Abstract
The metabotropic glutamate (mGlu) receptors and, in particular, mGlu5 are crucially involved in multiple forms of synaptic plasticity that are believed to underlie explicit memory. MGlu5 is also required for information transfer through neuronal oscillations and for spatial memory. Furthermore, mGlu5 is involved in extinction of implicit forms of learning. This places this receptor in a unique position with regard to information encoding. Here, we explored the role of this receptor in context-dependent extinction learning under constant, or changed, contextual conditions. Animals were trained over 3 days to take a left turn under 25% reward probability in a T-maze with a distinct floor pattern (Context A). On Day 4, they experienced either a floor pattern change (Context B) or the same floor pattern (Context A) in the absence of reward. After acquisition of the task, the animals were returned to the maze once more on Day 5 (Context A, no reward). Treatment with the mGlu5 antagonist, 2-methyl-6-(phenylethynyl) pyridine, before maze exposure on Day 4 completely inhibited extinction learning in the AAA paradigm but had no effect in the ABA paradigm. A subsequent return to the original context (A, on Day 5) revealed successful extinction in the AAA paradigm, but impairment of extinction in the ABA paradigm. These data support that although extinction learning in a new context is unaffected by mGlu5 antagonism, extinction of the consolidated context is impaired. This suggests that mGlu5 is intrinsically involved in enabling learning that once-relevant information is no longer valid. © 2014 The Authors. Hippocampus Published by Wiley Periodicals, Inc.
Keywords: extinction, hippocampus, MPEP, rat, spatial learning
Introduction
The Group I metabotropic glutamate (mGlu) receptor, mGlu5, plays a pivotal role in multiple aspects of hippocampal function: it mediates hippocampus-dependent short- and long-term spatial memory (Balschun and Wetzel, 2002; Naie and Manahan-Vaughan, 2004; Balschun et al., 2006) and is a key factor in diseases that affect cognition and memory such as fragile X syndrome (Dölen and Bear, 2008). Accordingly, dendritic protein synthesis is triggered by mGlu5 (Huber et al., 2001), suggesting that it is intrinsically involved in synaptic restructuring that underlies long-term memory. On a physiological level, mGlu5 enables long-term stability of place fields (Zhang and Manahan-Vaughan, 2014), learning-facilitated synaptic plasticity (Popkirov and Manahan-Vaughan, 2011), long-term potentiation (LTP) (Naie and Manahan-Vaughan, 2004), and long-term depression (LTD) (Popkirov and Manahan-Vaughan, 2011). It also mediates hippocampal neuronal oscillations that enable information transfer within the hippocampus (Bikbaev et al., 2008). However, the involvement of mGlu5 in memory-related processes is not restricted to explicit learning and memory: mGlu5 is also intrinsically involved in the acquisition of conditioned fear (Handford et al., 2014), conditioned reinforcement (O'Connor et al., 2010), and conditional emotional responses (George et al., 2009).
Aside from the explicit necessity to learn and retain new experiences and information, a key element to efficient cognitive functioning and survival is the ability to learn that information that was once relevant is no longer so. In effect, the original memory, or moreover the behavior that was associated with this memory must be suppressed, a process known as extinction learning. In terms of extinction of implicit memory, it has been reported that transgenic mice that lack mGlu5 exhibit impaired extinction of conditioned place preference to cocaine (Bird et al., 2014) and impaired extinction of operant responses (Chesworth et al., 2013). Furthermore, mGlu5 antagonists impair extinction of conditioned taste aversion (Simonyi et al., 2009).
Within the hippocampus, the N-methyl-d-aspartate receptor (NMDAR) (Morgado-Bernal, 2011) and mGlu5 receptor (Mukherjee and Manahan-Vaughan, 2013) comprise pivotal molecular components for the encoding of learning and memory, and a role for the hippocampus in extinction has been reported. Thus, the hippocampus is involved in the processing and retrieval of context both during extinction learning and recall of extinction in a fear context (Hobin et al., 2006), in appetitive extinction learning (Jarrard et al., 1986; Good and Honey, 1991; Chan et al., 2003), and under conditions of associative learning (Lissek et al., 2013). Hippocampal activity in extinction may be context-dependent (Kalisch et al., 2006), and the hippocampus may be specifically involved in the encoding of an association between a context, a conditioned stimulus (CS), and an unconditioned stimulus (US) (Alvarez et al., 2008; Lang et al., 2009). The hippocampus mediates object-context information processing both in humans (Sadeh et al., 2012) and rodents (Goh and Manahan-Vaughan, 2013a,b). It is likely that the hippocampus mediates both context-specific components to learning about the relationship of the CS and the US and may mediate context-dependent extinction learning and renewal (Corcoran et al., 2005; Ji and Maren, 2005, 2008; Koseki et al., 2009; Fujiwara et al., 2012; Lengersdorf et al., 2014; Wiescholleck et al., 2014).
MGlu5 contributes to context-specific spatial learning (Goh and Manahan-Vaughan, 2013b). Here, we examined the role of mGlu5 in context-dependent extinction learning when the context remains constant, or when extinction is facilitated by a context change. We observed that antagonism of mGlu5 prevents extinction of the learned context, although it does not affect the facilitation of extinction that occurs owing to a context change. This suggests that mGlu5 is intrinsically involved in information modification when the hippocampus registers that previously salient information is no longer relevant. Under these circumstances, changing the context elicits a transient suppression of the learned behavior but has no long-term influence on extinction learning. This indicates that modification of learned and consolidated information through extinction learning critically depends on mGlu5.
Materials and Methods
This study was carried out in accordance with the European Communities Council Directive of September 22, 2010 (2010/63/EU) for care of laboratory animals. All experiments were performed according to the guidelines of the German Animal Protection Law and were approved by the North Rhine-Westphalia State Authority (Bezirksamt, Arnsberg). All efforts were made to reduce the number of animals used.
