Abstract
Biomarkers may facilitate detection of gastric cancer at an earlier stage and reduce mortality. Here we sought to determine if the glycosylation profile of serum immunoglobulin G (IgG) could distinguish patients with non-atrophic gastritis (NAG), duodenal ulcer (DU) and gastric cancer (GC). Serum IgG was released and analyzed using nano-LC-TOF mass spectrometry. Statistically significant false discovery rate (FDR)-adjusted p-values were observed for 18 glycans, eight that differed significantly between NAG and GC, three that distinguished NAG from DU, and eight that differed between DU and GC. The IgG glycosylation signature may be useful as a predictive marker for gastric cancer.
Keywords: Gastric cancer, serum, N-glycan, gastritis, duodenal ulcer, nanoLC-MS
INTRODUCTION
Gastric cancer (GC) is the fourth most common malignancy, but the second most common cause of cancer-related death worldwide [1-3]. In the industrialized countries, the incidence of gastric cancer has diminished dramatically over the last 50 year, but in less developed countries in Eastern Europe, Latin America and Asia, the disease remains a major cause of morbidity and mortality [4]. Pathologically, there are two histological types of gastric cancer: the diffuse- and the intestinal-type (DGC and IGC, respectively) [5]. While DGC is not well characterized, IGC is known to progress through a series of histologic stages starting with gastritis and progressing over decades to atrophy (loss of glands), intestinal metaplasia, dysplasia, and finally adenocarcinoma [6].
Helicobacter pylori is a bacterium that infects the gastric epithelium of approximately 50% of the world’s population, and is designated by the World Health Organization as a Type I (definite) carcinogen. Individuals that are infected with H. pylori nearly always develop a non-atrophic gastritis (NAG), which in itself is largely asymptomatic, but in some cases progresses to gastric cancer. Alternatively, H. pylori infection may also cause duodenal ulcer (DU), but these patients usually do not develop gastric cancer [7], and so it is thought that these individuals develop a host response to H. pylori infection that is different from that in gastric cancer patients.
Mortality from gastric cancer is high because it produces no known specific symptoms in its early stages when it is surgically curable. If gastric cancer is detected at an early stage, the 5-year survival is approximately 90% [8], but most cases present with locally advanced or metastatic disease, which has a median survival of only 24 months and a 5-year survival of less than 15%. Therefore, early detection and preventative strategies are critical to decrease mortality from gastric cancer [3]. The identification of signature molecules for the early detection of gastric cancer would thus be highly valuable. One of the emerging fields for biomarker discovery is protein glycosylation [9]. Several studies have identified altered glycosylation patterns with varying health and disease states, such as liver cirrhosis [10], rheumatoid arthritis [11], pregnancy [12] and aging (e.g.[13-15]), but also various types of cancer (e.g.[16-20]), including gastric cancer [21]. This indicates that protein glycosylation may reflect one’s balanced physiological state, and is affected by most disease states.
The majority of glycomic studies performed to date have focused on the global glycomic analysis of plasma or serum [20], including our resent studies on gastric cancer [21]. These approaches rely on the comprehensive release of glycans from proteins, but ignore any protein correlation. It cannot be determined whether altered glycosylation profiles are due to altered protein concentrations or to differential glycan expression. Both site-specific and protein-specific glycosylation information are lost. Protein-specific glycosylation analysis would provide more insight into the actual changes in glycosylation, and would allow the establishment of hypotheses regarding causes and effects of proteins with altered glycosylation. Immunoglobulin G (IgG) is the most abundant glycoprotein in plasma and serum. It is generally believed that glycans on IgG contribute significantly to the global serum glycome.
The large abundance of IgG and its role as a representative protein of the immune system makes it an ideal protein to examine the role of protein-specific glycosylation in cancer. Several recent studies have focused on observing the relationship between health states and IgG glycosylation. Altered glycosylation patterns of the Fc region of IgG have been associated with several physiological states [22], including autoimmune diseases [11, 23, 24], upon vaccination [25] and with aging [26-28]. However, studies of IgG glycosylation in cancer patients, and thus the role of the humoral immune response in this disease, are scarce. Recently, studies determined altered IgG glycosylation patterns with ovarian [29, 30] and gastric [31] cancer. Clearly altered glycosylation patterns were observed in both studies.
Mass spectrometry is often the method of choice for glycosylation studies and our group recently introduced the use of chip-based nano-liquid chromatography with time of flight mass spectrometry (nLC-chip-TOF-MS) on a porous graphitic carbon stationary phase for the analysis of N-glycans [32, 33]. This method has been shown to provide good separation, good sensitivity and was shown to be highly stable [34]. In this report, we analyzed the glycosylation pattern of IgG, taking both Fab and Fc glycosylation into account, of a sample cohort consisting of non-atrophic gastritis (NAG), duodenal ulcer (DU), intestinal-type gastric cancer (IGC) and diffuse-type gastric cancer (DGC) patients. Glycans that showed altered levels with the different disease states were identified in this pilot study.
