Abstract
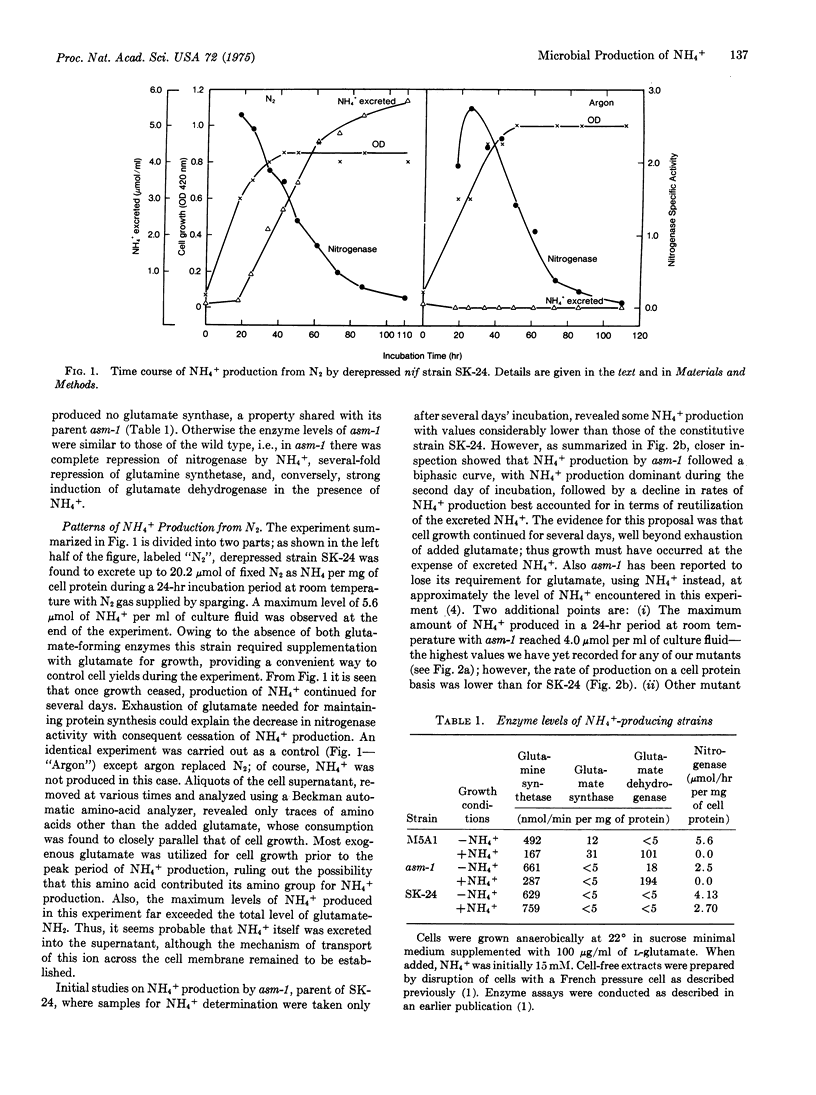
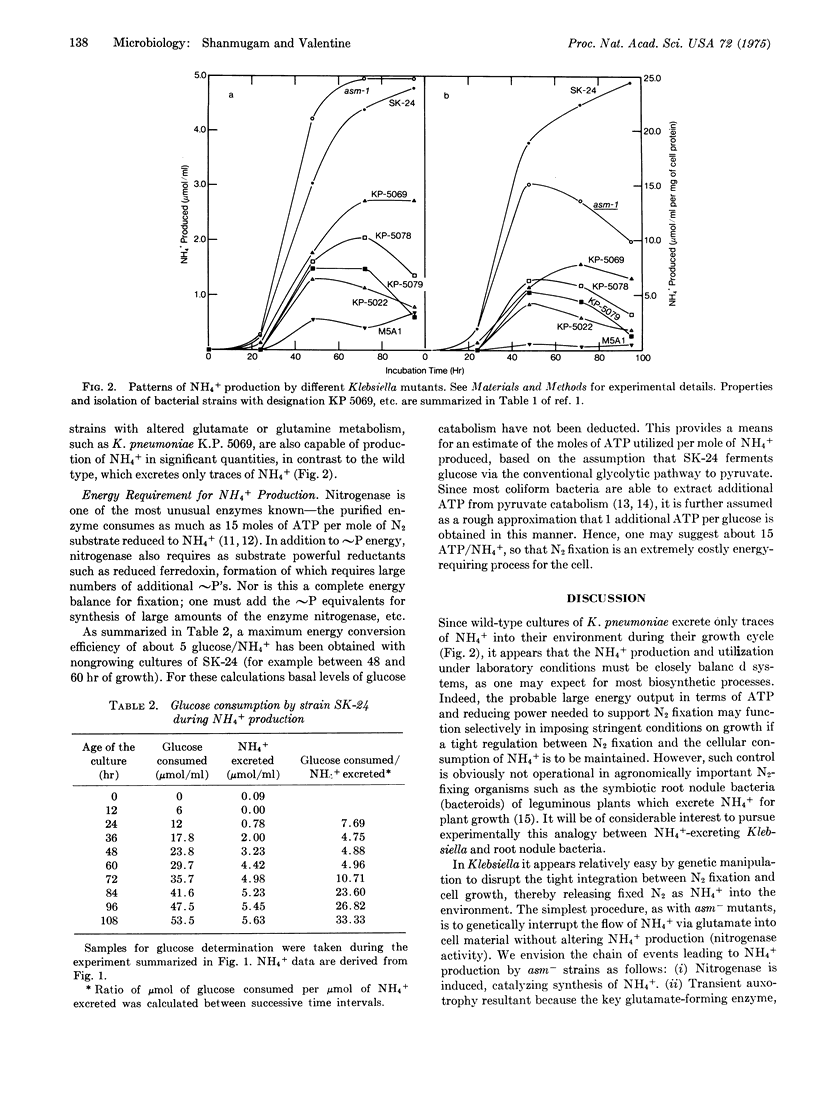
Genetic manipulation of nitrogenase and key glutamate-forming enzymes can provide mutants that excrete fixed N2 as NH4+. A derepressed N2 fxation mutant (SK-24) has been isolated , which excretes up to 20.2 mumol of fixed N2 as NH4+ per mg of cell protein in 24 hr at room temperature. Biochemical analysis shows that this mutant, which requires glutamate for growth, releases fixed N2 as NH4+ into the environment because of (i) constitutive synthesis of nitrogenase and (ii) genetic blocks resulting in losses of glutamate synthase [L-glutamine:2-oxoglutarate aminotransferase (NADPH oxidizing), EC 2.6.1.53] and glutamate dehydrogenase [L-glutamate:NADP oxidoreductase (deaminating), EC 1.4.1.4] activities, enzymes essential for NH4+ assimilation into cell material. The parent strain (asm-1), missing only glutamate synthase activity, also actively excretes NH4+ during early phases of its growth but eventually reutilizes the NN4+. A miximum yield of 4.0 mumol of NH4+/ml per 24 hr has been noted for asm-1 only during the growth period. Biosynthesis of NH4+ PROCEEDS AT THE EXPENSE OF A Variety of fermentable sugars, such as sucrose or glucose, with a maximum energy conversion efficiency of about 5 glucose degraded per NH4+ formed. The use of microbes for production of NH4+ fertilizer is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Knappe J., Schacht J., Möckel W., Höpner T., Vetter H., Jr, Edenharder R. Pyruvate formate-lyase reaction in Escherichia coli. The enzymatic system converting an inactive form of the lyase into the catalytically active enzyme. Eur J Biochem. 