Male Wistar rats (7–8 weeks old) were housed in groups of four and maintained on a 12-h light/12-h dark cycle. For the extinction task the animals were given sufficient food to maintain 85% of their free-feeding weight and ad libitum access to water. They were handled individually for 20 min/ day for 2 days before the behavioral tests started. Each group consisted of 13–15 rats.
Extinction Task
Extinction experiments were conducted in a T-maze that was composed of a starting box (25 cm × 20 cm) that was separated from the main corridor (100 cm × 20 cm) by a sliding door and two side corridors (40 cm × 10 cm) positioned perpendicular to the other end of the main corridor (Fig. 1a). The walls were 40 cm high. In each side corridor, 1 cm in front of the end wall and in the middle of the floor, a small round cup was placed, where a reward could be hidden.
FIGURE 1.
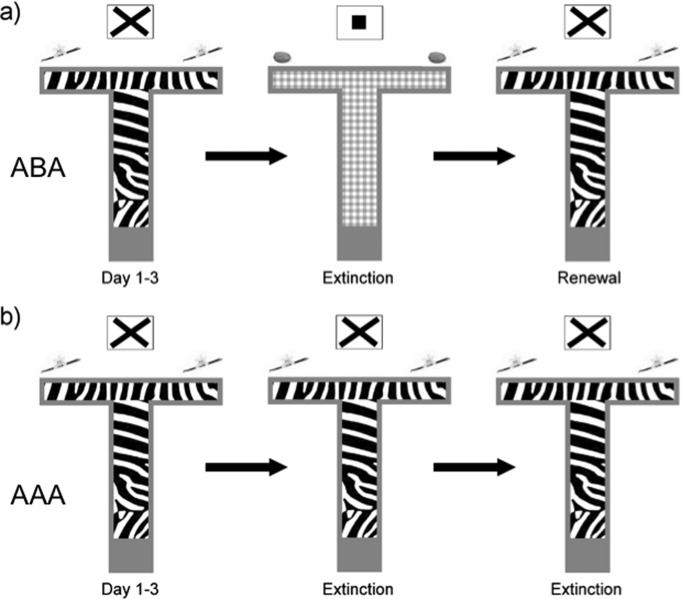
Paradigm for context-dependent extinction in the T-maze. Animals were trained to choose a specific goal arm (e.g., left) in a T-maze that contained both visuospatial and olfactospatial cues. Specifically the floor of the maze contained a specific pattern, extramural cues were present, and the goal arms each contained a faint odor (e.g., vanilla) at the ends of the arms. In the “AAA” paradigm these contextual conditions were kept constant throughout. In the “ABA” paradigm″ extinction learning was assessed in a context that contained a different floor pattern, different extramural cues, and a different odor in the goal arms. Animals underwent 3 days of acquisition comprising of 20 trials per day (D1–3). By Day 3, reward probability for correct arm choices was reduced to 25%. A correct choice level of 80% in the last 10 trials on Day 3 was deemed as the criterion for successful acquisition of the task. On Day 4 (D4) animals were either returned to the context in the absence of any reward (AAA paradigm) or were exposed to a new context (ABA paradigm, illustrated here, also unrewarded). On Day 5 (D5), animals were returned to the A context in the absence of reward. Here, renewal was expected in the first 10 trials, followed by extinction as the animals realized that no reward will be received.
The context of the maze was changeable by three aspects (Fig. 1a): (1) exchanging the plastic floor of the maze—the floors had distinct visual patterns such as zebra stripes, checkered patterns, or geometric lines; (2) exchanging the odors—1 µl of almond or vanilla (food aroma, Dr. Oetker, Bielefeld, Germany) at the end of the two arms; and (3) exchanging the extra-maze cue cards (Din A5 white paper with a black cross or a black-filled square) that were positioned 40 cm above the end of the main corridor (Fig. 1). To determine the influence of contextual cues on extinction, we tested one group in an AAA design, in which training and all of the extinction sessions were conducted in the same context. The second group was tested in an ABA renewal design, in which training was conducted in Context A while the extinction session was conducted in Context B. The final extinction session was conducted again in the training Context A to examine renewal of appetitive responses.
Two days before the beginning of the behavioral training, the rats were weighed and food availability was reduced to achieve 85% of the previously determined body weight. This weight was sustained until the end of the experiment.
Every day, each rat underwent a learning session consisting of 20 consecutive trials. The trial began when the door to the starting box was opened and the animal could enter the maze. It ended when the animal entered an arm of the T-maze or when 30 s had elapsed without arm entry. In each trial, the animals were expected to search for a food pellet placed at the end of a predetermined arm. From Day 1 through 3 reward probability was decreased stepwise from 100 to 25% to increase extinction resistance. Otherwise, testing contextual changes during repeated extinction trials would not have been possible. Concomitantly, the time allowed to reach the arm was decreased in a stepwise manner from 2 min to 30 s. Learning criterion was reached when the animal successfully entered the correct arm on eight of the last 10 trials on the first day. Between each trial, the maze was wiped with a wet sponge to mix the odor trail that the animal could have left behind. Between animals, the maze was cleaned with alcohol, rinsed with water, and dried. On Day 4, extinction learning was evaluated. Here, the rats were introduced in the T-maze for 20 trials during which the context (floor, odor, and cue card) was changed and no reward was present in any arm. On the last Day 5, renewal (RN) was assessed. Here, the animal was reintroduced to the original T-maze context for 20 trials with no food rewards.
Analysis of Decision Time
As the confidence of the animal increases during the gradual acquisition of the T-maze task, the time taken to make a decision which arm to choose can be expected to become reduced (Luce, 1986; Avila and Lin, 2014). We assessed this by monitoring the time taken by the animal to move from the departure area in the T-maze to its arm of choice. We assessed this for every choice (not just correct choices). This was used to give us a measure of the confidence of the animal that it knew which arm to go to.