MATERIALS AND METHODS
Sample collection
Patients
Human sera were obtained from the Gastroenterology Unit of the Mexico General Hospital, Secretaria de Salud and the Oncology Hospital, Instituto Mexicano del Seguro Social, both in Mexico City, from October 1999 to July 2002. All patients were at least 30 years old and presented for endoscopy because of clinical indications. The protocol was approved by the Research and Ethics Committees of each hospital and informed consent letters were signed by all study participants.
Clinical and Histopathology Diagnosis
Gastric biopsies were obtained systematically from six defined locations in the gastric antrum, corpus, and transitional zone and also from the location of a lesion, if one was identified during endoscopy. Biopsies from each location were formalin fixed, paraffin embedded, and stained with hematoxylin and eosin for histopathologic evaluation and classification according to the updated Sydney system by a single experienced pathologist [35]. Final diagnosis was that of the most severe histologic lesion or based on endoscopy findings in the cases of duodenal ulcer.
Serology
A 5 mL blood sample was drawn from each patient; serum was obtained and frozen at -80° C. Serum samples were tested by ELISA for IgG antibodies against H. pylori whole cell antigens as previously described [36].
Isolation of IgG from Serum
Immunoglobulin G was captured from 5 μL of serum using a Protein G affinity purification step as previously published [37]. Briefly, 5 μL of serum was added to 25 μl Protein G coated beads in 195 μl Dulbecco’s phosphate buffered saline (DPBS) in a 96-well filter plate (Orochem, Lombard, IL). The IgG was allowed to bind at room temperature for 1h while shaking continuously, after which the flow-through was collected using a vacuum manifold. IgG bound to Protein G beads was washed four times using DPBS, followed by two times using micropurified water to remove excess salt. IgG was eluted using 200 μL of 100 mM formic acid in water and collected using a vacuum manifold. The IgG fraction was dried under vacuum and subsequently reconstituted in 100 μL of a 100mM ammonium bicarbonate solution with 5 mM DTT for N-glycan release.
N-glycan release
N-glycan release of IgG fractions was performed as described previously [38], with slight modifications. Briefly, 50 μL of the captured IgG fractions were denatured using six cycles alternating between 100°C and room temperature for 10 seconds each. One μL of PNGaseF (New England Biolabs, Ipswich, MA) was added to the samples, and enzymatic glycan release was performed overnight at 37°C.
N-glycan purification using graphitized carbon SPE
Oligosaccharides released by PNGaseF were purified using 96-well porous graphitic carbon (PGC) filter plates (40 μL PGC, Glygen, Columbia, MD) [16, 33, 39]. Briefly, wells of the SPE plate were conditioned using 2 × 200 μL of 80% acetonitrile (ACN) containing 0.05% trifluoroacetic acid (TFA), followed by 2 × 200 μL of water containing 0.05% TFA. Oligosaccharide samples were diluted using 100 μL of water and subsequently loaded onto the wells. Wells were washed using 4 × 200 μL of water and N-glycans were eluted using 2 × 200 μL of 40% ACN containing 0.05% TFA. All eluates were dried in vacuo prior to analysis.
nHPLC-chip-TOF-MS analysis
N-glycans were analyzed using an Agilent (Santa Clara, CA) 6200 series nanoHPLC-chip-TOF-MS, consisting of an autosampler, which was maintained at 8°C, a capillary loading pump, a nanopump, HPLC-chip-MS interface and an Agilent 6210 TOF mass spectrometer [34]. The microfluidic chip (glycan chip II, Agilent) contained a 9 × 0.075 mm i.d. enrichment column coupled to a 43 × 0.075 mm i.d. analytical column, both packed with 5 μm porous graphitized carbon (PGC). N-glycans from IgG were reconstituted in 50 μL of water and 1 μL of sample was used for injection. Upon injection, the sample was loaded onto the enrichment column using 3% ACN containing 0.1% formic acid (FA, Fluka, St. Louis, MO). After the analytical column was switched in-line, the nano-pump delivered a gradient of 3%ACN with 0.1% FA (solvent A) and 90% ACN with 0.1% FA (solvent B). The mass spectrometer was operated in the positive mode, and ions were scanned over a mass range from m/z 400-3000.
Data processing
Data analysis was performed using Masshunter® qualitative analysis (version B.03.01, Agilent) and Microsoft® Excel® for Mac 2011 (version 14.1.3, Microsoft) [34]. Data was loaded into Masshunter qualitative analysis, and glycan features were identified and integrated using the Molecular Feature Extractor algorithm. First, signals above a signal to noise threshold of 5.0 were considered. Then, signals were deconvoluted using a tolerance of 0.0025 m/z ± 10 ppm. The resulting deconvoluted masses were subsequently annotated using a retrosynthetic theoretical glycan library [40], where a 15 ppm mass error was allowed. Glycan compositions and volume were exported to csv-format for further evaluation.
