Abstract
The intricate relationships that associate pain, stress responses and emotional behavior have been well established. Acute stressful situations can decrease nociceptive sensations and conversely, chronic pain can enhance other pain experiences and heighten the emotional and behavioral consequences of stress. Accordingly, chronic pain is comorbid with a number of behavioral disorders including depression, anxiety abnormalities and associated stress-related disorders including post traumatic stress disorder (PTSD). The central nucleus of the amygdala (CeA) represents a convergence of pathways for pain, stress and emotion, and we have identified pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in fiber elements in the lateral capsular division of the CeA (CeLC). The PACAP staining patterns colocalized in part with those for calcitonin gene related peptide (CGRP); anterograde fiber tracing and excitotoxic lesion studies demonstrated that the CeLC PACAP/CGRP immunoreactivities represented sensory fiber projections from the lateral parabrachial nucleus (LPBn) along the spino-parabrachioamygdaloid tract. The same PBn PACAP/CGRP fiber system also projected to the BNST. As in the BNST, CeA PACAP signaling increased anxiety-like behaviors accompanied by weight loss and decreased feeding. But in addition to heightened anxiety-like responses, CeA PACAP signaling also altered nociception as reflected by decreased latency and threshold responses in thermal and mechanical sensitivity tests, respectively. From PACAP expression in major pain pathways, the current observations are novel and suggest that CeA PACAP nociceptive signaling and resulting neuroplasticity via the spino-parabrachioamygdaloid tract may represent mechanisms that associate chronic pain with sensory hypersensitivity, fear memory consolidation and severe behavioral disorders.
Keywords: Parabrachial nucleus, Central amygdala - lateral capsular division, Parabrachialamygdaloid tract, PACAP, CGRP, Hypersensitivity
The intricate relationships that associate pain, stress responses and emotional behavior have been well established. Acute stressful situations can decrease nociceptive sensations and conversely, chronic pain can enhance other pain experiences and heighten the emotional and behavioral consequences of stress. Accordingly, chronic pain is comorbid with a number of behavioral disorders including depression, anxiety abnormalities and associated stress-related disorders including post traumatic stress disorder (PTSD). The central nucleus of the amygdala (CeA) represents a convergence of pathways for pain, stress and emotion, and we have identified pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in fiber elements in the lateral capsular division of the CeA (CeLC). The PACAP staining patterns colocalized in part with those for calcitonin gene related peptide (CGRP); anterograde fiber tracing and excitotoxic lesion studies demonstrated that the CeLC PACAP/CGRP immunoreactivities represented sensory fiber projections from the lateral parabrachial nucleus (LPBn) along the spino-parabrachioamygdaloid tract. The same PBn PACAP/CGRP fiber system also projected to the BNST. Similar to the anxiogenic effects of BNST PACAP signaling, PACAP injections into the CeA increased anxiety-like behaviors as reflected by decreased open arm entries on elevated plus-maze tests accompanied by weight loss and decreased feeding. But in addition to heightened anxiety-like responses, CeA PACAP signaling also altered nociception as reflected by decreased latency and threshold responses in thermal and mechanical sensitivity tests, respectively. From PACAP expression in major pain pathways, the current observations are novel and suggest that CeA PACAP nociceptive signaling and resulting neuroplasticity via the spino-parabrachioamygdaloid tract may represent mechanisms that associate chronic pain with sensory hypersensitivity, fear memory consolidation and severe behavioral disorders.
1. Introduction
Chronic neuropathic pain alters sensory responses and carries an emotional subtext that can have severe effects on behavior. Persistent pain can heighten pain experiences from hyperalgesia and allodynia (Rouwette et al., 2012; Veinante et al., 2013). Further, patients suffering from chronic pain are more prone to experience depression, sleep dysregulation, panic disorders, obsessive compulsive behavior, anxiety abnormalities and stress-related disorders including post-traumatic stress disorder (PTSD) (Asmundson and Katz, 2009). The intricate relationship between pain and behavior has been well studied and among brain regions, the amygdala is centrally situated to integrate the many descending and ascending signals to modulate the sensory and emotional components of pain. Highly processed descending polymodal nociceptive information is conveyed from the somatosensory cortex and thalamus to the basolateral amygdala (BLA) which in turn projects to the central nucleus of the amygdala (CeA). The resulting CeA efferents signals are relayed to other central nuclei, including those traveling with hypothalamic - periaqueductal grey projections for autonomic control and anti-nociception to dampen pain stimuli (Veinante et al., 2013). Among several ascending pathways carrying pain transmission to the CeA, the most prominent is the spino-parabrachioamygdaloid tract (Bernard et al., 1996; Gauriau and Bernard, 2002; Rouwette et al., 2012; Veinante et al., 2013). Peripheral nociceptive signals carried via primary sensory A[.asteriskmath]- and C-fibers terminate in the dorsal horn where second order neurons send projections via the spino-parabrachial pathway to pontine lateral and external medial parabrachial nuclei (PBn) (Todd, 2010). Hence the PBn collects cutaneuous (mechanical and thermal), deep (muscular and articular) and visceral nociceptive signals and relays the information in a highly organized topographical manner principally to lateral capsular division of the CeA (CeLC). The roles of the CeA/CeLC in nociceptive processing have been examined from a number of vantages. In vivo electrophysiological studies have shown that noxious stimuli and chronic pain paradigms increase spontaneous and evoked CeA neuronal activity (Bernard et al., 1992; Ji and Neugebauer, 2009; Neugebauer and Li, 2003), and synaptic transmission at Pbn-CeA and BLACeA synapses (Ikeda et al., 2007; Neugebauer et al., 2003). Visceral, inflammatory and chronic neuropathic pain can induce CeA neuron stress peptide and c-fos expression (Bon et al., 1998; Nakagawa et al., 2003; Suwanprathes et al., 2003; Ulrich-Lai et al., 2006; Rouwette et al., 2011) and increase glutaminergic NR1 receptor phosphorylation in CeA neurons (Bird et al., 2005). Further, human brain imaging studies have implicated the amygdala in pain (Simons et al., 2012). Hence the neurocircuit intersections in the CeA can modulate the sensory, emotional and affective responses to pain.
Pituitary adenylate cyclase activating polypeptide (PACAP) is a well studied neural and endocrine pleiotropic peptide important in the development and homeostatic regulation of many physiological systems (reviewed in Vaudry et al., 2009). In the central and peripheral nervous systems, PACAP is neurotrophic to promote neuronal survival, proliferation and differentiation in development and regeneration, participates in sensory and autonomic signaling, is important in hippocampal learning and memory processes and regulates a variety of hypothalamic/limbic stress-related behavioral responses. PACAP binds to several G protein-couple receptor subtypes (Braas and May, 1999; Harmar et al., 2012; Spengler et al., 1993). PACAP binds selectively at the PAC1 receptor; both PACAP and VIP bind the VPAC receptors with equal high affinity. Recently, the expression of PACAP and its cognate PAC1 receptor has been shown to be upregulated in specific limbic regions by chronic (Hammack et al., 2009). PACAP infusions into the bed nucleus of the stria terminalis (BNST) is anxiogenic, and altered blood PACAP levels and PAC1 receptor polymorphism have been associated with PTSD and other stress-related disorders (Almli et al., 2013; Chen et al., 2013; Ressler et al., 2011; Uddin et al., 2013; Wang et al., 2013). In sum, these observations have implicated limbic PACAP/PAC1 receptor signaling in stress- and anxiety-related behaviors.
In evaluating PACAP expression in other limbic structures, we noted high levels of PACAP immunoreactivity in fiber terminals and varicosities the CeLC, suggesting that the CeLC may be a target of distant PACAP projections. The CeLC is heavily innervated by the lateral Pbn (LPBn) and PACAP has been localized to many sensory pathways. From these observations, we have hypothesized that LPBn PACAP signaling to the CeLC has both sensory and behavioral consequences. In examining the localization and roles of PACAP to the CeLC, our current work demonstrates that PACAP is a component of the parabrachioamygdaloid pathway and that PACAP/PAC1 receptor signaling in the CeA elicits nociceptive and behavioral responses. The integration of these nociceptive and emotion pathways may represent a set of neural circuits that mediate the adverse sensory and emotional consequences of chronic pain.
