Abstract
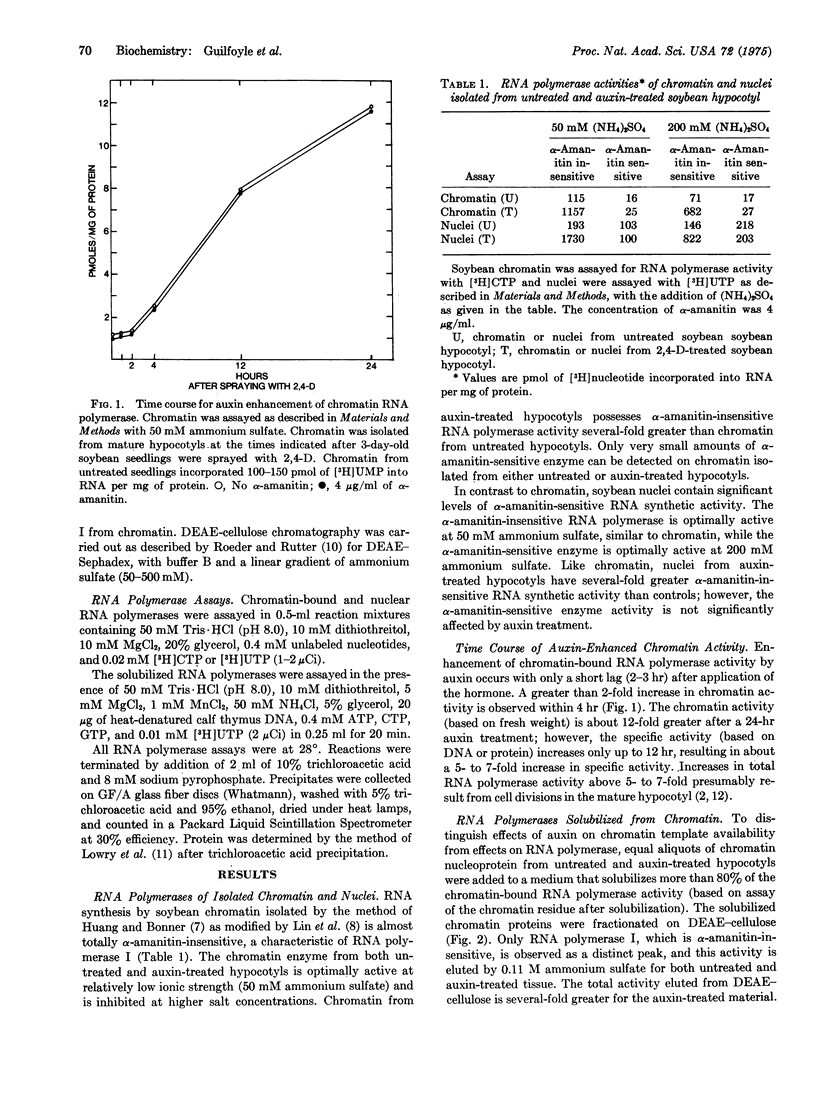
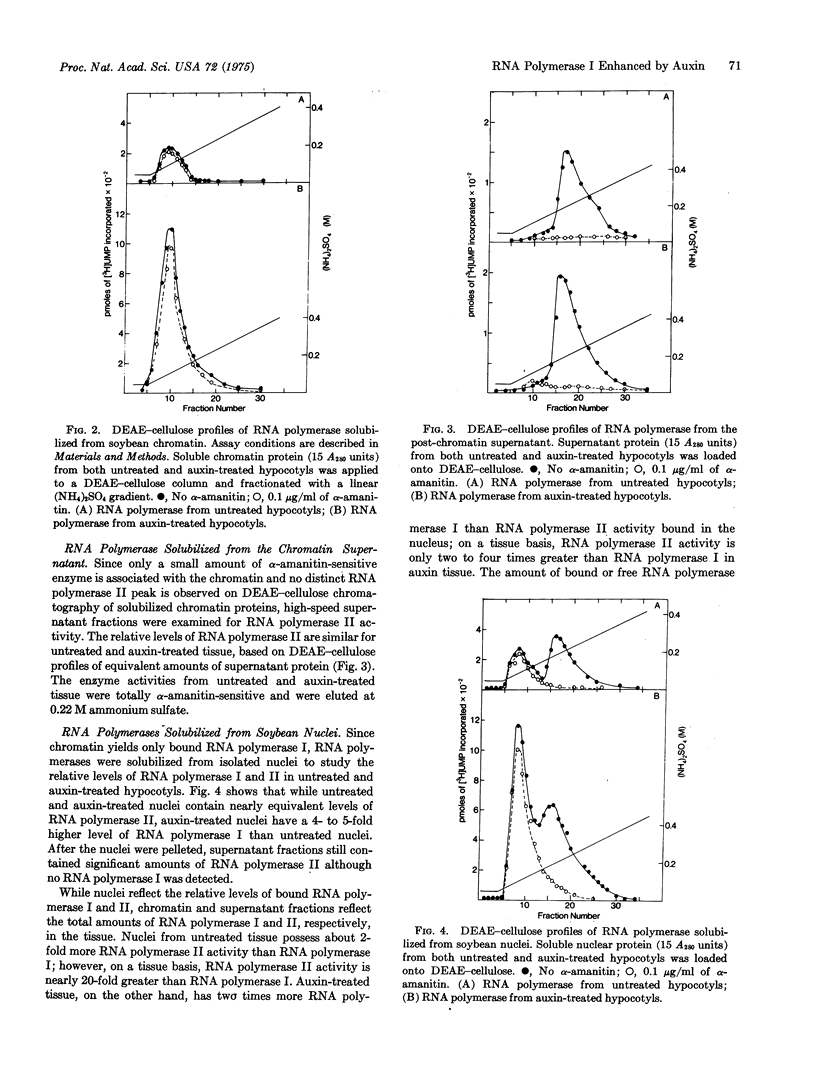
When etiolated soybean seedlings are treated with the synthetic auxin, 2,4-dichlorophenoxy-acetic acid, cells of the mature hypocotyl become swollen and proliferate abnormally. This abnormal growth induced by auxin coincides with a 5- to 8-fold increase in the alpha-amanitin-insensitive RNA polymerase associated with isolated chromatin or nuclei. The alpha-amanitin-sensitive RNA polymerase activity of the auxin-treated hypocotyl was similar to that of control tissue. The increase in RNA polymerase I activity of chromatin and nuclei was maintained after solubilization and fractionation on DEAE-cellulose. Auxin thus appears to enhance RNA synthetic activity (i.e., ribosomal RNA) in mature soybean tissue by altering RNA polymerase I directly rather than by altering RNA polymerase I directly rather than by altering the chromatin template.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J., Gorski J. Uterine ribonucleic acid polymerase. Effect of estrogen on nucleotide incorporation into 3' chain termini. Biochemistry. 1971 Jun 8;10(12):2384–2390. doi: 10.1021/bi00788a032. [DOI] [PubMed] [Google Scholar]
- Beato M., Seifart K. H., Sekeris C. E. The effect of cortisol on the binding of actinomycin D to and on the template activity of isolated rat liver chromatin. Arch Biochem Biophys. 1970 May;138(1):272–284. doi: 10.1016/0003-9861(70)90308-5. [DOI] [PubMed] [Google Scholar]
- Fuhrman S. A., Gill G. N. Hormonal control of adrenal RNA polymerase activities. Endocrinology. 1974 Mar;94(3):691–700. doi: 10.1210/endo-94-3-691. [DOI] [PubMed] [Google Scholar]
- GORSKI J. EARLY ESTROGEN EFFECTS ON THE ACTIVITY OF UTERINE RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Mar;239:889–892. [PubMed] [Google Scholar]