Drug Treatment
The negative allosteric mGlu5 modulator 2-methyl-6-(phenylethynyl) pyridine (MPEP; Tocris) was dissolved in 0.9% NaCl. MPEP or vehicle was injected intraperitoneally (i.p.) according to body weight (10 mg/kg or an equivalent volume of vehicle: 10 ml/kg). MPEP or vehicle (0.9% NaCl) were given 30 min before commencement of the extinction session on Day 4 to ensure adequate time for the drug to reach the brain and to observe for any effect of the injection procedure.
Data Analysis
Correct answers were defined as trials in which the animals went first to the target arm. For analysis purpose, each 20-trial session was divided into 2 × 10 trials (first 10 and last 10 trials) in order to allow a better insight into progression of learning, extinction, and renewal. The time needed to reach the end of the first arm visited was calculated for each trial. For analysis purpose, each session was divided into four sets of five trials of which the times were averaged. Data were analyzed using analysis of variance (ANOVA) with repeated-measures including two within-subject factors (Day and Session) and two between-group factors (Treatment and Experimental Design) to assess for differences between control and MPEP-treated animals. Differences between trial blocks or between trials days of a specific group (control or MPEP-treated animals) were assessed using Bonferroni post hoc tests. Except where “ANOVA” is mentioned explicitly, all P values in the Results section correspond to values determined from the Bonferroni test. The level of significance was set at P < 0.05.
Results
Significant Extinction Learning Occurs in a T-Maze Task, Using an AAA Paradigm
Extinction learning and extinction retrieval were tested in a context-dependent T-maze paradigm (Fig. 1). During the first 3 days animals were trained to take a constant turn (e.g., left) in a T-maze that contained a specific floor pattern. Two sets of contiguous 10 trials were conducted per day. Reward probability was systematically reduced in the first 3 training days. By Day 3 the reward probability was 25% and animals were expected to reach the criterion of 80% correct arm choices. A significant difference in performance was evident between Day 1 and Day 2, reflecting successful acquisition of the task (P < 0.001). No significant difference was evident in performance within the first and second 10 trial block on Day 3. At this point correct choice performance was close to 100% (Fig. 2).
FIGURE 2.
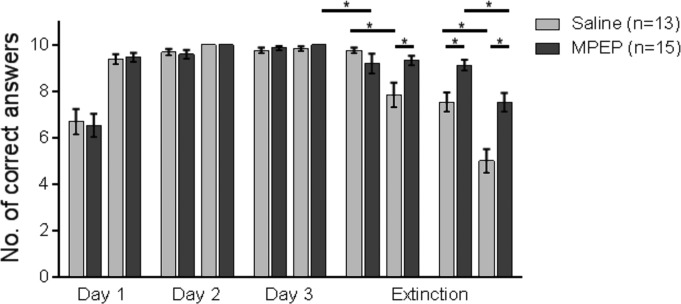
Antagonism of mGlu5 prevents extinction in the AAA paradigm. Animals underwent 20 contiguous trials per day of training in the AAA paradigm. Bar charts represent the percentage of correct arm choices in the first and second set of 10 trials on each test day. Animals participated in 3 days of acquisition training in the AAA paradigm, ending on Day 3 with a 25% reward probability. Control animals were treated with vehicle before re-exposure to the context on Day 4, in the absence of reward. Here, by the second set of 10 trials no significant extinction was evident. Upon being returned to the same context on Day 5 (without reward) an initial recovery of the learned CS–US response was evident in the first set of 10 trials that was followed by significant extinction of the CS–US response. Treatment of animals with the mGlu5 antagonist, MPEP, before re-exposure to the A context in the absence of reward on Day 4 significantly impaired extinction learning. A return to the same context on Day 5 resulted in no significant difference in the level of correct choices (in the first 10 trials) compared to the last 10 trials on Day 4, but under these circumstances significant extinction became evident by the second set of trials. An asterisk indicates a significant effect of at least P < 0.01 between the trials indicated by the bar.
On Days 4 and 5 the animals were returned to the same context but received no reward. There was no difference between performance levels in the last 10 trials of Day 3 and the first 10 trials of the extinction day (Day 4) (P = 0.89). But performance was significantly poorer, when the second 10 trials of Day 3 were compared to the last 10 trials on the first extinction day (P < 0.001). One day later animals were once more returned to the same context in the absence of reward. Here, performance declined further: although performance in the first 10 trials was equivalent to the (last 10 trials of the) day before (P = 0.641), by the second set of trials performance was significantly poorer (P < 0.001) (Fig. 2).
Thus, in control animals significant extinction occurred in this AAA paradigm: whereby A signifies the context and “AAA” signifies the A context on Days 1–3, Day 4, and Day 5.
Extinction in a T-Maze Task, Using an AAA Paradigm, Is Prevented by Antagonism of mGlu5
To assess the effects of mGlu5 receptor antagonism on extinction, animals were treated with MPEP before the extinction trials on Day 4, and performance was compared to animals that received vehicle on Day 4. Before this was done, we assessed that learning performance and behavior of the two animal groups was equivalent on the first 3 days of the experiment. Here, we confirmed that significant learning occurred in the animal groups between Day 1 and Day 2 (P < 0.001). No significant difference in the animals' performance was evident on Days 1, 2, or 3 when the two cohorts were compared [ANOVA: F(1.141, 29.666) = 0.131; P = 0.754].
In contrast to control animals, we observed that when animals were treated with MPEP (10 mg/kg, i.p.) before exposure to the “A” context on Day 4, no significant decline in correct choice performance was evident within the MPEP group (comparison of last 10 trials on Day 3 with first 10 trials of Day 4: P = 0.461; first 10 trials of Day 4 versus last 10 trials of Day 4, P = 0.07). Thus, extinction was impaired by prior treatment with an mGlu5 antagonist.
When we compared the performance of the vehicle-treated animals and the MPEP-treated animals during extinction learning (Day 4, first and second trial blocks) a significant difference was also evident [ANOVA: F(1, 26) = 11.843; P = 0.002], whereby extinction in the second trial block was poorer in MPEP-treated animals compared to controls (P < 0.05).