2. Materials and methods
2.1 Animals
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were habituated to the animal facility for 1 week before experimentation. Rats were single-housed and maintained on a 12 h light/dark cycle (lights on at 0700 h). Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
2.2 Chronic variate stress
Following acclimation, each animal was randomly assigned to either a control or chronically stressed group. Control group animals were handled and remained in their home cages until euthanasia. The chronically stressed group of animals underwent a chronic variate stress paradigm in which rats were exposed to one of 5 different stressors (oscillation, forced swim, restraint, pedestal standing and footshock) each day for 7 days, as described previously (Hammack et al., 2009; Roman et al., 2012; Roman et al., 2014). All animals within the group were exposed to the same order of stressors for the same duration.
2.3 Immunocytochemistry
The brains from perfusion fixed animals were postfixed in 4% paraformaldehyde at 4 C for 24 h, washed and equilibrated in 30% surcrose before embedding in Tissue-Tek OCT compound for cryosectioning. The sections (30 :m) were mounted onto subbed slides, permeabilized with 0.3% Triton X-100, blocked with 1% BSA and incubated in primary antibody for 48 h at 4 C. CRH immunoreactivity was localized using an affinity purified rabbit antibody (1:100, No. G-019-06, Phoenix Pharmaceuticals, Burlingame, CA). CGRP immunoreactivity was examined using a polyclonal antibody raised against the full length CGRP(1-37) peptide (1:1500, Ian Dickerson, Univ Rochester) for visualization with AlexaFluor 488 conjugated donkey anti-rabbit IgG (1:200, Jackson Immunoresearch). PACAP immunoreactivity was detected using a mouse PACAP monoclonal antibody (1:10, Jens Hannibal, Bisperg Hospital, Copenhagen, Denmark) followed by tyramide signal amplification (Hannibal, 2002). Following primary PACAP antibody incubation, the tissues were incubated in biotinylated horse anti-mouse antibody (1:200, 2 h; Vector Laboratories, Burlingame, CA) and treated streptavidin-HRP (1:200, 30 min) before application of tyramide-biotin reagent (1:100, 10 min; Perkin Elmer, Waltham, MA). After extensive washing, the PACAP immunoreactivity was localized with Cy3-conjugated streptavidin (1:200, 2 h; Jackson Immunoresearch, West Grove, PA). In dual localization studies, the sections were incubated in PACAP and CGRP or CRH antisera concurrently. Tissues sections from BDA anterograde tracing (Section 2.5.1.) and excitotoxic lesion (Section 2.5.2.) studies were also processed for immunocytochemistry using the same procedures. Images from immunocytochemistry, excitotoxic lesion and anterograde tracing experiments were acquired sequentially with appropriate filter sets using a Nikon E800 point scanning confocal microscope. Image analyses were performed using NIH ImageJ (Schneider et al., 2012) to threshold, determine signal area (pixel number in staining area) and calculate Pearson's and Mander's correlation coefficients. In within subject excitotoxic lesion studies, the area of immunoreactivity on the side of the lesion was compared to the vehicle control contralateral side.
2.4 Transcript analyses
Quantitative PCR (QPCR) was performed exactly as described previously (Girard et al., 2002, Girard et al., 2006; Hammack et al., 2009). Briefly, after euthanasia by rapid decapitation, the coronal rat brain sections were prepared using a rodent brain matrix (Ted Pella, Inc. Redding, CA) and the micropunched amygdala tissues were quickly frozen on dry ice for total RNA extraction using STAT-60 RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX). All RNA were reverse transcribed simultaneously using random hexamer primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA) to obviate variability. Real-time QPCR was performed as described using SYBR Green I detection (Girard et al., 2002; Girard et al., 2006; Hammack et al., 2009). Briefly, cDNA templates were diluted 5-fold to minimize the inhibitory effects of the reverse transcription reaction components and assayed on an ABI Prism 7500 Fast Real -Time PCR System (Applied Biosystems, Foster City, CA) using SYBR Green I JumpStartTM Taq ReadyMix (Sigma, St. Louis, MO) containing 3.5 mM MgCl2, 200 :M dATP, dGTP, dCTP and dTTP, 0.64 U Taq DNA polymerase and 300 nM of each primer in a final 25 :l reaction volume. Oligonucleotide primer sequences were: PACAP (S) 5’-CATGTGTAGCGGAGCAAGGTT-3’ (AS) 5’-GTCTTGCAGCGGGTTTCC-3’; CRH (S) 5’-TGGATCTCACCTTCCACCTTCTG-3’ (AS) 5’-CCGATAATCTCCATCAGTTTCCTG-3’. The melting profiles for amplified DNA fragments were performed to verify unique product amplification in the quantitative PCR assays. For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence (Girard et al., 2002; Girard et al., 2006). The increase in SYBR Green I fluorescence intensity ()Rn) was plotted as a function of cycle number and the threshold cycle (CT) was determined by the software as the amplification cycle at which the )Rn first intersects the established baseline. The transcript levels in each sample were calculated from the CT by interpolation from the standard curve to yield the relative changes in expression. For each target sequence, all samples from the same brain region were amplified together in the same assay to minimize variability. All data were normalized to 18S RNA.
2.5 Surgical Procedures
2.5.1. Anterograde tracing
Rats were anesthetized with isoflurane (1.5 - 3.5%), and secured into a stereotactic apparatus (David Kopf Instruments, Tunjunga, CA). The skull was exposed from a midline incision and a micropipette (30 - 50 :m tip diameter) filled with 10% biotinylated dextran amine (BDA; 10 kDa) was lowered into the LPBn using coordinates (from bregma in mm) AP: -9.3 ML: -2.3 DV: -8.0, for iontophoretic tracer application (5 :A, 7 sec on and 7 sec off, 20 min total). The process was repeated on the contralateral LPBn. After 14 days, the 4% paraformaldehyde perfusion - fixed rat brains were processed and the cryosections incubated in 1:200 streptavidin-Cy2 (Jackson Immunoresearch) for BDA tracer localization. The anterograde tracing studies were sometimes performed in conjunction with peptide immunocytochemistry for concurrent localizations (Section 2.3). As for most peptide antisera, the PACAP antibody preferentially labeled fibers than soma which precluded immunocytochemistry of retrogradely labeled LPBn neurons from the CeLC.
2.5.2. Excitotoxic lesion
Adult male rats were surgically prepared as above and a microsyringe (1 :l, Hamilton Co., Reno, NV) was unilaterally placed into the LPBn (from bregma in mm, AP: -9.3 ML: -2.3 DV: -7.9) for automated pump infusion of 2 :g NMDA in 200 nl over 4 min. The syringe was left in place for an additional 4 min and following postsurgical recovery the rats were returned to their home cages and for 7 days. NMDA excitotoxic lesion at the targeted site was verified by processing the brain cryosections for neuron specific nuclear protein (anti-NeuN,1:1500) immunoreactivity as visualized using Cy3-coupled secondary antisera (Roman et al., 2012). Only brains that displayed LPbn neuronal loss were used for further analyses.
2.5.3. Intra-amygdalar PACAP Infusion
Rats were anesthetized and secured in a stereotactic apparatus as described above. Four screws were secured into the exposed skull and two stainless steel cannulae (22 GA, PlasticsOne, Roanoke, VA) were targeted to the CeA bilaterally using coordinates (from bregma in mm) AP: -2.6, ML: +4.5, DV: -7.2. A dental cement skullcap was formed to secure the cannula and during the 7 day postsurgical recovery the rats were routinely wrapped in a towel to habituate handling. For treatments, the rats were similarly restrained in a towel and PACAP or vehicle (0.05% BSA in saline) was slowly infused (1 :g/0.5 :l each side) at 0.25 :l/min (Harvard Apparatus, Holliston, MA) through an internal cannula that projected 1 mm from the guide cannulae; the PAC1 receptor specific agonist maxadilan (from Ethan Lerner, Harvard/Massachusetts General Hospital) was similarly infused in some studies. The peptide concentrations and treatment procedures were similar to those described in previous work (Hammack et al., 2009; Koch-Schellenberg et al., 2014; Roman et al., 2014). The infusion cannula were left in place for an additional minute before removal. Animal body weights were determined before and 24 h after infusions for all experiments (Kocho-Schellenberg et al., 2014). At the end of each study, the rats were perfused with 4% paraformaldehyde and the brains cryosectioned for cresyl violet staining to confirm cannulae placement. Only data from correct CeA cannulae placements are described in Results. PACAP infusions into misplaced targets outside of the CeA, including the basolateral amygdala, had no effects on stress-related behavior, body weight, food consumption and water intake.