Statistics
Missing values for individual glycan compositions were filled in on the log scale by using a weighted k-nearest neighbor algorithm [41]. The values for the composition groups (high mannose, etc.) were computed by adding together the appropriate compositions on the raw scale, then converting back to the log scale. The subjects were randomly split into 6 groups, as balanced as possible with respect to diagnosis and age group (30–39, 40–49, 50–59, 60+). In all six groups combined, then with each of the groups successively left out, glycan compositions were tested for significance using a 3-way analysis of variance (ANOVA) with sex, age group, and diagnosis class as the explanatory variables and the logarithmic compositions as responses. To account for multiple testing, the method of Benjamini and Hochberg[42] was applied to control the false discovery rate (FDR) at 10%. A glycan was declared significantly different among the three diagnosis groups if the FDR adjusted p-value of the F-test for inclusion of diagnosis in the model was less than 0.1. Once diagnosis was declared statistically significant, differences among the diagnosis classes were identified by the Tukey-Kramer method [43].
RESULTS
Subject characteristics
A cohort of serum samples from 66 individuals with NAG (n=18), DU (n=17), IGC (n=15) and DGC (n=16) was selected. H. pylori infection is known to nearly always cause gastritis [7]. Because the prevalence of H. pylori infection is very high in Mexico and other Latin American countries [44], and virtually all H. pylori-infected individuals develop gastritis, the NAG group serves as the most appropriate control. Like the other groups, they are infected with H. pylori, but do not develop clinical disease. The seroprevalence of H. pylori was 77% in the cohort studied here (Table 1). Individuals that develop DU are highly unlikely to develop GC, and may therefore create a physiological environment that is protective of GC. Therefore, a group of individuals with DU was included in the study cohort. The groups of NAG and DU were matched for age and sex. Age- and sex- matched samples were not available for the GC group since these patients are predominantly older and of male sex.
Table 1.
Patient demographics and clinical profiles
| Diagnosis | N | Mean Age (range) | Sex
|
H. pylori (+) | |
|---|---|---|---|---|---|
| M | F | ||||
|
| |||||
| Non-Atrophic Gastritis | 18 | 46.1 (30-68) | 10 | 8 | 15 |
|
| |||||
| Duodenal Ulcer | 17 | 53.2 (31-80) | 8 | 9 | 15 |
|
| |||||
| Gastric Cancer | |||||
|
| |||||
| - Intestinal | 15 | 69.3 (48-86) | 12 | 3 | 7 |
|
| |||||
| - Diffuse | 16 | 57.5 (36-81) | 11 | 5 | 14 |
|
| |||||
| Total | 66 | ||||
Glycans detected on IgG
To evaluate the N-glycan profiles originating from immunoglobulin G (IgG, for a schematic overview see Supplementary Figure S1) as a possible biomarker for gastric cancer, IgG was immunopurified from the serum samples. The purity of the obtained IgG fractions was assessed by SDS-PAGE (Supplementary Figure S2) and mass spectrometry. In a tryptic digest of the captured fraction, IgG heavy and light chains were identified with high confidence. Low levels of albumin were also observed, but human albumin is not glycosylated and does therefore not interfere with the analysis. These results indicate that highly pure IgG fractions were obtained and that the observed glycans are from IgG.
Previous mass spectrometry based studies of IgG glycosylation patterns in disease states have been performed using tryptic IgG glycopeptides, which do not readily allow identification of glycans on the variable region of IgG. To get a more complete overview of the IgG glycosylation pattern and its associations with gastric cancer, N-glycans were released from the intact protein. A typical chromatogram obtained from IgG glycans is depicted in Figure S3 in the supplementary information. A total of 48 glycan compositions from IgG could be observed consistently (>70% of the samples) throughout the sample set, and they are listed in Table S4 in the supplementary information. Several glycan species, particularly high mannose- (#27,28,35,36,38 and 48 in Table S4) and hybrid- (#39, 40, 42, 44 and 47 in Table S4) type glycans, have not been found on IgG in previous studies, and are therefore, most likely linked to the Fab portion of the antibody.
This is, to our knowledge, the first report of the total IgG glycosylation profile in human serum, and this study thus allows the determination of the abundance and variability of the individual glycan compositions on IgG. The glycan compositions were ordered according to their average relative abundance in the samples in supplementary Table S4. To evaluate the inter-individual variation in IgG glycosylation, the standard deviation was calculated (Table S4). A relative S.D. of approximately 25% was observed for the higher abundant glycans and a relative S.D. of approximately 50% was observed for lower abundant glycans. The total plasma glycosylation pattern is highly variable with an average relative S.D. of 24% [45], and the results presented here show that the variation in glycosylation on IgG is similar to the overall plasma profile. The increased S.D. observed for lower abundant glycans in this study is most likely a reflection of the analytical variation, which is relatively larger for lower abundant glycans.