- Guilfoyle T. J., Hanson J. B. Greater Length of Ribonucleic Acid Synthesized by Chromatin-bound Polymerase from Auxin-treated Soybean Hypocotyls. Plant Physiol. 1974 Jan;53(1):110–113. doi: 10.1104/pp.53.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T. J., Hanson J. B. Increased Activity of Chromatin-bound Ribonucleic Acid Polymerase from Soybean Hypocotyl with Spermidine and High Ionic Strength. Plant Physiol. 1973 Jun;51(6):1022–1025. doi: 10.1104/pp.51.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. E., Key J. L. Inhibition of Auxin-induced Deoxyribonucleic Acid Synthesis and Chromatin Activity by 5-Fluorodeoxyuridine in Soybean Hypocotyl. Plant Physiol. 1971 May;47(5):606–608. doi: 10.1104/pp.47.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T. Mammalian RNA polymerases. Prog Nucleic Acid Res Mol Biol. 1973;13:93–126. doi: 10.1016/s0079-6603(08)60101-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Purves W. K. Ribonucleic Acid Synthesis by Cucumber Chromatin: Developmental and Hormone-induced Changes. Plant Physiol. 1970 Oct;46(4):581–585. doi: 10.1104/pp.46.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri M. M., Varner J. E. Enhancement of RNA synthesis in isolated pea nuclei by gibberellic acid. Proc Natl Acad Sci U S A. 1968 Jan;59(1):269–276. doi: 10.1073/pnas.59.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin C. Y., Guilfoyle T. J., Chen Y. M., Nagao R. T., Key J. L. The separation of RNA polymerases I and II achieved by fractionation of plant chromatin. Biochem Biophys Res Commun. 1974 Sep 23;60(2):498–506. doi: 10.1016/0006-291x(74)90268-x. [DOI] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Cortisone stimulation of nucleolar RNA polymerase activity. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2177–2180. doi: 10.1073/pnas.68.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh J. E., Haussler M. R., Lindell T. J. Rapid enhancement of chick intestinal DNA-dependent RNA polymerase II activity by 1 alpha, 25-dihydroxyvitamin D3, in vivo. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2337–2341. doi: 10.1073/pnas.71.6.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]