2.6 Behavioral Assessments
2.6.1. Elevated Plus Maze
The plus maze was elevated 75 cm from the floor and consisted of two opposing open and two opposing closed arms (each arm 50 cm long and 10 cm wide) that extended perpendicularly from a central square platform (10 × 10 cm). The length of the closed arms were walled with black opaque plastic panels 30 cm in height. Illumination using a red bulb was 6 lux at the center of the maze. The rats were first room habituated for 10 min and then individually placed in the center of the maze facing a closed arm for free exploration for 5 min. A ceiling mounted camera digitally captured all movements during each session for analyses.
2.6.2. Mechanical sensitivity testing
Mechanical sensitivity assessment was performed using von Frey monofilaments (Stoelting, Wood Dale, IL). All rats were first habituated in the clear acrylic testing chamber 20 min/day for 4 days with a fan to generate ambient noise. On day of testing, the rats were placed in the acrylic testing chamber on top of a metal mesh floor (IITC Life Science Inc., Woodland Hills, CA) and habituated again for 10 min before the application of von Frey filaments to the lateral plantar surface of the hindpaw. In ascending diameter thickness, each filament was applied until bent at 30 degrees for 5 - 7 sec. The smallest filament that evoked a paw withdrawal in 3 of 5 trials was used as the mechanical threshold for that trial. Thresholds from both the left and the right hindpaws were measured.
2.6.3. Thermal sensitivity testing
Responses to thermal stimuli were tested using a Hargreave's apparatus (Plantar Analgesia Meter, IITC Life Science Inc., Woodland Hills, CA). Prior to behavioral testing, the rats were first habituated in the acrylic testing chamber for 4 days. On day of testing, the rats were placed in an elevated clear acrylic testing chamber on top of a glass floor with an internal heating element that heated the glass to a consistent 30 C. Using a guide light to target the hindpaw, a beam of focused radiant light (4 × 6 mm, set to 25% of active intensity) from the apparatus beneath the glass floor was delivered to the plantar surface of the paw. Upon rat awareness of the heat stimuli, as indicated by withdrawal or licking of the hindpaw, the heat source was immediately terminated and the reaction time automatically recorded. An automatic cut-off timer set at 30 sec was built into the system to prevent tissue damage. Each time point represented the latency average of 3 trials from both the left and right hindpaw separated by 5 min inter-trial intervals. The PACAP, maxadilan and vehicle treatment groups exhibited comparable average baseline latency scores (PACAP, 12.9 sec; maxadilan, 12.5 sec; vehicle, 12.3 sec).
2.7 Experimental treatment and testing procedures
2.7.1. Experiment 1 - Behavioral effects of amygdala PACAP infusions on elevated plus maze
Adult male rats were cannulated for amygdala infusions as described in Surgical procedures (Section 2.5.3). The rats were handled daily for habituation and after 7 day postsurgery recovery, the rats were randomly assigned to vehicle or PACAP groups (n = 10 per group). On experimental day, the rats were weighed for baseline measures and bilaterally injected with vehicle or PACAP38 as described in random order. The injection needle was left in place for 1 min after which the rats were returned to their home cages for 30 min and habituated in the testing room (10 min) before evaluation on the elevated plus maze (Section 2.6.1.). The rats were allowed to freely roam the maze for 5 min and all data were captured digitally. At the same time the following day, the vehicle and PACAP-treated rats were re-weighed to assess weight change over 24 h; food and water consumption were also measured. All weight change measures in this and subsequent experiments were performed between 0900 and 1000 h. All behavioral tests were completed between 0900 and 1500 h; behavioral testing was randomized and counter balanced for order and time of testing.
2.7.2. Experiment 2 - Nociceptive effects of PACAP after amygdala infusions
Adult male rats were surgically prepared and handled as described in Sections 2.5.3. and Experiment 1 above. The rats received 2 days of baseline thermal and mechanical sensitivity testing, and on experiment day, the rats were weighed and received either vehicle or PACAP38 amygdala infusions (n = 6 per group) as described in random order. After 30 min, the rats were tested for mechanical sensitivity using von Frey filaments (Section 2.6.2.) and evaluated for thermal sensitivity on a Hargreave's apparatus (Section 2.6.3.) at subsequent time points (1 h, 4 h and 24 h). As before, weight change in the vehicle and PACAP-infused rats was assessed after 24 h; food and water consumption was also determined. As robust PACAP-induced thermal sensitivity was noted at 1 h, a separate study was prepared to better establish amygdala PACAP thermal nociception onset and persistence (30 min and 72 h time points) using exactly the same procedures (n = 7 - 8 per group). The thermal sensitivity data at the different time points from the two cohorts were combined for analyses in a linear mixed model using an autoregressive covariate structure as described in statistical methods (Section 2.8).
2.7.3. Experiment 3 - Nociceptive effects of amygdala maxadilan infusions
Adult male rats were surgically prepared, handled and treated exactly as described for the first study in Experiment 2 except for the application of maxadilan (n = 7 - 8 per group). Thirty min after amygdala maxadilan infusion, the rats were tested for mechanical sensitivity using von Frey monofilaments; at subsequent time points the rats were evaluated on a Hargreave's apparatus for thermal sensitivity.
2.8 Statistics
Statistical Student's t-tests were performed using GraphPad PRISM v.6. For analyses of thermal withdrawal thresholds, a linear mixed model using an autoregressive covariate structure was employed to allow combined analysis of two cohorts with differing timepoints, followed by pairwise comparisons between groups using Sidak-Holmes correction for multiple comparisons (MIXED procedure of the SAS System for Windows version 9.2; SAS Institute Inc, Cary, NC). All values represent the mean change ∀ SEM. P < 0.05 was considered significant.
3. Results
3.1. PACAP and CGRP are expressed in the CeA and BNST
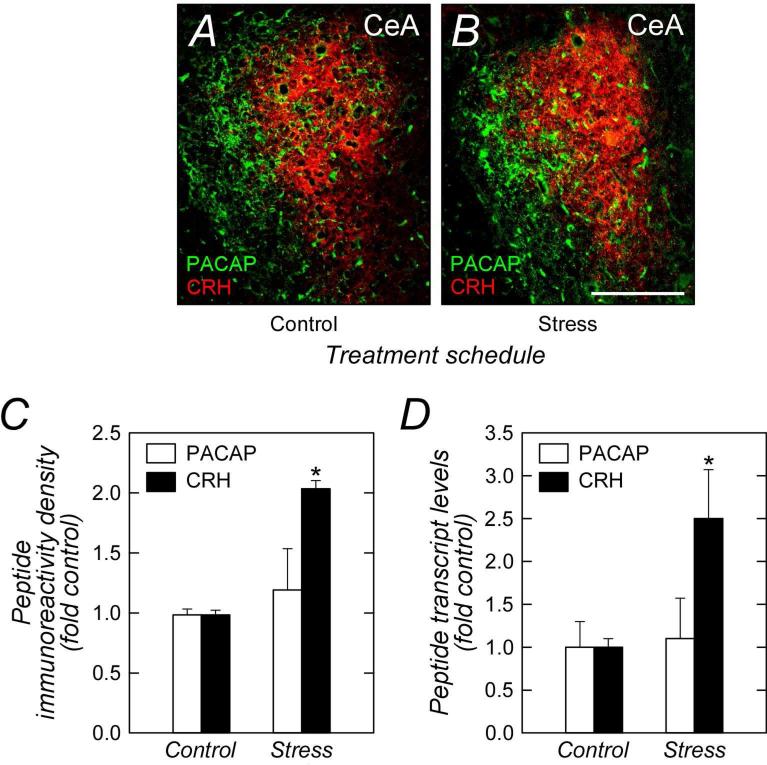
Our previous studies identified regulated PACAP expression in the BNST (Hammack et al., 2009). In evaluating PACAP expression in other limbic structures, we observed significant levels of PACAP immunoreactivity restricted to the lateral capsular division of the CeA (CeLC; Figure 1). A number of neuropeptides have been identified in the CeA including corticotropin releasing hormone (CRH) which has been shown to regulated by psychological stressors (Makino et al., 1999). However, unlike PACAP in the CeLC, CRH immunoreactivity in the amydala was prominent in the adjacent lateral (CeL) and medial (CeM) subdivisions of the CeA, recapitulating the apparent dichotomy of PACAP and CRH peptidergic pathways in the limbic system (Roman et al., 2014). Further, the pattern of PACAP and CRH expression following repeated stress appeared converse of that in the BNST. Whereas BNST PACAP was augmented after stress (Hammack et al., 2009; Roman et al., 2014), chronic stress increased CRH immunoreactivity levels in the CeA approximately 2-fold without altering CeA PACAP expression (Figure 1A - 1C). The stress-mediated changes PACAP and CRH staining in the CeA mirrored transcript expression patterns (Figure 1D) and in aggregate were suggestive of their distinct but complementary roles in stress pathways and behaviors.