IgG glycans as candidate markers for the detection of gastric cancer
To evaluate whether IgG glycans can be used to segregate cases of gastric cancer, we first conducted ANOVA analysis on the glycans grouped according to their structural features: high mannose, truncated, hybrid, biantennary, triantennary, bisected, fucosylated and sialylated glycans. No significant differences were observed between, IGC and DGC (data not shown). Therefore, the two cancer groups were merged into a single GC group, and ANOVA was performed with diagnosis classes NAG, DU and GC. Significant differences were observed among the diagnostic groups for the truncated, biantennary and sialylated glycan groups, with FDR-adjusted p-values of 0.021, 0.017 and 0.039, respectively (Figure 1). Further evaluation using a Tukey test indicated that significantly increased levels of truncated glycans were observed in the GC group compared to the NAG group, while levels of biantennary glycans were decreased in GC compared to NAG. Sialylated glycans were significantly decreased between the DU group and the GC group (Table 2). No significant differences were observed between H. pylori positive and H. pylori negative individuals. These results indicate a different IgG glycosylation profile between the different disease groups and justified further analysis on single glycans.
Figure 1. Glycan groups show altered abundances with gastric cancer.
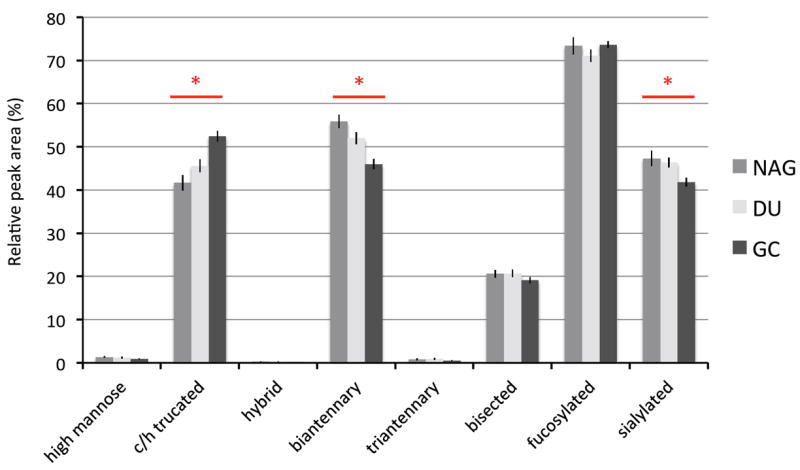
Bars represent the mean (± SEM) relative abundance of the glycan features in each group. Glycan features for a given group do not sum to 100% because there are glycans that have more than a single feature. For example, if a glycan is fucosylated and sialyated, it is counted is both subgroups. Significant differences (denoted by *) were found among the diagnostic groups for truncated, biantennary, and sialyated glycans with FDR-adjusted p-values of 0.021, 0.017 and 0.039, respectively
Table 2.
Tukey test p-values and differences for glycans declared significant by FDR-adjusted p-value.
| Composition | GC VS NAG | DU vs NAG | GC vs DU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Difference | Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Difference | Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
|
| Truncated | 14.8% | 3.3% | 27.6% | 0.0074 | 3.1% | -8.6% | 16.4% | 0.8120 | 11.3% | -0.1% | 24.0% | 0.0514 |
| Biantennary | -13.7% | -21.7% | -4.8% | 0.0019 | -6.4% | -16.3% | 4.7% | 0.3357 | -7.7% | -16.5% | 2.0% | 0.1369 |
| Sialylated | -7.8% | -15.4% | 0.5% | 0.0703 | 1.4% | -8.1% | 11.8% | 0.9411 | -9.0% | -16.7% | -0.7% | 0.0322 |
| H3N3F1 | 29.3% | -0.9% | 68.7% | 0.0603 | 26.0% | -7.0% | 70.6% | 0.1689 | 2.6% | -21.7% | 34.5% | 0.9709 |
| H3N4F1 | 36.7% | 14.4% | 63.3% | 0.0002 | 13.4% | -7.4% | 38.9% | 0.3040 | 20.5% | 0.6% | 44.5% | 0.0416 |
| H3N5 | 31.2% | 0.0% | 72.0% | 0.0495 | 28.5% | -5.7% | 75.0% | 0.1343 | 2.1% | -22.5% | 34.5% | 0.9820 |
| H3N5F1 | 31.9% | 1.8% | 70.9% | 0.0337 | 12.7% | -16.1% | 51.5% | 0.5964 | 17.0% | -10.1% | 52.4% | 0.3317 |
| H4N3F1 | -2.9% | -32.1% | 38.9% | 0.9787 | -39.8% | -60.0% | -9.4% | 0.0114 | 61.2% | 11.9% | 132.1% | 0.0072 |
| H4N4F1 | 3.1% | -11.7% | 20.3% | 0.8854 | -14.9% | -28.6% | 1.6% | 0.0808 | 21.1% | 3.4% | 41.7% | 0.0136 |
