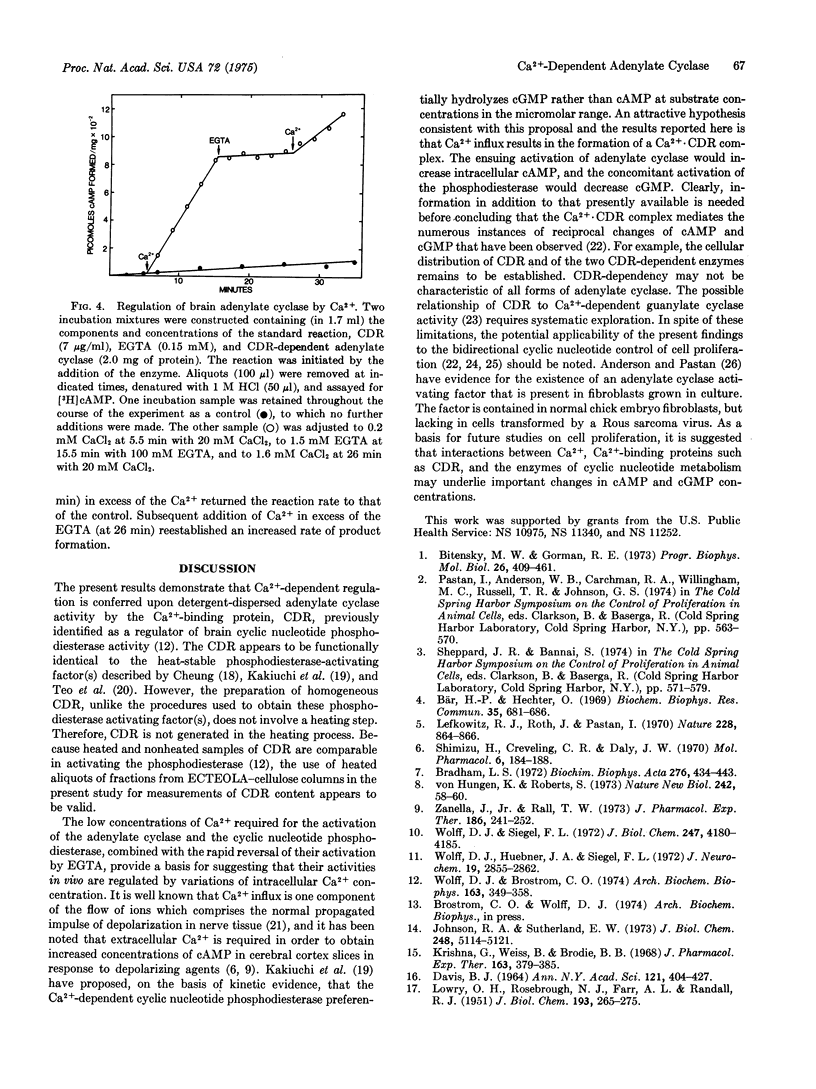
An activating factor of adenylate cyclase (EC 4.6.1.1) HAS BEEN OBTAINED FROM DETERGENT-DISPERSED PREPARATIONS OF PORCINE CEREBRAL CORTEX BY COLUMN CHROMATOGRAPHY ON ECTEOLA-cellulose. The factor was identified by acrylamide gel electrophoresis and by enzyme activation studies as the Ca2+-binding protein that regulates the activity of a brain cyclic nucleotide phosphodiesterase. This Ca2+-binding protein confers a Ca2+-dependent activation upon the adenylate cyclase, which is reversed by the subsequent addition of egta in excess of the free Ca2+. It is proposed that this Ca2+-dependent regulator controls enzymatic activities responsible for the synthesis of adenosine 3':5'-monophosphate and for the hydrolysis of guanosine 3':5'-monophosphate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E. Cellular responses to cyclic AMP. Prog Biophys Mol Biol. 1973;26:409–461. doi: 10.1016/0079-6107(73)90023-0. [DOI] [PubMed] [Google Scholar]
- Bradham L. S. Comparison of the effects of Ca 2+ and Mg 2+ on the adenyl cyclase of beef brain. Biochim Biophys Acta. 1972 Aug 28;276(2):434–443. doi: 10.1016/0005-2744(72)91005-4. [DOI] [PubMed] [Google Scholar]
- Bär H. P., Hechter O. Adenyl cyclase and hormone action. 3. Calcium requirement for ACTH stimulation of adenyl cyclase. Biochem Biophys Res Commun. 1969 Jun 6;35(5):681–686. doi: 10.1016/0006-291x(69)90459-8. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Sutherland E. W. Detergent-dispersed adenylate cyclase from rat brain. Effects of fluoride, cations, and chelators. J Biol Chem. 1973 Jul 25;248(14):5114–5121. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K. Regulation of nucleoside cyclic 3':5'-monophosphate phosphodiesterase activity from rat brain by a modulator and Ca2+. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3526–3530. doi: 10.1073/pnas.70.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pastan I. Effects of calcium on ACTH stimulation of the adrenal: separation of hormone binding from adenyl cyclase activation. Nature. 1970 Nov 28;228(5274):864–866. doi: 10.1038/228864a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Sano M. Studies on guanylate cyclase. A new assay method for guanylate cyclase and properties of the cyclase from rat brain. J Biol Chem. 1974 Jul 10;249(13):4207–4211. [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. W. Cyclic adenosine 3',5'-monophosphate formation in brain slices: stimulation by batrachotoxin, ouabain, veratridine, and potassium ions. Mol Pharmacol. 1970 Mar;6(2):184–188. [PubMed] [Google Scholar]
- Teo T. S., Wang T. H., Wang J. H. Purification and properties of the protein activator of bovine heart cyclic adenosine 3',5'-monophosphate phosphodiesterase. J Biol Chem. 1973 Jan 25;248(2):588–595. [PubMed] [Google Scholar]
- Von Hungen K., Roberts S. Catecholamine and Ca 2+ activation of adenylate cyclase systems in synaptosomal fractions from rat cerebral cortex. Nat New Biol. 1973 Mar 14;242(115):58–60. doi: 10.1038/newbio242058a0. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Calcium-binding phosphoprotein from pig brain: identification as a calcium-dependent regulator of brain cyclic nucleotide phosphodiesterase. Arch Biochem Biophys. 1974 Jul;163(1):349–358. doi: 10.1016/0003-9861(74)90486-x. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Huebner J. A., Siegel F. L. Calcium-binding phosphoprotein of pig brain: effects of cations on the calcium binding. J Neurochem. 1972 Dec;19(12):2855–2862. doi: 10.1111/j.1471-4159.1972.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Siegel F. L. Purification of a calcium-binding phosphoprotein from pig brain. J Biol Chem. 1972 Jul 10;247(13):4180–4185. [PubMed] [Google Scholar]
- Zanella J., Jr, Rall T. W. Evaluation of electrical pulses and elevated levels of potassium ions as stimulants of adenosine 3', 5'-monophosphate (cyclic AMP) accumulation in guinea-pig brain. J Pharmacol Exp Ther. 1973 Aug;186(2):241–252. [PubMed] [Google Scholar]