One day later (Day 5), when animals were returned to the same context in the absence of reward, and presumably little MPEP remained bound to mGlu5 receptors (Walker et al., 2001), an extinction effect became apparent (comparison of last 10 trials on Day 4 with first 10 trials of Day 4: P < 0.01; first 10 trials of Day 5 versus last 10 trials of Day 5: P < 0.001). In contrast to the untreated group, the MPEP group showed a renewal effect during the first trial block on Day 5 (Fig. 2) (P < 0.01). The extinction effect was significantly poorer than that seen in controls however, as indicated by the data obtained in the second trial block on Day 5 (Fig. 2) (P < 0.001). The performance on Day 5 was different when the control and MPEP animals were compared [ANOVA: F(1, 26) = 20.495; P < 0.001, first and second trial blocks]. In addition, a significant difference in overall performance (Days 1–5) was found when the untreated animals were compared to the MPEP-treated animals [ANOVA: F(2.828, 73.53) = 7.493; P < 0.001].
A Context Change Reveals Improved Extinction in a T-Maze Task, Using an ABA Paradigm
Extinction of, for example, fear memory, is typically facilitated by a context change (Bouton, 2004). Here, we explored if a context change in the T-maze paradigm also facilitates extinction. The protocol was identical to that described above, with the only difference being that on Day 4 the floor pattern was changed, as were the odor reinforcements and extramural cues (B context, no reward). On Day 5, the animals were returned to the “A” context that they had experienced on Days 1–3, except here no reward was given.
In control animals, significant extinction occurred (on Day 4) under these conditions, which was significantly better than extinction effects in the AAA paradigm (Fig. 3) (P = 0.046). Performance in the second 10 trials on Day 4 was significantly poorer than in the first 10 trials (P > 0.001) in line with the occurrence of significant extinction. Re-exposure to Context A on Day 5 (unrewarded) revealed significant retrieval effects in the first 10 trials compared to the last 10 trials of Day 4 (P < 0.001). Extinction of this retrieval effect became evident during the second set of 10 trials (first versus second 10 trials, Day 5: P < 0.001).
FIGURE 3.
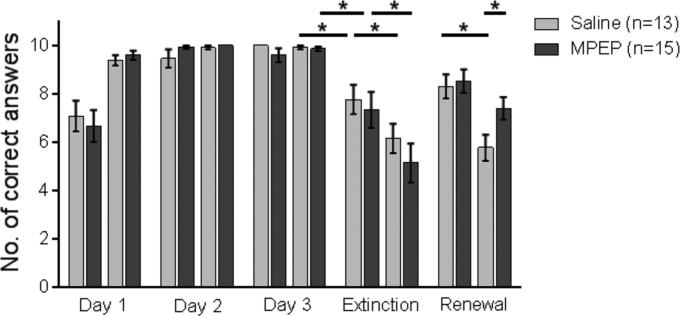
Antagonism of mGlu5 selectively prevents extinction of the A context in the ABA paradigm, although extinction in the B context is unaffected. Animals underwent 20 contiguous trials per day of training in the ABA paradigm. Bar charts represent the percentage of correct arm choices in the first and second set of 10 trials on each test day. Animals participated in 3 days of acquisition training in the ABA paradigm, ending on Day 3 with a 25% reward probability. Control animals were treated with vehicle before exposure to the novel Context “B” on Day 4, in the absence of reward. Here, by the second set of 10 trials significant extinction was evident that was also significantly better than extinction learning under the same conditions in the “A” context. Upon being returned to the same context on Day 5 (without reward) an initial recovery of the learned CS–US response was evident in the first set of 10 trials that was followed by significant extinction of the CS–US response. Treatment of animals with the mGlu5 antagonist, MPEP, before novel exposure to the “B” context in the absence of reward on Day 4 had no effect on extinction learning. A return to the same context on Day 5 resulted in an initial recovery of the learned CS–US response (in the first 10 trials), but no extinction of the recovered response was evident, i.e., an inhibition of extinction learning of the “A” context occurred. An asterisk indicates a significant effect of at least P < 0.01 between the trials indicated by the bar.
Improvement of Extinction by a New Context (ABA Paradigm) is Unaffected by Antagonism of mGlu5, and Extinction of the Old Context is Impaired
Here, we explored if antagonism of mGlu5 affects extinction learning if exposure to Context A on Day 4 is replaced by a new floor context (B) (unrewarded). Despite treatment of animals with the mGlu5 antagonist, MPEP (10 mg/kg, i.p.) before exposure to the “B” context on Day 4, significant extinction occurred (Fig. 3). Here, compared to vehicle-treated controls responses in the first 10 trials on Day 4 (P = 0.642) and in the second set of 10 trials on Day 4 were equivalent (P = 0.299). ANOVA of performance across the trial blocks on Day 4 also revealed no significant difference in vehicle and MPEP-treated animals [ANOVA: F(1,26) = 0.818; P = 0.375, first and second trial blocks]. Significant extinction was also evident if the first and second 10 trials within the MPEP-treated group were compared (P < 0.001).
Strikingly, although significant renewal occurred in the A context on Day 5 (Fig. 3) (P < 0.001, first 10 trials, Day 5 versus last 10 trials, Day 4), it was not followed by significant extinction (first 10 trials, Day 5 versus last 10 trials, Day 5, P = 0.285). These data suggest that extinction in the new context (B) has no bearing on the inhibition of extinction in the old context (A) by mGlu5 antagonism.
The modulation by MPEP of extinction behavior in the AAA and ABA paradigms was significantly different. When we compared performance levels across trial blocks on Day 4 (extinction) in the presence of MPEP, a significant difference was evident between AAA and ABA groups [ANOVA, treatment × paradigm: F(1,28) = 20.368; P > 0.001]. This difference was also evident when we compared overall performance in the ABA and AAA paradigms in MPEP-treated animals across Days 3–5 [ANOVA, days × treatment × paradigm: F(1,46) = 12.568; P < 0.001] and when we compared performance relative to trial blocks over this period [ANOVA, trials × treatment × paradigm: F(1,46) = 7.426; P = 0.004]. Taken together, we conclude that antagonism of mGlu5 results in a significantly different extinction outcome when performance in the AAA and ABA conditions is compared.