| H4N5S1 | 51.8% | -2.4% | 136.2% | 0.0680 | 119.8% | 32.7% | 264.1% | 0.0012 | -30.9% | -56.0% | 8.4% | 0.1273 |
| H4N5F1S1 | 46.3% | 4.9% | 104.0% | 0.0212 | 57.0% | 7.4% | 129.5% | 0.0160 | -6.8% | -33.6% | 30.8% | 0.8714 |
| H5N3 | 156.0% | 8.4% | 504.8% | 0.0289 | 17.7% | -55.9% | 214.0% | 0.9157 | 117.4% | -9.4% | 421.9% | 0.0918 |
| H5N4 | -20.0% | -37.6% | 2.6% | 0.0870 | -0.2% | -24.8% | 32.5% | 0.9999 | -19.8% | -37.7% | 3.2% | 0.0976 |
| H5N4F1 | -23.8% | -42.4% | 0.8% | 0.0582 | -24.7% | -45.2% | 3.7% | 0.0917 | 1.1% | -23.9% | 34.4% | 0.9951 |
| H5N4F1S1 | -16.8% | -29.9% | -1.2% | 0.0333 | -12.2% | -27.8% | 6.9% | 0.2573 | -5.3% | -20.5% | 12.9% | 0.7387 |
| H5N4F1S2 | -24.5% | -42.3% | -1.1% | 0.0392 | -5.6% | -30.5% | 28.4% | 0.8952 | -20.0% | -39.2% | 5.2% | 0.1317 |
| H5N5 | -21.1% | -43.7% | 10.5% | 0.2164 | 11.3% | -24.3% | 63.6% | 0.7831 | -29.1% | -49.8% | -0.1% | 0.0493 |
| H5N5S1 | -29.8% | -52.2% | 2.9% | 0.0752 | 13.1% | -26.9% | 75.2% | 0.7763 | -38.0% | -58.0% | -8.4% | 0.0126 |
| H5N5F1 | -34.9% | -55.1% | -5.5% | 0.0202 | -3.9% | -37.1% | 47.0% | 0.9730 | -32.3% | -53.6% | -1.0% | 0.0429 |
| H6N5S3 | -23.2% | -54.1% | 28.3% | 0.4360 | 59.7% | -11.2% | 187.2% | 0.1421 | -52.0% | -71.5% | -18.9% | 0.0038 |
| H6N5F1S2 | -14.3% | -40.5% | 23.4% | 0.5689 | 47.4% | -2.7% | 123.4% | 0.0722 | -41.8% | -59.9% | -15.7% | 0.0024 |
Three-way ANOVAs were performed on individual glycan compositions. Statistically significant FDR-adjusted p-values were observed for 18 glycans. A Tukey test was performed to further evaluate the significant glycans and the results are depicted in Table 2. There were eight glycans that differed significantly between GC and both NAG and DU, while three differed significantly between NAG and DU, (Figure 2). Of the eight glycans that differed between NAG and GC, levels of five non-galactosylated structures (H3N4F1, H3N5, H3N5F1, H5N3 and H4N5F1S1, where H=number of hexoses, N=hexosamines, F=fucoses, and S=NeuAc sialic acids) were increased in GC, while levels of three fully galactosylated glycans (H5N4F1S1, H5N4F1S2 and H5N5F1) were decreased, indicating a loss of galactoses on the IgG of GC patients. These results are illustrated in Figure 3. Interestingly, one of the significant glycans is a hybrid type glycan that is likely located on the Fab part of IgG as its presence has not been reported using glycopeptide analysis. Further direct analysis is needed to confirm the location of the individual glycans.
Figure 2. Levels of Individual glycans differ with disease state.
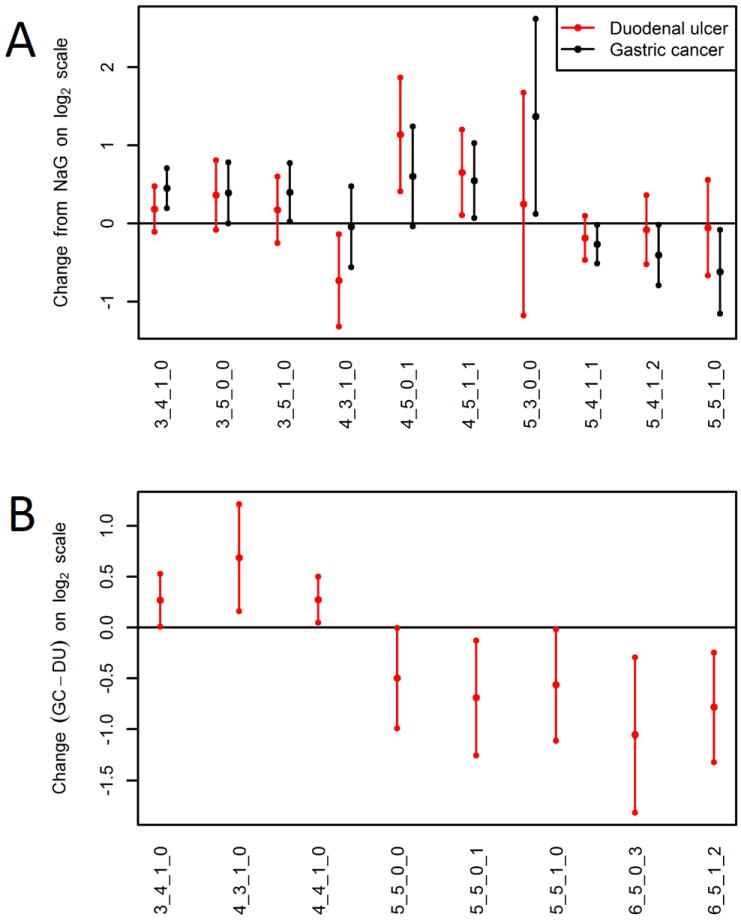
Mean difference as well as 95% confidence interval is displayed for glycans that differ significantly between (A) NAG versus DU (black) and NAG versus GC (grey), and (B) DU versus GC. Levels of glycans are significantly altered when the 95% C.I. does not include 0. Positive values indicate increased levels, while negative values indicate decreased levels of a certain glycan. X-axis labels represent the glycan composition (reading left to right): number of hexoses, N-acetylhexosamines, fucoses, and sialic acids. For example 3_4_1_0 indicates 3 hexoses, 4 N-acetylhexosamines, 1 fucose, and 0 sialic acids.
