Abstract
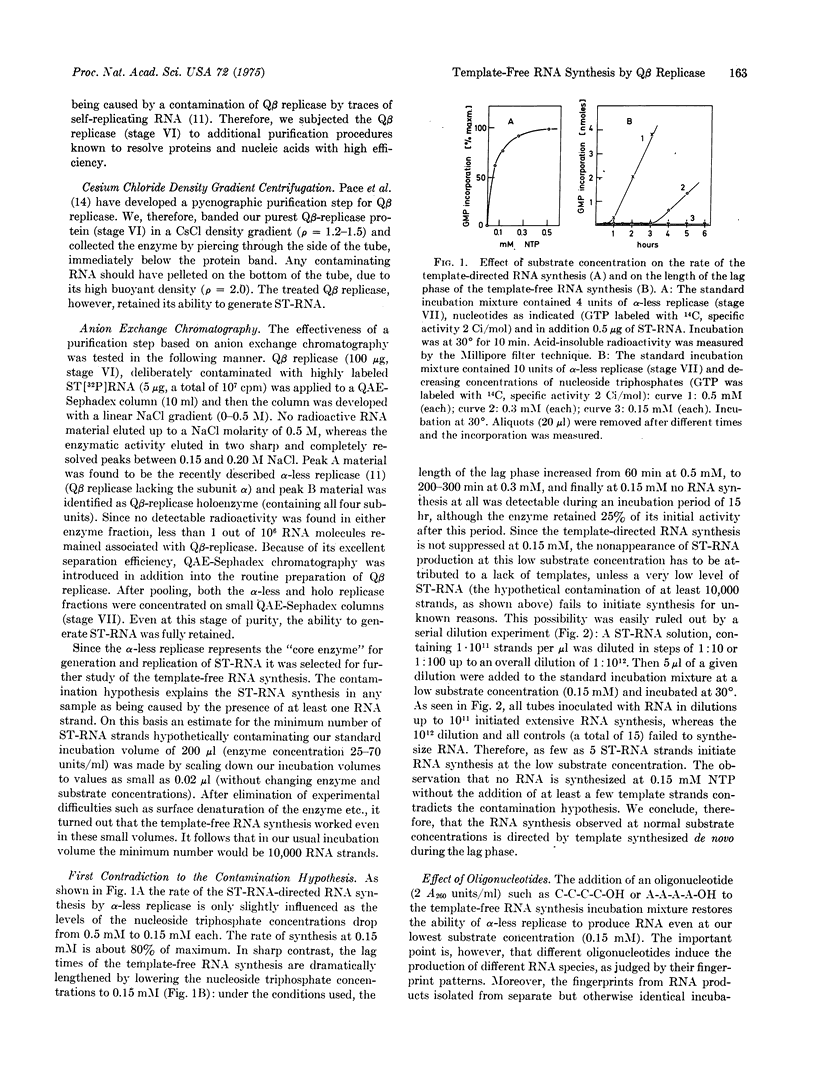
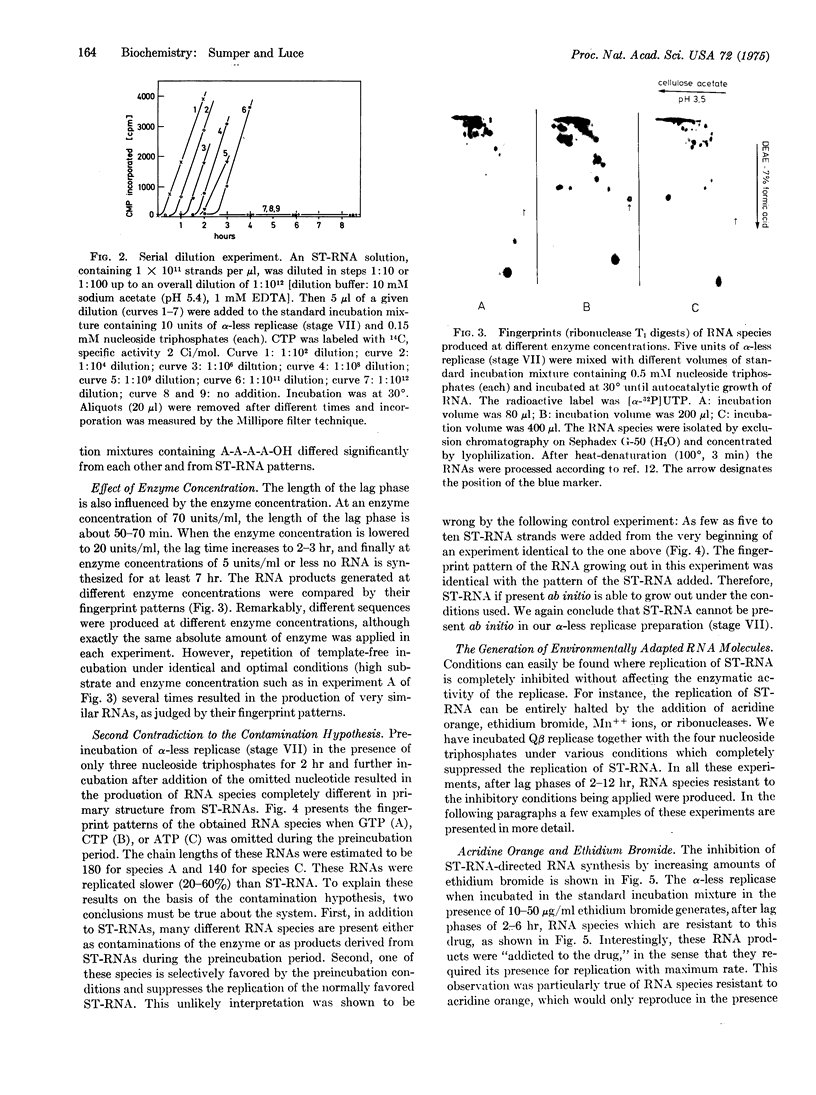
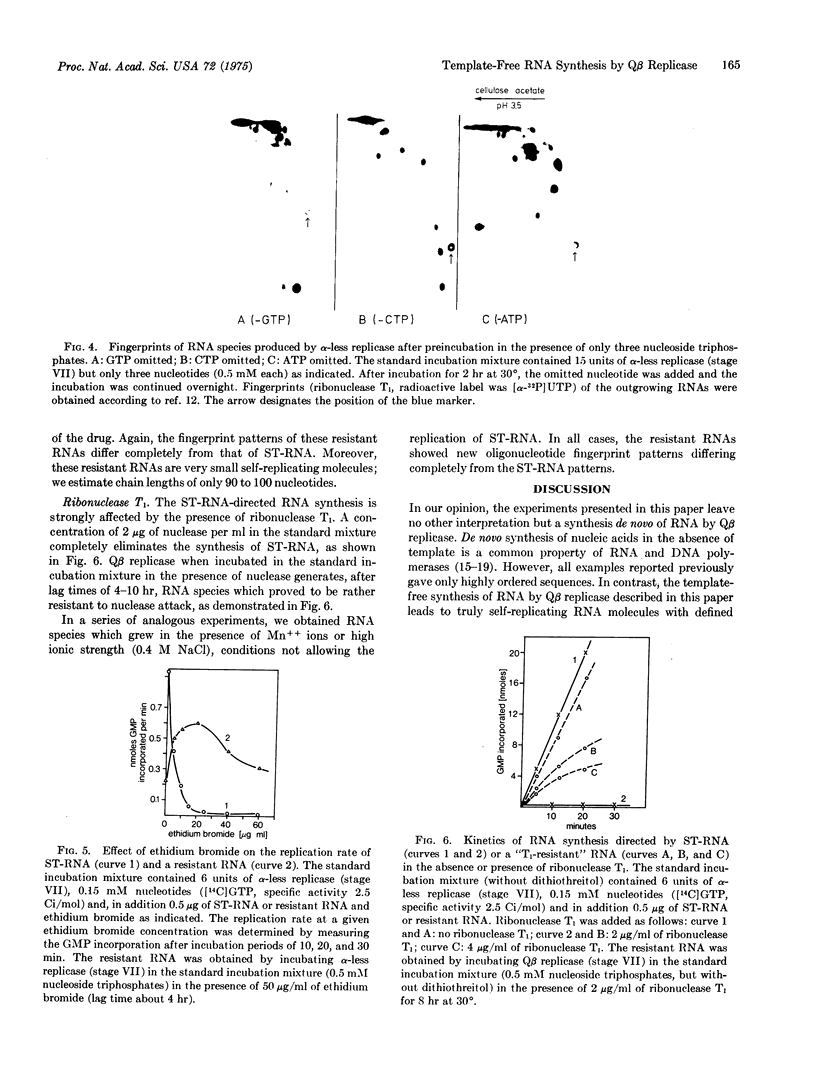
Highly purified coliphage Qbeta replicase when incubated without added template synthesizes self-replicating RNA species in an autocatalytic reaction. In this paper we offer strong evidence that this RNA production is directed by templates generated de novo during the lag phase. Contamination of the enzyme by traces of RNA templates was ruled out by the following experimental results: (1) Additional purification steps do not eliminate this RNA production. (2) The lag phase is lengthened to several hours by lowering substrate or enzyme concentration. At a nucleoside triphosphate concentration of 0.15 mM no RNA is produced although the template-directed RNA synthesis works normally. (3) Different enzyme concentrations lead to RNA species of completely different primary structure. (4) Addition of oligonucleotides or preincubation with only three nucleoside triphosphates affects the final RNA sequence. (5) Manipulation of conditions during the lag phase results in the production of RNA structures that are adapted to the particular incubation conditions applied (e.g., RNA resistant to nuclease attack or resistant to inhibitors or even RNAs "addicted to the drug," in the sense that they only replicate in the presence of a drug like acridine orange). RNA species obtained in different experiments under optimal incubation conditions show very similar fingerprint patterns, suggesting the operation of an instruction mechanism. A possible mechanism is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Rensing U., August J. T. Replication of RNA viruses. X. Replication of a natural 6 s RNA by the Q-beta RNA polymerase. J Mol Biol. 1969 Oct 28;45(2):181–193. doi: 10.1016/0022-2836(69)90098-9. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Feix G., Pollet R., Weissmann C. Replication of viral RNA, XVI. Enzymatic synthesis of infectious virual RNA with noninfectious Q-beta minus strands as template. Proc Natl Acad Sci U S A. 1968 Jan;59(1):145–152. doi: 10.1073/pnas.59.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMATOS P. J., KRUG R. M., TAMM I. ENZYMIC SYNTHESIS OF RNA WITH REOVIRUS RNA AS TEMPLATE. I. CHARACTERISTICS OF THE REACTION CATALYZED BY THE RNA POLYMERASE FROM ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:193–207. doi: 10.1016/s0022-2836(64)80100-5. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Specific template requirments of RNA replicases. Proc Natl Acad Sci U S A. 1965 Aug;54(2):579–587. doi: 10.1073/pnas.54.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Eoyang L., Banerjee A. K., August J. T. Replication of RNA viruses. V. Template activity of synthetic ribopolymers in the Q-beta RNA polymerase reaction. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1790–1797. doi: 10.1073/pnas.57.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., BERTSCH L. L., JACKSON J. F., KHORANA H. G. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID, XVI. OLIGONUCLEOTIDES AS TEMPLATES AND THE MECHANISM OF THEIR REPLICATION. Proc Natl Acad Sci U S A. 1964 Feb;51:315–323. doi: 10.1073/pnas.51.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R. A new method for the purification of Q RNA-dependent RNA polymerase. Biochim Biophys Acta. 1972 Feb 23;262(1):88–100. doi: 10.1016/0005-2787(72)90221-3. [DOI] [PubMed] [Google Scholar]
