Abstract
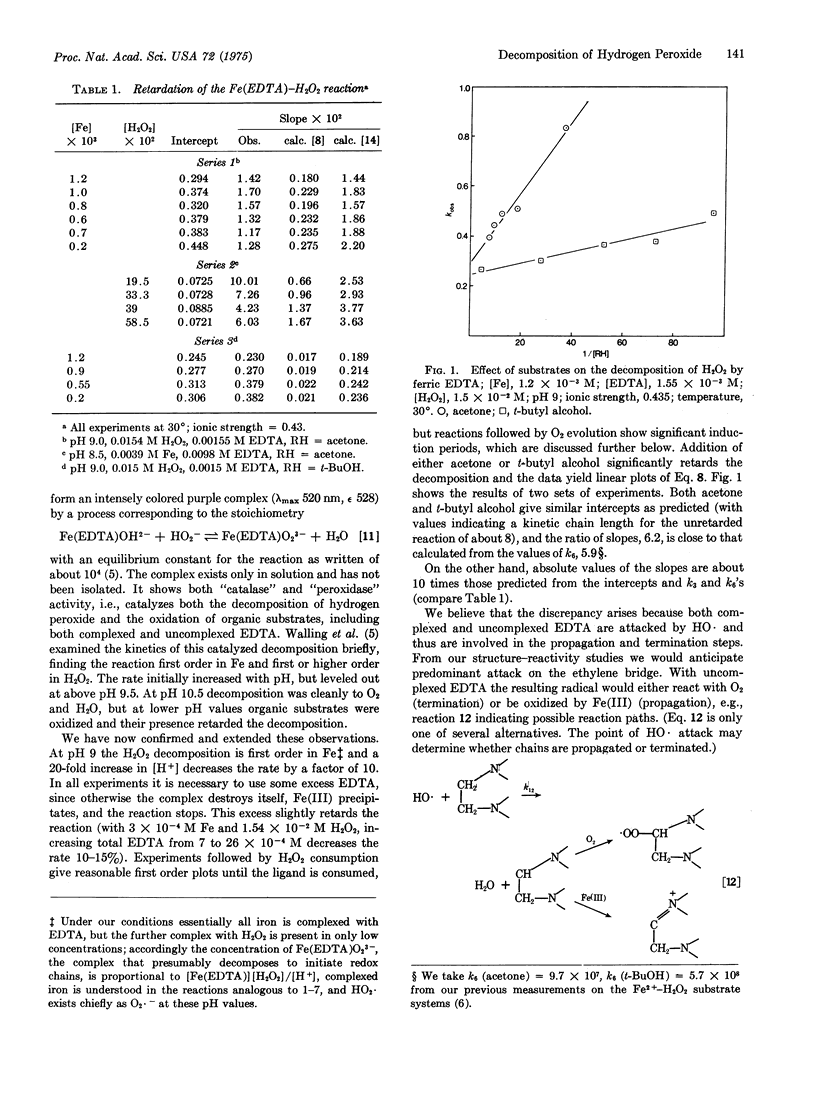
Added substrates, acetone and t-butyl alcohol, strongly retard the decomposition of H2O2 brought about by ferric ethylenediaminetetraacetate (EDTA) at pH 8-9.5. Their relative effectiveness and the kinetic form of the retardation are consistent with their interruption of a hydroxyl radical chain that is propagated by HO· attack both upon H2O2 and on complexed and uncomplexed EDTA. Similar retardation is observed with decompositions catalyzed by ferric nitrilotriacetate and hemin, and it is proposed that such redox chains may be quite a general path for transition metal ion catalysis of H2O2 decomposition.
Keywords: hydroxyl radicals, metal ion catalysis, ferric complexes, catalase, peroxidase
Full text
PDF