Figure 3. The altered IgG glycosylation profile that is associated with gastric cancer.
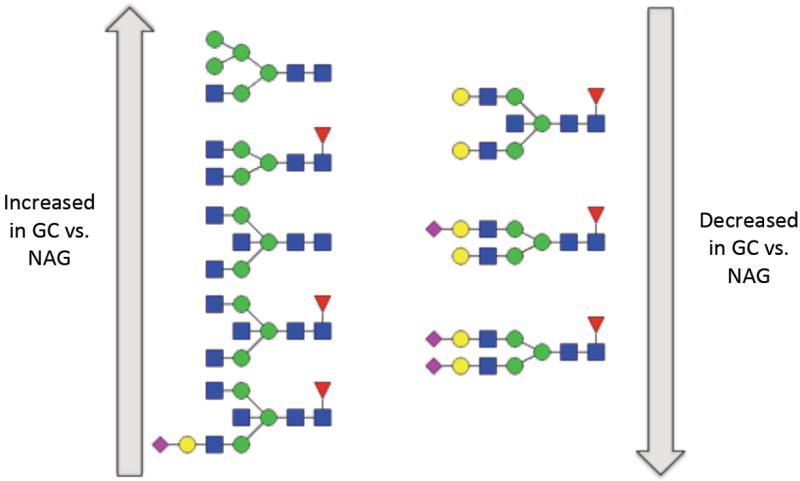
Increasing glycans are shown on the left, while decreasing glycans are shown on the right. Symbol key: blue square: N-acetyl glucosamine, green circle: mannose, yellow circle: galactose, red triangle: fucose and purple diamond: sialic acid.
While the direction of the change was usually similar for both DU and GC relative to NAG, eight glycan compositions altered significantly between DU and GC, of which six were not significant between NAG and GC. Here non- and mono-galactosylated glycans containing a fucose (H3N4F1, H4N3F1 and H4N4F1) were increased in GC, while fully galactosylated glycans with a bisecting GlcNAc and triantennary sialylated glycans were decreased (H5N5, H5N5S1, H5N5F1, H6N5S3 and H6N5F1S2, see Figure 2B). These findings hold a promise for a predictive marker for gastric cancer, which may be able to distinguish between the non-malignant DU condition and GC.
DISCUSSION
Protein-specific glycan biomarkers represent a new paradigm for the diagnosis of cancer. The research presented here provides results regarding the differentiating potential of IgG-glycosylation signatures for the detection of gastric cancer from human blood. In this pilot study it was observed that levels of several truncated glycans, where the galactose is missing on at least one arm, are increased with gastric cancer, while levels of several fully galactosylated bi- and tri- antennary structures were decreased (Table 2). We recently reported the association of increased levels of non-galactosylated biantennary glycans with gastric cancer in total serum in the same cohort [21]. The results reported here differ in that we have now identified glycan profiles specifically on IgG that are associated with gastric cancer. Similar results were also obtained in a previous study [31], which focused only on the analysis of the IgG Fc glycosylation site. Together these results strongly suggest that the altered levels of non-galactosylated biantennary glycans observed in serum is mostly caused by the altered IgG glycosylation signature.
Further validation studies in additional sample cohorts will be needed to evaluate whether the glycosylation pattern of IgG has sufficient predictive power and could indeed serve as a biological marker for GC, whether alone or in combination with other proteins and glycans [46]. It has to be noted that the current studies have focused on detection in individuals that have already developed GC. Such markers would be a tremendous improvement over current diagnostics, but the development of markers for individuals with precancerous lesions, e.g. atrophy, metaplasia and dysplasia would be even more beneficial. Further studies should, therefore, include specimens from these disease classes.