Figure 1.
PACAP and CRH immunoreactivities are differentially distributed and regulated in the CeA. Tissue sections from control (A) and chronically stressed (B) rats were examined for CeA PACAP (Cy2, green) and CRH (Cy3, red) staining patterns. In both groups, CeA fiber PACAP immunoreactivity was predominantly in the lateral capsular region (CeLC) with diffuse staining extending into the lateral division (CeL); CRH immunoreactivity was localized predominantly to the CeL. From quantitative image analyses, only CRH immunoreactivity was augmented by chronic variate stress (C, n = 3). These results complemented quantitative PCR measurements which also demonstrated increased CRH transcript expression after stress (D, n = 6). Data represent mean ∀ SEM. Asterisk, significantly different from control at p < 0.05. Scale bar, 250 :m
A number of neuropeptides exhibit distinct expression patterns within the CeA (Cassell et al., 1986). From staining patterns the immunoreactivity for PACAP in the CeLC was largely punctate which appeared characteristic of terminals and varicosities of neuronal PACAP fiber projections from distal nuclei. As the CeLC is heavily innervated by the PBn in the spinoparabrachioamygdaloid tract (Bernard et al., 1996; Gauriau and Bernard, 2002; Rouwette et al., 2012; Veinante et al., 2013) and PACAP is highly expressed in sensory neurons in many pathways (Beaudet et al., 1998; Mulder et al., 1994; Pettersson et al., 2004b; Zhang et al., 1995), we examined whether the PACAP immunoreactivity in the CeLC reflected parabrachioamygdaloid projections. Further, as fibers in the CeLC have been described to contain CGRP immunoreactivity (Dobolyi et al., 2005), we also compared the relative distribution of PACAP and CGRP in the parabrachioamygdaloid tract.
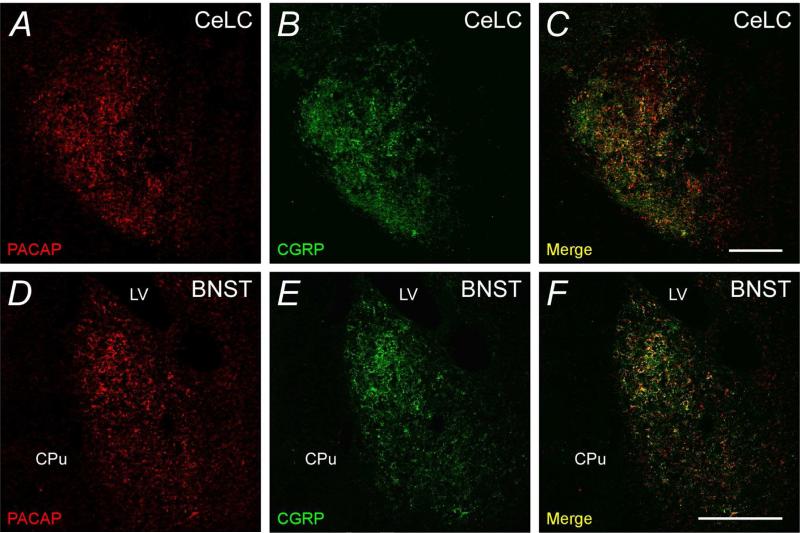
In these studies, PACAP and CGRP immunoreactivities displayed considerable overlap in fiber elements (Figure 2A - 2C) that appeared to form basket-like networks suggestive of axosomatic innervation of CeLC neurons. Given the heavy density of the peptide immunoreactivites, both Pearson's and Mander's correlation coefficients were determined for the acquired images to assess the extent of CeLC PACAP and CGRP colocalization. For both measures, scores closer to 1 represent greater degrees of overlap and from 4 independent studies, Pearson's r was > 0.7 and Mander's coefficient was > 0.6 (Mander's CGRP/PACAP ratio = 0.625; PACAP/CGRP ratio = 0.631).
Figure 2.
PACAP and CGRP immunoreactivities can be colocalized in the CeLC and BNST. Tissue sections for the amygdala (A - C) and BNST (D - F) were processed for dual PACAP (Cy3, red) and CGRP (Alexa488, green) immunocytochemical localization. The merged micrographs demonstrate that in both regions, PACAP and CGRP immunoreactivities were largely colocalized (yellow) in the same fiber structures. Amygdala, representative micrograph from 4 independent experiments; BNST, representative micrograph from 3 experiments. LV, lateral ventricle; CPu, caudate-putamen. Correlation coefficients described in text. Scale bar, 200 :m for corresponding tissues.
Since the bed nucleus of the stria terminalis (BNST) is part of the central extended amygdala and has been described to display both PACAP and CGRP expression and function (Hammack et al., 2009; Sink et al., 2011), the relationship between PACAP and CGRP within the BNST was also investigated. BNST PACAP and CGRP expression was highest within the oval nucleus (BNSTov) and as in the CeLC, PACAP and CGRP immunoreactivities were coexpressed in a majority of the fiber elements (Figure 2D - 2F). Image analyses were performed as before and from 3 independent experiments, Pearson's coefficient for PACAP and CGRP colocalization was approximately 0.7, and Mander's coefficient was approximately 0.6 (Mander's CGRP/PACAP ratio = 0.57; PACAP/CGRP ratio = 0.56). Hence the two statistical measures were in good agreement and suggested that more than half of the PACAP or CGRP neuronal fibers projecting to the CeLC and BNSTov expressed both peptides.
3.2. PACAP and CGRP immunoreactives in the CeLC and BNST are localized to projection fibers from pontine parabrachial nucleus (PBn)
From several considerations, our evaluations for the potential origins of the PACAP- and CGRP-expressing neurons projecting to the CeLC and BNSTov narrowed to the LPBn. The external LPBn contains a large population of PACAPergic neurons that may transmit signals to the amygdala (Das et al., 2007; Hannibal, 2002; Resch et al., 2013). Further, CGRP expression in the CeLC and BNSTov has been suggested previously to originate from PBn neurons (Dobolyi et al., 2005). Hence from these observations, we examined whether PACAP- and CGRP-expressing fibers to the CeLC and BNSTov were components of the parabrachioamygdaloid tract.
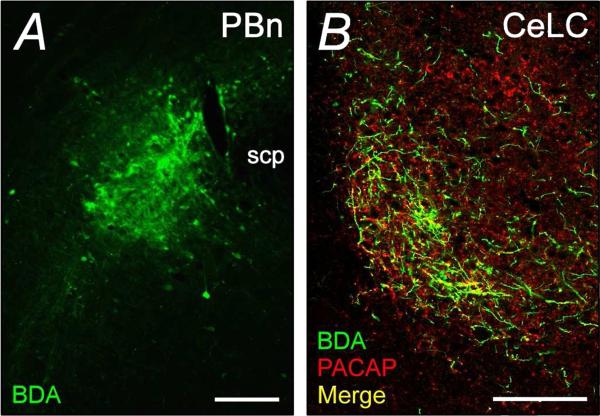
For these studies, we first evaluated whether anterograde fibers from the LPBn to the amygdala and BNST expressed PACAP. From injection site analyses, the BDA infusions into the LPBn was confined to a small area (Figure 3A). In the amygdala, the neuroanatomical tracer was confined to the CeLC and upon immunocytochemical processing, a subset of the BDA-labeled fibers in the CeLC expressed PACAP-immunoreactivity (Figure 3B). Although these results provided evidence for CeLC PACAP immuonoreactivity originating from the LPBn, the small focal size of the PBn BDA injection resulted in a modest number of labeled fibers in the CeLC. Hence the number of BDA labeled fibers was not as extensive as that observed for PACAP-immunoreactivity which precluded estimations of the relative contribution of CeLC PACAP immunoreactive fibers originating from the PBn. From the same limitations, the BDA-labeled fibers from the PBn to the BNST appeared low (data not shown).