Animal Behavior (Accuracy) Was Equivalent in Both Groups on Days 1–3, but Not on Days 3–5
In general, the performance (accuracy) levels of the vehicle-treated and MPEP-treated animal groups were equivalent on Days 1–3 (the days before treatment) [ANOVA: F(1.299, 67.565) = 0.27; P = 0.666] (Fig. 3) reflecting a comparable learning performance in both treatment groups and paradigm. In both groups, a significant difference in the number of correct choices became evident when the performances on Day 3 were compared with performances on Days 4 and 5, in line with extinction learning having occurred (decrease of the number of correct answers/no. of arm visits) [ANOVA: F(1.701, 85.036) = 42.639; P < 0.001, Bonferroni: P < 0.001 and P < 0.001 for comparison between Days 3–4 and 3–5, respectively]. Performance in MPEP-treated animals and vehicle-treated animals on Days 3–5 was significantly different between groups however [ANOVA: F(1.701, 85.036) = 4.436; P = 0.013], reflecting the significant impairment by MPEP of extinction on Day 5 (P < 0.001).
Antagonism of mGlu5 Prevents the Increase of Decision Time Associated With the Decrease of Correct Answers During Extinction and Renewal
The time required to enter a goal arm can be regarded as decision time (Luce, 1986; Avila and Lin, 2014). It reflects confidence in the knowledge of how to pursue the task successfully (Smith et al., 2003). Typically, as control animals begin to acquire the task the time required to make a decision decreases as the number of correct choices increases (Fig. 4) [ANOVA: F(11, 583) = 46.01; P < 0.001]. During extinction learning, decision time increases in conjunction with an attrition in the number of correct arm choices. During renewal (Day 5) decision time continues to increase (Fig. 4a) in the ABA condition even though renewal occurs (Fig. 3). In the AAA condition extinction learning on Days 4 and 5 was accompanied by a steady increase in the decision time (Fig. 4b).
FIGURE 4.
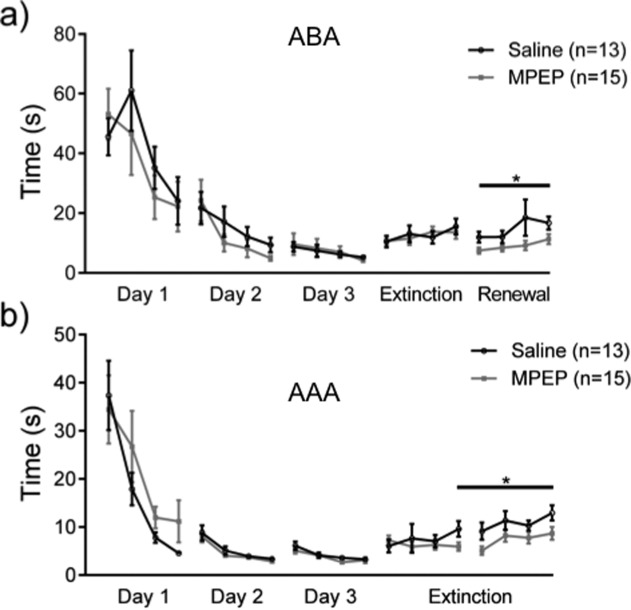
Antagonism of mGlu5 prevents the increase of decision time associated with the decrease of correct answers during extinction and renewal. Both graphs represent the amount of time that was needed to reach the end of an arm (both correct and incorrect choices) after the opening of the door. The ABA paradigm is represented in panel a and the AAA paradigm is represented in panel b. During the learning of the task, the time needed to reach the end of an arm steadily decreased while the correct answers increased, until a basal level of correct answers was reached on Day 3 that reflected animal reaching the 80% criterion of correct arm choices. Treatment with the mGlu5 antagonist, MPEP, before testing on Day 4 had no effect on decision time during extinction learning on Day 4, but it significantly reduced decision time on Day 5 compared to controls. This suggests that MPEP may elicit impaired consolidation of extinction learning on Day 4.
Antagonism of mGlu5 using MPEP before extinction had no effect on the increase in decision time on Day 4 (Fig. 4), neither in the ABA (Fig. 4a) or AAA (Fig. 4b) paradigm [ANOVA: F(3,153) = 0.68; P = 0.56]. However in the ABA paradigm, it significantly prevented the increase of decision time in the renewal state compared to control animals (Fig. 4a) in the AAA paradigm [ANOVA: F(1, 25) = 3.98; P = 0.05]. Thus, treatment with MPEP increased the choice-making confidence of the animals. This suggests that consolidation of the extinction experience was impaired by MPEP treatment.
Discussion
In this study, we observed that although extinction learning of an appetitive task within an unchanged context (AAA) significantly occurs, it is improved by a context change (ABA). Furthermore, we observed that antagonism of the mGlu receptor, mGlu5, significantly prevents extinction in the AAA paradigm. In contrast, mGlu5 antagonism does not alter improved extinction learning in the “B” context of the ABA paradigm but significantly impairs subsequent extinction following renewal of the conditioned response in the “A” context. These data suggest that mGlu5 is intrinsically involved in context-dependent extinction learning, whereby, its contribution pertains to extinction learning of a conditioned response, rather than to mechanisms that underlie facilitated extinction due to a context change.