- Kamen R. Characterization of the subunits of Q-beta replicase. Nature. 1970 Nov 7;228(5271):527–533. doi: 10.1038/228527a0. [DOI] [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Karstadt M. Azotobacter vinelandii ribonucleic acid polymerase. IV. Unprimed synthesis of rIC copolymer. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2094–2101. doi: 10.1073/pnas.58.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- MEHROTRA B. D., KHORANA H. G. STUDIES ON POLYNUCLEOTIDES. XL. SYNTHETIC DEOXYRIBOPOLYNUCLEOTIDES AS TEMPLATES FOR RIBONUCLEIC ACID POLYMERASE: THE INFLUENCE OF TEMPERATURE ON TEMPLATE FUNCTION. J Biol Chem. 1965 Apr;240:1750–1753. [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Peterson R. L., Spiegelman S. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc Natl Acad Sci U S A. 1967 Jul;58(1):217–224. doi: 10.1073/pnas.58.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J., Henes C. The function of pseudouridylic acid in transfer ribonucleic acid. II. Inhibition of amino acyl transfer ribonucleic acid-ribosome complex formation by ribothymidylyl-pseudouridylyl-cytidylyl-guanosine 3'-phosphate. J Biol Chem. 1969 Nov 25;244(22):6241–6253. [PubMed] [Google Scholar]
- RADDING C. M., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XIII. Kinetics of primed and de novo synthesis of deoxynucleotide polymers. J Biol Chem. 1962 Sep;237:2877–2882. [PubMed] [Google Scholar]
- SCHACHMAN H. K., ADLER J., RADDING C. M., LEHMAN I. R., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VII. Synthesis of a polymer of deoxyadenylate and deoxythymidylate. J Biol Chem. 1960 Nov;235:3242–3249. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Hayashi H., Miura K. I. Functional sites of transfer RNA for the binding to messenger RNA-ribosome complex. J Biochem. 1970 Mar;67(3):373–387. doi: 10.1093/oxfordjournals.jbchem.a129261. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]