Figure 3.
PBn projection fibers to the CeLC demonstrate PACAP immunoreactivity. Biotinylated dextran amine (BDA, 10 kD; 10%) was injected iontophoretically into the LPBn for anterograde transport into the CeLC over 14 days. BDA at the LPBn injection site (A) and in the projection fibers to the CeLC (B) were detected using streptavidin-conjugated Cy2 (green). Processing of the same CeLC sections for PACAP immunoreactivity (Cy3, red) demonstrated that the LPBn projection fibers can contain PACAP (B, merge in yellow). Representative data from 3 separate preparations. scp, superior cerebellar peduncle. Scale bar, 200 :m for corresponding tissues.
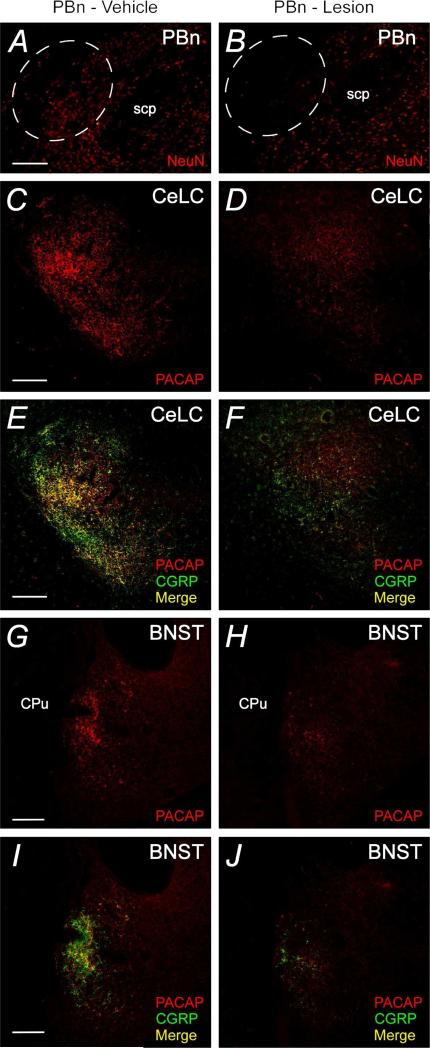
As an independent means of assessing peptide expression in LPBn projection fibers and to facilitate dual PACAP and CGRP immunocytochemistry in the same tissues, the LPBn was lesioned before amygdala and BNST immunocytochemistry (Figure 4). As the BDA anterograde fiber labeling studies demonstrated that the external lateral PBn only projected only to the ipsilateral amygdala, only one side of the PBn was lesioned so that the contralateral LPBn and limbic structures could remain intact and serve as vehicle controls. Accordingly, one side the LPBn was lesioned by excitotoxic NMDA injection (2 :g NMDA in 0.2:l) and after postsurgery recovery for 7 days, coronal brain cryosections were prepared to assess the extent of PBn lesion and altered peptide immunocytochemistry in the ipsilateral CeLC/BNST compared to staining patterns on the contralateral side. Only brain lesions with neuronal loss in the external LPBn as identified by diminished neu-N staining (Figure 4A and 4B) were used in subsequent analyses.
Figure 4.
Excitotoxic LPBn lesions diminish PACAP and CGRP fiber immunoreactivities in the CeLC and BNST. The LPBn was unilaterally lesioned with NMDA as described in Methods; the contralateral LPBn received vehicle. After 7 days, the PBn sections were processed for neuron-specific nuclear NeuN immunoreactivity (Cy3, red) to assess the specificity and extent of the lesion. Whereas vehicle injections had no apparent effects (A), NMDA injections produced substantial LPBn neuronal loss (B, dashed circled area). Representative vehicle treated and contralateral NMDA excitotoxic lesioned PBn in the same animal are shown; the lesioned image was flipped to facilitate comparison. CeA and BNST tissue sections from the NMDA excitotoxic lesioned animals were processed for dual PACAP and CGRP immunocytochemical localizations. Similar to Figure 2, tissue sections ipsilateral to LPBn - vehicle injections (left panels) demonstrated substantial PACAP (Cy3, red) and CGRP (AlexaFluor 488, green) colocalization in the CeLC (C and E) and BNST (G and I); colocalization in merged micrographs illustrated in yellow. By contrast, the same CeLC and BNST regions in the contralateral half that received LPBn NMDA excitotoxic lesion (PBn - lesion) demonstrated marked decreases in both PACAP and CGRP immunoreactivities. Again, micrographs from the stained CeLC and BNST regions from the PBn - lesioned side were flipped for comparisons with the control vehicle -injected side from the same animals to facilitate comparisons. These data were consistent with the colocalization of PACAP and CGRP in Figure 2. scp, superior cerebellar peduncle; LV, lateral ventricles; CPu, caudate putamen. Representative figures from 3 separate animals. Scale bar, 200 :m in corresponding tissues.
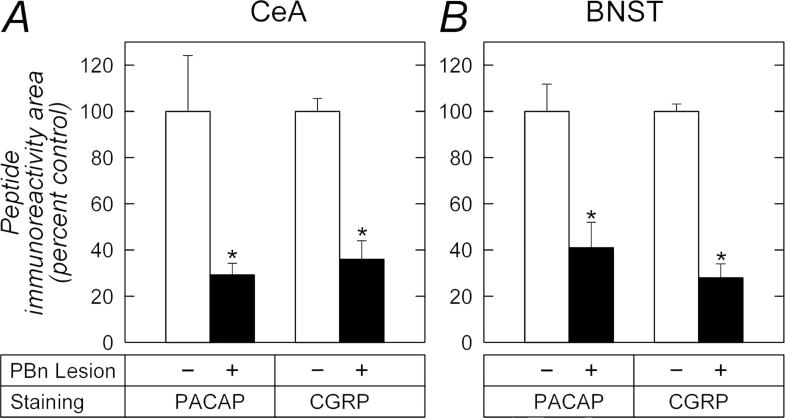
Following external LPBn lesion, PACAP and CGRP immunoreactivities in both the CeLC and BNSTov were greatly reduced. The tissue sections were simultaneously processed for PACAP and CGRP immunoreactivities and within subjects, CeLC PACAP immunoreactivity was diminished 70% [.universal] 5% on the side ipsilateral to the PBn lesion compared to staining levels in the contralateral CeLC in which the corresponding PBn received vehicle injection (t(2) = 4.41, p = 0.048; Figures 4C - 4D, and 5A). The same changes were observed in the BNSTov. PACAP staining levels in the BNSTov ipsilateral to the PBn lesion were diminished 59% ∀ 11% compared to the contralateral BNSTov with PBn vehicle injections (t(2) = 5.77, p = 0.029; Figures 4G - 4H, and 5B). As PACAP and CGRP demonstrated significant colocalization in these structures (Figure 2), a similar change in CGRP staining was therefore anticipated. From analyses, LPBn lesions resulted in a 64% ∀ 8% loss in CGRP immunoreactivity in the CeLC (t(2) = 7.49, p = 0.017) and 72% ∀ 6% in the BNSTov (t(2) = 8.90, p = 0.012) compared to contralateral structures with vehicle injections into the PBn (Figures 4E - 4F, 4I - 4J, 5A and 5B). Hence the anterograde labeling/lesion studies complement the immunocytochemical data to demonstrate that PACAP and CGRP can be colocalized in LPBn neurons and that their projections represent substantial components in the fiber tracts innervating the CeLC and BNSTov.
Figure 5.
CeA and BNST peptide immunoreactivities are diminished after PBn lesions. PACAP and CGRP immunoreactivities in the CeA (A) and BNST (B) from studies described in Figure 4 were subjected to image analyses as described in Methods. The PBn lesions decreased PACAP and CGRP immunoreactivities in the limbic regions to a comparable extent compared to levels on the contralateral hemisphere with PBn - vehicle injections. n = 3, data represent mean ∀ SEM. *, different from vehicle control at p < 0.05.