Fontanez-Nuin et al. (2010) reported that although intraperitoneal MPEP injection in rats before extinction learning of conditioned fear had no effect on extinction acquisition, it impaired extinction retrieval on the following day. In subsequent experiments, they showed that local injections of MPEP into the infralimbic component of the prefrontal cortex revealed a similar, albeit less pronounced pattern of results. On the basis of behavioral and electrophysiological results, Fontanez-Nuin et al. (2011) concluded that mGlu5-mediated infralimbic burst firing is a constituent of the consolidation of extinction learning but that further nonprefrontal neural entities must also act synergistically via mGlu5. Several strands of evidence make it likely that the hippocampus is one of these entities. First, alterations of hippocampal activity can alter extinction learning (Corcoran et al., 2005; de Carvalho et al., 2013; Psotta et al., 2013). Second, extinction learning is accompanied by changes of theta oscillation coupling between the hippocampus, the amygdala, and the prefrontal cortex (Lesting et al., 2011). Third, contextual extinction learning is accompanied by hippocampal activation and the amount of this activity is predictive for the extent of the subsequent renewal effect (Lissek et al., 2013). Fourth, hippocampal inactivation during the acquisition of extinction results in low extinction retrieval rates (Corcoran et al., 2005; Sierra-Mercado et al., 2011). Fifth, the hippocampus plays an essential role in all kinds of context-dependent conditioning tasks (Bouton et al., 2006; Hobin et al., 2006; Ji and Maren, 2008; Milad and Quirk, 2012). Thus, we are inclined to believe that at least a part of the effects observed in this study were mediated by the hippocampus. This contribution is possibly time-dependent, as the hippocampus contribution to context-related acquisition of fear conditioning decreases over time (Marschner et al., 2008).
Nonetheless, other structures are likely to have contributed to the acquisition of the task and may have been affected by the antagonism of mGlu5. The striatum is particularly interesting in this regard, as it is not only involved in the extinction of operant conditioning, fear conditioning, and drug addiction (Ichikawa et al., 2004; Raczka et al., 2011; Schwabe and Wolf, 2011; Rodriguez-Romaguera et al., 2012; Knackstedt et al., 2014) but also believed to contribute increasingly to a spatial task when it transitions from being unfamiliar to familiar, and thus includes more procedural components (Chang and Gold, 2003). However, other studies reported that during learning and extinction (transfer of the reward to the other arm) of a procedural memory task in a T-maze, where performance was rewarded to 100% in all trials, the striatum showed neuronal activation only during initial learning or initiation of extinction (Rueda-Orozco et al., 2008), suggesting that the striatum becomes active only when new motor or procedural learning needs to take place. MGlu5 receptors are expressed in the striatum (Shigemoto, 1993; Romano et al., 1996; Tallaksen-Greene et al, 1998). Thus, these data suggest that the striatum could also have contributed to the changes in learning behavior we observed after mGlu5 antagonism. If these changes were decisive, we would have expected to see equivalent alterations in correct choice performance in the AAA and ABA contexts, as both demanded identical motor and procedural adaptation. This was not the case however.
Packard and McGaugh (1996) reported that under conditions of overtraining in a cross-maze, the initial strategy of place learning mediated by the hippocampus shifts to a strategy of response learning mediated by the caudate nucleus. In other words, the hippocampus is no longer involved in this kind of information processing. Although we cannot entirely exclude that a procedural strategy contributed to the acquisition of the task in this study, it is unlikely that overtraining occurred. The performance criterion (of 80% correct choices) was reached by the second trial block on Day 1 and was sustained through the second trial block on Day 3. However, reward probability was systematically reduced in our study from 100% during the first 10 trials on Day 1 to 25% by the end of the 3-day training period. On Day 4, extinction learning was tested. In the study by Packard and McGaugh (1996), a shift away from hippocampus-dependent information processing first became evident after 15 days of training. Furthermore, no change in reward probability was implemented.
To examine context-dependent extinction learning under nonaversive conditions, we used a T-maze paradigm that implemented low reward probability with context dependency in the acquisition phase. The context comprised a specific floor pattern that was reinforced by a faint odor that was located at the ends of the goal arms and constant extramural cues. When the context was changed, the odor, the floor pattern, and the extramural cues were distinct from the original context. This strategy was chosen because we have observed in the past that visuospatial and olfactospatial information serve as potent cues for both spatial learning, hippocampal synaptic plasticity, and place field formation (Kemp and Manahan-Vaughan, 2007; André and Manahan-Vaughan, 2013; Zhang and Manahan-Vaughan, 2014). We observed that although extinction learning in the A context (AAA, in the absence of food reward) was effective and significant, it was greatly improved by a context change (ABA). This is in line with findings by others in humans that the hippocampus exhibits a higher degree of activation in ABA trials when compared with AAA trials (Lissek et al., 2013).
We observed that antagonism of mGlu5 prevented extinction learning in the AAA context. Extinction occurred only when the animals were returned to the T-maze in the AAA paradigm on Day 5, by which time little MPEP would be expected to have remained bound to mGlu5 receptors: reversal of hyperalgesia by high systemic doses of MPEP wear off within 24 h (Walker et al., 2001) in line with effective metabolism of the ligand in this period. MGlu5 is important for the acquisition and stabilization of long-term memory (Rodrigues et al., 2002; Naie and Manahan-Vaughan, 2004), but application of an antagonist of mGlu5 receptors after memory consolidation has occurred has no effect on the previously formed memory trace (Rodrigues et al., 2002). In the present circumstances, however, a revision of the previously formed memory was expected to occur in the form of a detectable suppression of the conditioned response. This failed to occur in the presence of the mGlu5 antagonist. This suggests that mGlu5 contributes to the mechanisms underlying extinction learning. Interestingly, it has been reported that mGlu5 is not required for extinction of fear conditioning in a foot shock paradigm (Simonyi et al., 2007). However, in the latter study, renewal effects were not examined, and several days of repeated exposure to the paradigm in the absence of foot shock were necessary to detect an extinction effect in controls. Furthermore, the mGlu5 antagonist applied after the first extinction trial, which makes the approach and the outcome hard to compare with ours. It may however be the case that different mechanisms may underlie context-dependent extinction learning under nonaversive and aversive conditions.