1969 Dec;11(2):316–327. doi: 10.1111/j.1432-1033.1969.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. ATP hydrolysis and electron transfer in the nitrogenase reaction with different combinations of the iron protein and the molybdenum-iron protein. Biochim Biophys Acta. 1972 Jul 12;275(1):93–101. doi: 10.1016/0005-2728(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Magasanik B., Prival M. J., Brenchley J. E., Tyler B. M., DeLeo A. B., Streicher S. L., Bender R. A., Paris C. G. Glutamine synthetase as a regulator of enzyme synthesis. Curr Top Cell Regul. 1974;8(0):119–138. doi: 10.1016/b978-0-12-152808-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Nagatani H., Shimizu M., Valentine R. C. The mechanism of ammonia assimilation in nitrogen fixing Bacteria. Arch Mikrobiol. 1971;79(2):164–175. doi: 10.1007/BF00424923. [DOI] [PubMed] [Google Scholar]
- Streicher S., Gurney E., Valentine R. C. Transduction of the nitrogen-fixation genes in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1174–1177. doi: 10.1073/pnas.68.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S. Glutamine synthetase and ammonium regulation of nitrogenase synthesis in Klebsiella. Nature. 1974 Oct 11;251(5475):481–485. doi: 10.1038/251481a0. [DOI] [PubMed] [Google Scholar]