3.3. PACAP signaling in the amygdala alters emotional behaviors and pain responses
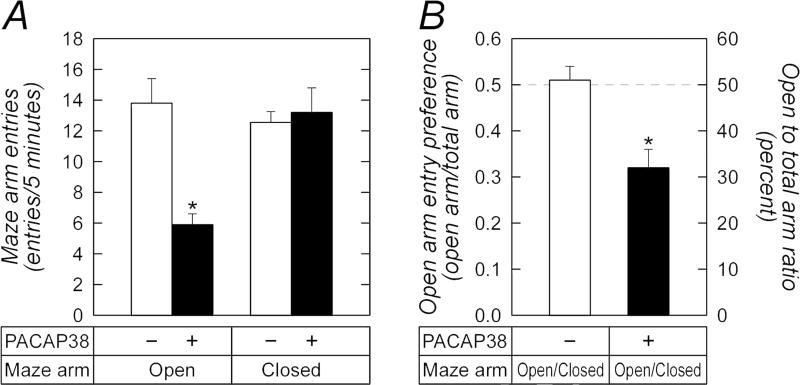
Our previous work demonstrated that PACAP signaling in the BNST enhances anxiety-related responses including increased baseline startle responses, decreased open arm entries on the elevated plus maze, decreased open field crossings, decreased exploratory behavior in novelty tests and decreased weight gain (Hammack et al., 2009; Kocho-Schellenberg et al., 2014; Roman et al., 2014). To examine whether PACAP expression and signaling in the central amygdala produced similar stress-related behavioral responses, we implanted bilaterally cannulae targeting the CeA for PACAP infusions (1 :g/0.5 :l) following previous treatment protocols (Hammack et al., 2009; Koch-Schellenberg et al., 2014; Roman et al., 2014; Experiment 1, Section 2.7.1.). Similar to PACAP-elicited responses in the BNST, amygdala PACAP infusions induced anxiety-like responses as shown by decreased open arm time (54.2 vs 88.3 sec, t(18) = 2.71; p = 0.01) and open arm entries (5.9 vs 13.8; t(18) = 4.39, p = 0.0003) compared to vehicle-treated animals on the elevated plus maze (Figure 6A). Unlike the BNST where PACAP had no apparent effects on locomotor activity, PACAP injections into the CeA appeared to produce a small but significant decrease in total distance traveled during the test period not attributed to spontaneous freezing behavior. To mitigate this potential confound, open arm preference (open : total arm entries) was calculated for each animal as this measure is less prone to locomotor vagaries. Whereas vehicle control animals had no preference for either open or closed arms (open : total arm entries = 0.51 ∀ 0.03), CeA PACAP-infused animals demonstrated diminished open arm preference (Figure 6B, open : total arm entries = 0.32 ∀ 0.04; t(18) = 3.70, p = 0.0015). These PACAP-mediated changes were comparable to those observed following BNST PACAP injections suggesting that PACAP signaling in the BNST and CeA can contribute to stress-related behaviors.
Figure 6.
PACAP infusions into the CeA decrease open arm entries on the elevated plus maze. Adult rats were cannulated as described in Methods for CeA PACAP infusions. Thirty minutes after PACAP injection, the animals were placed in the center square of the elevated plus maze, facing a closed arm, for behavior testing during a 5 min period. All movements were tracked digitally for data analyses. Total open arm entries (A) and open arm preference (B, open arm entries/total arm entries) were calculated. CeA PACAP signaling significantly increased anxiety-like behavior reflected by decreased number of open arm entries and open arm preference. There were no changes in the number of closed arm entries and there were no indications of freezing behaviors. n = 10 per group, data represent mean ∀ SEM, *, different from vehicle control p < 0.05.
Similar to stress-mediated behaviors, BNST PACAP infusions were also capable of inducing anorexia-like responses resulting dramatic animal weight loss over the next 24 h which approximated 5 - 8% of body weight and was reflected by decreased food consumption. Accordingly, animal weight changes were also monitored during the CeA PACAP infusion studies (Experiments 1 and 2, Sections 2.7.1. and 2.7.2.). After 24 h, animals with CeA PACAP injections demonstrated a small (~1%) but significant decrease in body weight compared vehicle treated animals (t(45) = 2.63, p = 0.012). Given the small weight changes, we sought to establish these observations using the PAC1 receptor selective agonist maxadilan (Experiment 3, Section 2.7.3.). CeA maxadilan infusions again produced a small decrease in body weight (1.5% decrease; t(13) = 2.81, p = 0.014) which was accompanied by diminished food intake (17.5% decrease; t(13) =2.66, p = 0.018) without apparent changes in water consumption (t(13) = 1.47, p = 0.163). These changes largely reflected the propensity for vehicle treated animals to gain a small amount of weight during the 24 h period while the PACAP treated animals experienced a slight weight loss. Hence, in apparent contrast to the BNST, the effects of CeA PACAP signaling on stress related anxiety-like responses did not appear to be strongly associated with weight and feeding changes.
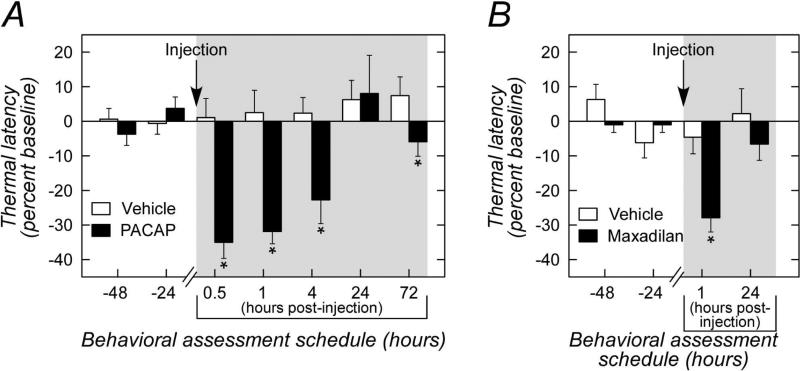
The fiber projections from the LPBn to the CeLC are part of the spino-parabrachial amygdaloid pathway conveying nociceptive information from the dorsal horn to the amygdala. PACAP has been identified at many sensory pathway intersections including the dorsal root ganglion, layers 1 and 2 of the dorsal horn, and from previous and current work, the LPBn. The CeLC responds to noxious stimuli and in modulating pain perception may contribute to the affective component of the pain experience. Hence from its attributes as a sensory peptide and its localization in the CeLC, we examined whether PACAP signaling in the amygdala also altered spinal pain-associated reflexes. As before, cannulae were placed into the amygdala bilaterally and following recovery, the rats were habituated for Hargreave's thermal nociception tests (Experiment 2, Section 2.7.2.). A baseline latency for hindpaw thermal withdrawal was first determined for each rat; PACAP was subsequently infused into the CeA and the temporal changes in hindpaw withdrawal to the same thermal stimuli were examined over the next 72 h. Following PACAP infusion, there was a significant reduction in paw withdrawal latency at 30 min (35% decrease in latency; veh, 13.0 ∀ 1.0 sec vs PACAP 9.1 ∀ 0.9 sec, p = 0.002; Figure 7A) and at 1 h (31% decrease in latency; veh, 11.8 ∀ 0.9 sec vs PACAP, 7.9 ∀ 0.6 sec, p = 0.011). The PACAP-induced responses persisted at 4 h (21% decrease in latency; veh, 11.3 ∀ 0.8 sec vs PACAP 8.9 ∀ 0.7 sec; p = 0.015) and returned to baseline by 24 h. There was a small but significant decrease in latency at 72 h post injection compared to the corresponding vehicle control group (p = 0.021); whether this reflected any PACAP-mediated plasticity in the CeA remains to be examined. Again, the thermal sensitivity responses were recapitulated with the PAC1 receptor-specific agonist maxadilan (Experiment 3, Section 2.7.3.). CeA maxadilan infusions decreased paw withdrawal latency approximately 24% (veh, 11.7 ∀ 0.9 sec vs maxadilan, 8.9 ∀ 0.6 sec; p = 0.002; Figure 7B) at 1 h which returned to baseline by 24 h. Overall, the PACAP and maxadilan results were robust and well reproducible across trials suggesting that intra-amgydalar PACAP signaling can facilitate thermal hyperalgesia.
Figure 7.