Extinction learning proceeded very fast in the “B” context, both for MPEP-treated animals and controls. This is possibly due to Context “A” becoming excitatory when being associated with reward (Rescorla and Wagner, 1972). During extinction, the Context “B” possibly became inhibitory, as it is associated with nonreward. Therefore, extinction in the old “A” context developed much slower as the excitatory context is still present, although reinforcement is no longer delivered (Parades-Olay and Rosas, 1999). Renewal refers to the recovery of a previously learned response that was suppressed during extinction learning (Bouton and Bolles, 1979). This is particularly evident in the ABA paradigm (Bouton and Bolles, 1979; Bouton, 2004), as was also seen in our study. The renewal effect indicates that an extinguished (CS–US) response has not been erased or forgotten, but rather a new competing association between the CS and US is learned, which in turn can be neglected in favor of the old CS–US response, if the animal is returned to the context in which this was learned, or given cues to help retrieve this memory (Myers and Davis, 2002; Bouton, 2004). Interestingly, we observed that antagonism of mGlu5 had no effect on extinction learning in the “B” context. A similar observation was made with regard to context-dependent extinction of fear conditioning (Toth et al., 2012).
The finding that mGlu5 antagonism differentiates between extinction learning of the conditioned response in the “A” context, and extinction in learning in the “B” context is intriguing. One possible interpretation is that the switch to the “B” context caused a significant, though not complete, generalization decrement (Capaldi, 1967,1994), which would explain the lower response levels in the ABA group during extinction compared to the AAA group. The generalization decrement could have been strong enough to make it difficult to detect an effect of mGlu5 antagonism in the ABA group, but there might have been sufficient generalization of extinction learning back to Context A to influence performance there during the renewal test. There is, however, good reason to assume that extinction may involve new learning (Daly, 1974; Mauk and Ohyama, 2004) and with regard to other hippocampus-dependent learning forms, mGlu5 is particularly important for the long-term stability of new memories, as determined by cognitive (Manahan-Vaughan and Braunewell, 2005; Naie and Manahan-Vaughan, 2004) and cellular (Naie and Manahan-Vaughan, 2004; Goh and Manahan-Vaughan, 2013b; Zhang and Manahan-Vaughan, 2014) analysis. The observations of this study are in line with this, and suggest that rapid learning within the 20 trials of exposure to the B paradigm during extinction testing is wholly unaffected by mGlu5 antagonism. Regardless of whether a new context was presented on Day 4 during extinction learning (ABA) or not (AAA), mGlu5 antagonism prevented extinction of the response learned in A. This is an important finding. It suggests that manipulations of mGlu5 could be used to specifically target extinction learning. It also adds to evidence that extinction learning in a novel context has no impact on the original learned CS–US response, rather it generates competition presumably through providing a new more salient learned response based on the altered context. Our data suggest that this process may be mechanistically distinct to the processes that underlined the acquisition of the original CS–US response. This finding also provides interesting correlates to disorders that involve mGlu5 dysfunction. Indeed, in patients who suffer from fragile X syndrome, enhanced cerebellum-dependent extinction has been reported (Smit et al., 2008). In fragile X syndrome, excess signaling by mGlu5 has been reported (Hays et al., 2011) and increased mGlu5 expression in the brains of fragile X patients has been described (Lohith et al., 2013).
In conclusion, we report here that mGlu5 is required for extinction learning. Although learning of a new context is unaffected by mGlu5 antagonism, extinction of the consolidated context is impaired. This suggests that acquisition of and extinction learning of the CS–US may require distinct cellular processes, and supports that mGlu5 is intrinsically involved in enabling learning that once-relevant information is no longer valid.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Jens Klausnitzer, Anne Borkowski, and Juliane Boege. They thank Nadine Kollosch for animal care and Sebastian Ocklenburg for statistical advice.
References
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: Cortical–hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André ME, Manahan-Vaughan D. Spatial olfactory learning facilitates long-term depression in the hippocampus. Hippocampus. 2013;23:963–968. doi: 10.1002/hipo.22158. [DOI] [PubMed] [Google Scholar]
- Avila I, Lin SC. Motivational salience signal in the basal forebrain is coupled with faster and more precise decision speed. PLoS Biol. 2014;12:e1001811. doi: 10.1371/journal.pbio.1001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn PJ, Nicoletti F, Manahan-Vaughan D. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS One. 2008;3:e2155. doi: 10.1371/journal.pone.0002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lohmann P, West B, Brown RM, Kirchhoff J, Raymond CR, Lawrence AJ. The mGlu5 receptor regulates extinction of cocaine-driven behaviours. Drug Alcohol Depend. 2014;137:83–89. doi: 10.1016/j.drugalcdep.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Capaldi EJ. Sequential versus nonsequential variables in partial delay of reward. J Exp Psychol. 1967;74:161–166. doi: 10.1037/h0024630. [DOI] [PubMed] [Google Scholar]
- Capaldi EJ. The relation between memory and expectancy as revealed by percentage and sequence of reward investigations. Psychon Bull Rev. 1994;1:303–310. doi: 10.3758/BF03213970. [DOI] [PubMed] [Google Scholar]
- Chan KH, Jarrard LE, Davidson TL. The effects of selective ibotenate lesions of the hippocampus on conditioned inhibition and extinction. Cogn Affect Behav Neurosci. 2003;3:111–119. doi: 10.3758/cabn.3.2.111. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Lawrence AJ. The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine-seeking in mice. PLoS One. 2013;8:e68371. doi: 10.1371/journal.pone.0068371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly HB. Arousal of frustration following gradual reductions in reward magnitude in rats. J Comp Physiol Psychol. 1974;86:1149–1155. doi: 10.1037/h0037643. [DOI] [PubMed] [Google Scholar]
- de Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proc Natl Acad Sci USA. 2013;110:1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Sawa K, Takahashi M, Lauwereyns J, Tsukada M, Aihara T. Context and the renewal of conditioned taste aversion: The role of rat dorsal hippocampus examined by electrolytic lesion. Cogn Neurodyn. 2012;6:399–407. doi: 10.1007/s11571-012-9208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Hutson PH, Stephens DN. Differential effects of MPEP and diazepam in tests of conditioned emotional response and Pavlovian-to-instrumental transfer suggests ‘anxiolytic’ effects are mediated by different mechanisms. Psychopharmacology (Berl) 2009;204:499–509. doi: 10.1007/s00213-009-1479-6. [DOI] [PubMed] [Google Scholar]