The structure of the IgG molecule is significantly affected by the glycans that are attached to it, and therefore the glycans play an important role in the affinity of the Fc region of the protein for the Fc receptors. Unglycosylated antibodies are severely impacted in their affinity for the Fc receptors and have much lower activities [47], which is likely due to a change in conformation of the IgG molecule. The removal of just the galactose residues off the IgG glycans, as observed in patients with gastric cancer, has also been shown to decrease the activity of antibodies, though to a much lesser extent [48]. Therefore, our findings indicate that the immune response is likely altered in gastric cancer patients.
It is now known that chronic inflammation is one of the characteristics of cancer [49], and multiple studies indicate that several of the carcinogens not only initiate the development of cancerous cells, but also trigger activation of the immune system that over time leads to chronic inflammation and provides the right tumor environment [50]. This effect is also true for H. pylori-induced GC, where the bacterial infection first causes inflammation prior to the development of precancerous lesions and later cancer [6]. It is currently not known whether the inflammatory process mostly plays an important role in the development of cancer, or whether the cancer induces an inflammatory response, or both. Altered IgG glycosylation profiles have been described in autoimmune and inflammatory diseases [11, 23, 51, 52]. Thus, altered IgG glycosylation profiles in gastric cancer may reflect the inflammatory process. However, since the control group in this study consisted of NAG, which is a chronic (albeit asymptomatic) inflammatory condition, it is more likely that the altered glycosylation profile is induced by the cancer. Further research towards the regulation of the altered glycosylation patterns on IgG as well as the specific effects on IgG functionality would provide more insight into the immune response that is associated with cancer in general and more specifically gastric cancer.
Overall, this study shows the human serum IgG glycosylation signature in gastric cancer, duodenal ulcer and non-atrophic gastritis patients. Eight glycans were expressed at altered levels between GC and NAG: truncated glycans were increased with gastric cancer, while fully galactosylated biantennary structures were decreased. When an individual develops DU they are highly unlikely to develop GC and it is speculated that a physiological environment is created that is protective of GC. Interestingly, when comparing DU to GC, similar glycans were significantly increased, while galactosylated glycans with a bisecting GlcNAc and triantennary sialylated glycans were decreased, indicating indeed a different response between DU and GC. The IgG glycosylation signatures that are presented here may provide predictive power for the detection of GC. Further studies are needed to determine their predictive potential as well as their biological background.
Supplementary Material
Highlights.
IgG glycosylation is monitored in serum of gastritis and gastric cancer patients (N=66).
An average variability and IgG glycosylation of 25% was observed, similar to overall serum.
Non-galactosylated glycans are increased on IgG in serum of gastric cancer patients.
A differential glycosylation signature is observed between duodenal ulcer and gastric cancer.
Significance.
Immunoglobulin G glycosylation patterns differ significantly between gastric cancer cases and controls. Therefore, these patterns may provide valuable markers for the detection of gastric cancer.
Acknowledgments
Funding was provided by the National Institutes of Health (R01 CA136647) and by the CONACYT, Mexico (2007-C01-69450). JT is a recipient of an exclusivity scholarship from Fundacion IMSS, Mexico.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2005;54:209–41. doi: 10.1016/j.critrevonc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter-Pylori Infection and the Risk of Gastric-Carcinoma. New Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 8.Ohta H, Noguchi Y, Takagi K, Nishi M, Kajitani T, Kato Y. Early gastric carcinoma with special reference to macroscopic classification. Cancer. 1987;60:1099–106. doi: 10.1002/1097-0142(19870901)60:5<1099::aid-cncr2820600530>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Packer NH, von der Lieth CW, Aoki-Kinoshita KF, Lebrilla CB, Paulson JC, Raman R, et al. Frontiers in glycomics: bioinformatics and biomarkers in disease. Proteomics; An NIH white paper prepared from discussions by the focus groups at a workshop on the NIH campus; Bethesda MD. September 11-13, 2006; 2008. pp. 8–20. [DOI] [PubMed] [Google Scholar]
- 10.Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–34. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 11.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–7. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 12.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruhaak LR, Uh HW, Beekman M, Hokke CH, Westendorp RG, Houwing-Duistermaat J, et al. Plasma protein N-glycan profiles are associated with calendar age, familial longevity and health. J Proteome Res. 2011;10:1667–74. doi: 10.1021/pr1009959. [DOI] [PubMed] [Google Scholar]
- 14.Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M, et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology. 2010;20:959–69. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- 15.Vanhooren V, Desmyter L, Liu XE, Cardelli M, Franceschi C, Federico A, et al. N-glycomic changes in serum proteins during human aging. Rejuvenation Res. 2007;10:521–31a. doi: 10.1089/rej.2007.0556. [DOI] [PubMed] [Google Scholar]