CeA PACAP/PAC1 receptor signaling increases thermal sensitivity. A, Rats were habituated in Hargreave's thermal sensitivity apparatus with 2 days of baseline assessments (24 and 48 h). PACAP was subsequently infused into the CeA (single injection) for thermal testing at the indicated time (shaded area). Whereas vehicle injection produced no apparent responses changes compared to baseline (white bars), CeA PACAP infusions consistently decreased thermal latency responses (black bars) up to 4 h post treatment. The responses dissipated by 24 h; the small but significant decrease in thermal latency at 72 h may reflect latent plasticity events. n = 6 - 8 per group, data represent mean response ∀ SEM, *, different from corresponding vehicle control, p < 0.025. B, the PACAP-induced decrease in thermal latency was mirrored in CeA infusions with the PAC1 receptor specific agonist maxadilan. The maxadilan responses observed at 1 h was again dissipated by 24 h. n = 7 - 8 per group, data represent mean response ∀ SEM, *, different from corresponding vehicle control, p = 0.002.
To assess whether CeA PACAP infusion would elicit similar changes on mechanical threshold, the same animals were also evaluated using von Frey hair stimulation tests (Experiment 2). From baseline tests, all animals demonstrated decreases in mechanical threshold after repeated trials over time. Although mechanical threshold in the PACAP-and maxadilan-treated rats appeared decreased compared to vehicle control animals after 30 min, analyses revealed a trend rather than statistical difference (PACAP, t(10) = 1.7, p = 0.11; maxadilan, t(13) =1.65, p = 0.12) which reflected in part the high variability within the assay. These apparent PACAP changes in mechanical threshold dissipated by 2 h post-peptide infusion. As thermal and mechanical pain are transduced by separate mechanisms, these differences may have contributed to the observed efficacy of PACAP between the two measures. Nevertheless, the ability for PACAP to modulate pain responses via amygdala signaling appears novel and suggests that it may carry nociceptive information to impact the behavioral and emotional aspects of pain.
4. Discussion
The central nucleus of the amygdala integrates nociceptive and stress-related signals that may be important for behavioral responses and the formation of emotional memory. In examining PACAP/PAC1 receptor expression and function in the limbic system, we identified high levels of fiber PACAP immunoreactivity in the CeLC. The CeLC is innervated by LPBn neurons that form part of the spino-parabrachioamygdaloid pathway and although PBn PACAP expression was previously described, the targets of these PBn PACAP neurons were not identified. Our current work identified PACAP immunoreactivity in anterogradely labeled LPBn projection fibers to the CeLC, and importantly, LPBn lesions significantly abolished PACAP immunoreactivity in the CeLC and BNST. These studies were also revealing in demonstrating the relationships between PACAP and other CeA peptides. Both CRH and CGRP share functional similarities with PACAP in mediating pain, stress and anxiety-like behaviors (Hammack et al., 2002; Koob and Heinrichs, 1999; Lee and Davis, 1997; Sink et al., 2011). Yet the dual localizations studies demonstrated a dichotomy in PACAP and CRH expression pattern; the localization of PACAP predominantly to the CeLC was distinct from CRH in the CeL which suggested separate but coordinate functions in intra-amygdalar neurocircuits. By contrast, PACAP and CGRP immunoreactivities in CeLC and BNST fibers were well colocalized from image analyses, and PBn lesions abolished much of the staining for both peptides in the CeLC and BNST to a comparable extent. Limbic PACAP and CGRP signaling share similarities in feeding and anxiety-like behaviors (Carter et al., 2013; Hammack et al., 2009; Kocho-Schellenberg et al., 2014; Sink et al., 2011); how their coordinate signaling modulates CeA and BNST functions however, remains to be evaluated.
Despite the extensive PACAP and CGRP colocalizations (60 - 70%), PACAP and CGRP may also exhibit independent CeLC and BNST functions. After LPBn lesions the remaining PACAP and CGRP immunoreactivities appeared largely dissociate (Pearson's coefficient 0.3 - 0.4) which may have represented endogenous CeLC/BNST peptide expression or PBn subpopulations expressing one of the peptides not affected by the lesion procedures. The former may be consistent with the upregulation of BNST PACAP transcripts by chronic stress (Hammack et al., 2009). PACAP and CGRP immunoreactivities in subpopulations of dorsal root ganglion (DRG) neurons for example can be separate and overlapping (Mulder et al., 1994), and comparable expression patterns may be present in the PBn and limbic structures.
The presence of PACAP in the parabrachioamygdaloid pathway has prominent implications in its roles modulating the sensory and emotional consequences of pain. The ability for the amygdala to integrate pain processes and the emotional aspects of behavioral has been well appreciated (Bernard et al., 1992; Gauriau and Bernard, 2002; Ulrich-Lai et al., 2006; Morano et al., 2008; Rouwette et al., 2011; Rouwette et al., 2012; Veinante et al., 2013) and among its many functions, the roles of PACAP as a sensory peptide are well recognized. PACAP and its PAC1/VPAC receptor subtypes are expressed in central and peripheral nervous system regions that mediate nociception. PACAP is found in small-diameter nociceptive DRG and in lamina I/II of the spinal cord neurons (Beaudet et al., 1998; Mulder et al., 1994; Pettersson et al., 2004a; Pettersson et al., 2004b), and neuropathic pain through axotomy, chemical induced cystitis or related models of nerve injury, can induce long-lasting upregulation of PACAP or PACAP receptor expression in these tissues (Dickinson et al., 1999; Mulder et al., 1994; Pettersson et al., 2004a; Vizzard, 2001). In the central nervous system, PACAP can be found in many regions such as the hypothalamus, limbic system, hippocampus, various brainstem nuclei including the PBn, and a number of thalamic and cortical regions implicated in pain processing (Das et al., 2007; Hannibal, 2002; Resch et al., 2013) .
However, the early investigations on PACAP in mediating pain were equivocal resulting in hyperalgesia in some experimental paradigms and hypoalgesia in others. These divergent responses likely reflected differences in the time course used in pain assessments in the different experimental models, and the peripheral vs central actions of PACAP. Peripheral intraplantar PACAP injections, for example, appeared to produce mechanical hypoalgesia in both the early and late stages of inflammatory pain (Sandor et al., 2009) whereas intrathecal injections were hyperalgesic (Ohsawa et al., 2002). In detailed studies, intrathecal PACAP administration resulted in an immediate analgesic response as measured by tail flick latencies, but transitioned into a long lasting hyperalgesia as demonstrated by increased aversive responses (Shimizu et al., 2004). By contrast, the studies using PACAP and PAC1 receptor knockout mice demonstrated unequivocally a role for PACAP signaling in the development of persistent pain. Mice deficient in PACAP or PAC1 receptor do not develop normal pain responses after arthritic pain or neuropathic pain (Jongsma et al., 2001; Mabuchi et al., 2004). PACAP knockout mice do not display thermal hyperalgesia or mechanical allodynia after intraplantar carrageenan injection or spinal nerve transection, but show normal acute nociceptive processes compared to wildtype mice (Mabuchi et al., 2004). In congruence, PAC1 receptor null mice exhibit dramatic decreases in thermal and mechanical nociceptive responses in the late phase of the formalin test, but preserve acute nociceptive processes in unchallenged states (Jongsma et al., 2001). Hence PACAP/PAC1 receptor signaling and resulting neuroplasticity appear critical in the central sensitization and development of persistent pain states.
Fibers from the lamina I spinal cord neurons carry thermal and mechanical noxious stimuli and project heavily via the spino-parabrachioamygdaloid tract to the lateral and external medial PBn (Gauriau and Bernard, 2002; Todd, 2010). From the convergence of these projections onto the PBn, the sensory representations on the PBn neurons are therefore necessarily large, covering several areas of the body. The majority of PBn neurons then project onto the lateral division of the BNST, the ventral medial hypothalamus (VMH), and the CeA; interestingly, as in the dorsal horn, high levels of PACAP expression are found within all of these regions. The LPBn prominently innervates the CeLC and consistent with the modalities conveyed by the tract, in vivo electrophysiological studies demonstrate that these CeLC neurons are selectively activated by thermal and mechanical nociceptive stimuli with receptive fields that can encompass the entire body. Hence from the broad body areas capable of stimulating the PBn and CeLC, the stimulus-response profiles, and the demonstration that spino-parabrachioamygdaloid tract lesions in the dorsolateral funiculus does not modify noxious stimuli response latency/threshold, the amygdala does not appear to mediate sensory discrimination but the affective-emotional and behavioral consequences of pain.