- Goh J, Manahan-Vaughan D. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex. 2013a;23:1118–1125. doi: 10.1093/cercor/bhs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J, Manahan-Vaughan D. Endogenous hippocampal LTD that is enabled by spatial object recognition requires activation of NMDA receptors and the metabotropic glutamate receptor, mGlu5. Hippocampus. 2013b;23:129–138. doi: 10.1002/hipo.22072. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behav Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Handford CE, Tan S, Lawrence AJ, Kim JH. The effect of the mGlu5 negative allosteric modulator MTEP and NMDA receptor partial agonist D-cycloserine on Pavlovian conditioned fear. Int J Neuropsychopharmacol. 2014;17:1521–1532. doi: 10.1017/S1461145714000303. [DOI] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci 2011. 2011;31:14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Izawa E, Matsushima T. Excitotoxic lesions of the medial striatum delay extinction of a reinforcement color discrimination operant task in domestic chicks; a functional role of reward anticipation. Brain Res Cogn Brain Res. 2004;22:76–83. doi: 10.1016/j.cogbrainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Feldon J, Rawlins JN, Sinden JD, Gray JA. The effects of intrahippocampal ibotenate on resistance to extinction after continuous or partial reinforcement. Exp Brain Res. 1986;61:519–530. doi: 10.1007/BF00237577. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15:244–251. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion of declarative memory processes. Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol. 2014;19:87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H, Matsumoto M, Togashi H, Miura Y, Fukushima K, Yoshioka M. Alteration of synaptic transmission in the hippocampal-mPFC pathway during extinction trials of context-dependent fear memory in juvenile rat stress models. Synapse. 2009;63:805–813. doi: 10.1002/syn.20657. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: Differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengersdorf D, Stüttgen MC, Uengoer M, Güntürkün O. Transient inactivation of the pigeon hippocampus or the nidopallium caudolaterale during extinction learning impairs extinction retrieval in an appetitive conditioning paradigm. Behav Brain Res. 2014;265:93–100. doi: 10.1016/j.bbr.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Glaubitz B, Uengoer M, Tegenthoff M. Hippocampal activation during extinction learning predicts occurrence of the renewal effect in extinction recall. Neuroimage. 2013;81C:131–143. doi: 10.1016/j.neuroimage.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Lohith TG, Osterweil EK, Fujita M, Jenko KJ, Bear MF, Innis RB. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol Autism. 2013;4:15. doi: 10.1186/2040-2392-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce RD. Response Times. New York: Oxford University Press; 1986. [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Ohyama T. Extinction as new learning versus unlearning: Considerations from a computer simulation of the cerebellum. Learn Mem. 2004;11:566–571. doi: 10.1101/lm.83504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Bernal I. Learning and memory consolidation: Linking molecular and behavioral data. Neuroscience. 2011;176:12–19. doi: 10.1016/j.neuroscience.2010.12.056. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Manahan-Vaughan D. Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology. 2013;66:65–81. doi: 10.1016/j.neuropharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: Relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Crombag HS, Mead AN, Stephens DN. The mGluR5 antagonist MTEP dissociates the acquisition of predictive and incentive motivational properties of reward-paired stimuli in mice. Neuropsychopharmacology. 2010;3:1807–1817. doi: 10.1038/npp.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Parades-Olay MC, Rosas JM. Within-subjects extinction and renewal in predictive judgements. Psicológica. 1999;20:195–210. [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psotta L, Lessmann V, Endres T. The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Neurobiol Learn Mem. 2013;103:34–38. doi: 10.1016/j.nlm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Raczka KA, Mechias ML, Gartmann N, Reif A, Deckert J, Pessiglione M, Kalisch R. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Transl Psychiatry. 2011;1:e12. doi: 10.1038/tp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci USA. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, van den Pol AN, O'Malley KL. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA splice variants, and regional distribution. J Comp Neurol. 1996;367:403–412. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E, Méndez-Díaz M, Prospéro-García O. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology. 2008;55:55–62. doi: 10.1016/j.neuropharm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Maril A, Bitan T, Goshen-Gottstein Y. Putting Humpty together and pulling him apart: Accessing and unbinding the hippocampal item-context engram. Neuroimage. 2012;60:808–817. doi: 10.1016/j.neuroimage.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav Brain Res. 2011;219:321–328. doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiol Learn Mem. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Parker KE, Ramsey AK, Schachtman TR. Metabotropic glutamate receptor 5 in conditioned taste aversion learning. Neurobiol Learn Mem. 2009;92:460–463. doi: 10.1016/j.nlm.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AE, van der Geest JN, Vellema M, Koekkoek SK, Willemsen R, Govaerts LC, Oostra BA, De Zeeuw CI, VanderWerf F. Savings and extinction of conditioned eyeblink responses in fragile X syndrome. Genes Brain Behav. 2008;7:770–777. doi: 10.1111/j.1601-183X.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Washburn DA. The comparative psychology of uncertainty monitoring and metacognition. Behav Brain Sci. 2003;26:317–339. doi: 10.1017/s0140525x03000086. [DOI] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Toth I, Dietz M, Peterlik D, Huber SE, Fendt M, Neumann ID, Flor PJ, Slattery DA. Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear. Neuropharmacology. 2012;62:1619–1626. doi: 10.1016/j.neuropharm.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Wiescholleck V, André MAE, Manahan-Vaughan D. Early age-dependent impairments of context-dependent extinction learning, object recognition, and object-place learning occur in rats. Hippocampus. 2014;24:270–279. doi: 10.1002/hipo.22220. [DOI] [PubMed] [Google Scholar]
- Zhang S, Manahan-Vaughan D. Place field stability requires the metabotropic glutamate receptor, mGlu5. Hippocampus. 2014 doi: 10.1002/hipo.22314. In press. doi: 10.1002/hipo.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]