- 16.Hua S, An HJ, Ozcan S, Ro GS, Soares S, DeVere-White R, et al. Comprehensive native glycan profiling with isomer separation and quantitation for the discovery of cancer biomarkers. Analyst. 2011;136:3663–71. doi: 10.1039/c1an15093f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirmiz C, Li BS, An HJ, Clowers BH, Chew HK, Lam KS, et al. A serum glycomics approach to breast cancer biomarkers. Mol Cell Proteomics. 2007;6:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, et al. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–64. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 19.Saldova R, Fan Y, Fitzpatrick JM, Watson RW, Rudd PM. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2011;21:195–205. doi: 10.1093/glycob/cwq147. [DOI] [PubMed] [Google Scholar]
- 20.Ruhaak LR, Miyamoto S, Lebrilla CB. Developments in the identification of glycan biomarkers for the detection of cancer. Mol Cell Proteomics. 2013;12:846–55. doi: 10.1074/mcp.R112.026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan S, Barkauskas DA, Ruhaak LR, Torres J, Cooke CL, An HJ, et al. Serum Glycan Signatures of Gastric Cancer. Cancer Prev Res (Phila) 2014;7:226–35. doi: 10.1158/1940-6207.CAPR-13-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913. doi: 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 23.Selman MHJ, Niks EH, Titulaer MJ, Verschuuren JJGM, Wuhrer M, Deelder AM. IgG Fc N-Glycosylation Changes in Lambed-Eaton Myasthenic Syndrome and Myasthenia Gravis. J Proteome Res. 2011;10:143–52. doi: 10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- 24.Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M, et al. Association between Galactosylation of Immunoglobulin G and Improvement of Rheumatoid Arthritis during Pregnancy Is Independent of Sialylation. J Proteome Res. 2013;12:4522–31. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- 25.Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics. 2012;11:M111 014563. doi: 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj J. 1998;15:683–9. doi: 10.1023/a:1006936431276. [DOI] [PubMed] [Google Scholar]
- 27.Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj J. 1997;14:401–5. doi: 10.1023/a:1018582930906. [DOI] [PubMed] [Google Scholar]
- 28.Ruhaak LR, Uh HW, Beekman M, Koeleman CA, Hokke CH, Westendorp RG, et al. Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. PLoS ONE. 2010;5:e12566. doi: 10.1371/journal.pone.0012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alley WR, Jr, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, et al. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11:2282–300. doi: 10.1021/pr201070k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Y, Wang Y, Zhang X, Zhou L, Zhang Z, Xu J, et al. Quantitative Analysis of Serum IgG Galactosylation Assists Differential Diagnosis of Ovarian Cancer. J Proteome Res. 2013 doi: 10.1021/pr4003992. [DOI] [PubMed] [Google Scholar]
- 31.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 32.Ruhaak LR, Deelder AM, Wuhrer M. Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2009;394:163–74. doi: 10.1007/s00216-009-2664-5. [DOI] [PubMed] [Google Scholar]
- 33.Chu CS, Ninonuevo MR, Clowers BH, Perkins PD, An HJ, Yin H, et al. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9:1939–51. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruhaak LR, Taylor SL, Miyamoto S, Kelly K, Leiserowitz GS, Gandara D, et al. Chip-based nLC-TOF-MS is a highly stable technology for large-scale high-throughput analyses. Anal Bioanal Chem. 2013;405:4953–8. doi: 10.1007/s00216-013-6908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol; International Workshop on the Histopathology of Gastritis, Houston 1994; 1996. pp. 1161–81. [DOI] [PubMed] [Google Scholar]
- 36.Torres J, Leal-Herrera Y, Perez-Perez G, Gomez A, Camorlinga-Ponce M, Cedillo-Rivera R, et al. A community-based seroepidemiologic study of Helicobacter pylori infection in Mexico. J Infect Dis. 1998;178:1089–94. doi: 10.1086/515663. [DOI] [PubMed] [Google Scholar]
- 37.Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, et al. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–81. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 38.Kronewitter SR, de Leoz ML, Peacock KS, McBride KR, An HJ, Miyamoto S, et al. Human serum processing and analysis methods for rapid and reproducible N-glycan mass profiling. J Proteome Res. 2010;9:4952–9. doi: 10.1021/pr100202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packer NH, Lawson MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj J. 1998;15:737–47. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- 40.Kronewitter SR, An HJ, de Leoz ML, Lebrilla CB, Miyamoto S, Leiserowitz GS. The development of retrosynthetic glycan libraries to profile and classify the human serum N-linked glycome. Proteomics. 2009;9:2986–94. doi: 10.1002/pmic.200800760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hrydziuszko O, Viant MR. Missing values in mass spectrometry based metabolomics: an undervalued step in the data processing pipeline. Metabolomics. 2011;8:161–74. [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 43.Miller R. Simultaneous Statistical Inference. Springer-Verlag; 1981. [Google Scholar]
- 44.Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701) Cancer Causes Control. 2013;24:209–15. doi: 10.1007/s10552-012-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, et al. Variability, Heritability and Environmental Determinants of Human Plasma N-Glycome. J Proteome Res. 2009;8:694–701. doi: 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- 46.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 47.Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989;259:347–53. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumpel BM, Rademacher TW, Rook GA, Williams PJ, Wilson IB. Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Hum Antibodies Hybridomas. 1994;5:143–51. [PubMed] [Google Scholar]
- 49.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1:966–9. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- 52.Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19:381–9. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