Many models of visceral, inflammatory and neuropathic pain have been shown to increase not only CeA neuronal excitability and PBn-CeA transmission, but also CeA c-fos expression and ERK activation (Veinante et al., 2013) which may play roles in pain-related neuroplasticity. Among bioactive peptides, CeA infusions with oxytocin, neurotensin and galanin have produced antinociceptive responses (Dobner, 2006; Jin et al., 2010; Robinson et al., 2002); interestingly, CRH and CGRP have been described to produce either nociceptive or antinociceptive processes which may have been related to dose and temporal parameters (Cui et al., 2004; Han et al., 2010; Ji et al., 2013; Xu et al., 2003). To facilitate understandings of PACAP roles in the CeLC, our current studies demonstrated that PACAP infusions into the CeA heightened noxious stimuli responses, especially in thermal reactivity tests. The effects of PACAP can be mediated by PAC1/VPAC receptors and notably, the PACAP-elicited CeA stress and nociceptive effects were recapitulated using maxadilan to implicate specific activation of the PAC1 receptor in these responses. Although the studies did not discriminate hyperalgesia from allodynia or spontaneous pain, the decrease in hindpaw withdrawal latency after CeA PACAP treatment was robust to clearly demonstrate altered sensory responses. The CeA PACAP effects in mechanical sensitivity assessments, however, appeared smaller which may have reflected assay variability in the testing protocol or neuronal responses to specific sensory modalities. The PBn responses to thermal stimuli are greater than those from mechanical stimuli (Bernard et al., 1996) and whether these mechanistic signals to the CeLC resulted in smaller PACAP-mediated mechanical responses remain to be established. The CeLC has major projections to the BNST, the dorsal substantia innominata and the medial CeA (CeM) which represents the major output of the CeA. The CeM has reciprocal projections to other nociceptive effector centers including thalamic nuclei, periaqueductal gray, lateral hypothalamus, ventromedial reticular formation, substantia nigra, rostral tegmental area, locus coeruleus, and dorsal raphe complex; hence in aggregate, the CeA is well integrated within ascending and descending pathways to influence nociceptive signal processing and responses.
The amygdala assigns emotional valence to extrinsic challenges and has been well studied with respect to fear. The prominent nociceptive inputs to the CeLC and in particular the high levels of PACAP expression carrying nociceptive information in the spinoparabrachioamygdaloid tract provide important mechanistic insights on how chronic pain can initiate and/or amplify stress-related behavioral abnormalities, including depression and anxiety disorders. As in the BNST, PACAP signaling in the amygdala promoted anxiety-like responses. CeA PACAP infusions decreased open arm time, entries and preference on the elevated plus maze which appeared comparable in efficacy compared to that observed from BNST signaling. Although CeA PACAP infusions may have induced nociceptive sensitivity to decrease locomotion and affect behavior, mitigating the potential confound by open arm preference analyses still demonstrated PACAP-mediated increases in anxiety-like behaviors. Conversely, there is also a small possibility that CeA PACAP-induced stress- and anxiety-related behaviors may have contributed to the heightened nociceptive responses described above; this consideration is being pursued in ongoing studies. However, unlike the overt BNST PACAP-elicited anorexia that accompanied the stress-related behavioral responses, CeA PACAP signaling had modest effects on feeding and weight change. These observations suggested that the PACAP effects on stress-related behaviors and feeding may be not be strongly associated mechanisms or circuits; the small changes in weight, for example, may have reflected PACAP effects on thermogenesis (Hawke et al., 2009). Interestingly, the PBn PACAP projections to the BNST also implicate direct nociceptive transmission to the BNST and in agreement, the anterolateral BNST has been shown to participate in pain and stress-induced nociceptive hypersensitivity (Morano et al., 2008; Rouwette et al., 2011; Tran et al., 2012). As in the BNST (Roman et al., 2014), preliminary experiments have shown that PACAP6-38, a PAC1/VPAC2 receptor antagonist, is capable of attenuating the effects of CeA PACAP signaling (data not shown). Although the neurocircuits and mechanisms underlying the CeA PACAP effects have not been examined extensively, one PACAP function has been suggested to potentiate excitatory transmission at the BLA - CeL synapse by enhancing post-synaptic AMPA receptor levels (Cho et al., 2012). The identities of PACAP targets in the CeLC, the functional mechanisms and consequences of PACAP CeLC signaling, and the functional relationships between PACAP and CGRP and CRH activities all remain to be investigated.
In summary, our results suggest that PACAP signaling via nociceptive fibers in the spino-parabrachioamygdaloid and associated tracts to the CeA and BNST may represent mechanisms that associate chronic pain with hypersensitivity and behavioral abnormalities including depression and anxiety-related disorders. Previous studies have shown that PACAP is a pleiotropic peptide with neurotransmitter, hormonal and neurotrophic functions which can facilitate neuroplasticity in development and regeneration after injury. PACAP signaling in chronic stress, fear and pain may facilitate the neuronal remodeling and plasticity in the limbic system that promote the maladaptive behavioral responses, and transition short-term memory to long term forms that appear necessary for fear memory consolidation associated with PTSD.
Highlights.
! Lateral parabrachial PACAP fibers project to the capsular central amygdala (CeA)
! The PACAP fibers can co-express CGRP
! CeA PACAP/PAC1 receptor signaling increases anxiety-like responses
! CeA PACAP signaling increases thermal/mechanical sensitivity
! CeA PACAP signaling may mediate the adverse emotional consequences of chronic pain
Acknowledgments
We thank Alan B. Howard for statistical data analyses in some of these studies.
Funding
This work was supported by grants MH-97988 (SEH/VM), MH-072088 (SEH), MH-096764 (VM), DK-051369, DK-060481 and DK-065989 (MAV), and funds from the University of Vermont (UVM) Center of Biomedical Research Excllence (Neuroscience COBRE, NCRR P30RR032135/NIGMS P30 GM103498), the National Alliance for Research on Schizophrenia and Depression (NARSAD) and UVM College of Arts and Sciences.
Abbreviations
- PACAP
pituitary adenylate cyclase activating polypeptide
- PAC1
PACAP selective receptor
- CGRP
calcitonin gene related peptide
- CRH
corticotropin releasing hormone
- PBn
parabrachial nucleus
- LPBn
lateral parabrachial nucleus
- CeA
central nucleus of the amygdala
- CeLC
CeA - lateral capsular division
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
References
- Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol. 2005;564:907–921. doi: 10.1113/jphysiol.2005.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon K, Lantéri-Minet M, Michiels JF, Menétrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP). Exp Brain Res. 1998;122:165–174. doi: 10.1007/s002210050504. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron NPY release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Baccarelli AA, Weeks DE, Kolls JK, Canino G, Celedón JC. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187:584–588. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WAJ, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci. 2012;32:14165–14177. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XY, Lundeberg T, Yu LC. Role of corticotropin-releasing factor and its receptor in nociceptive modulation in the central nucleus of amygdala in rats. Brain Res. 2004;995:23–28. doi: 10.1016/j.brainres.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol. 2007;500:761–776. doi: 10.1002/cne.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson T, Mitchell R, Robberecht P, Fleetwood-Walker SM. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology. 1999;38:167–180. doi: 10.1016/s0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Dobner PR. Neurotensin and pain modulation. Peptides. 2006;27:2405–2414. doi: 10.1016/j.peptides.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Girard BM, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y, El Meskini R, Ronnett GV, Waschek JA, May V. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase activating peptide in the rat central nervous system: an immunocytochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29:14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol Pain. 2013;9:2. doi: 10.1186/1744-8069-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol. 2009;102:2253–2264. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin WY, Liu Z, Liu D, Yu LC. Antinociceptive effects of galanin in the central nucleus of amygdala of rats, an involvement of opioid receptors. Brain Res. 2010;1320:16–21. doi: 10.1016/j.brainres.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Jongsma H, Pettersson LM, Zhang YZ, Reimer MK, Kanje M, Waldenstrom A, Sundler F, Danielsen N. Markedly reduced chronic nociceptive response in mice lacking the PAC1 receptor. Neuroreport. 2001;12:2215–2219. doi: 10.1097/00001756-200107200-00034. [DOI] [PubMed] [Google Scholar]
- Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E, Toufexis DJ, Braas KM, May V, Hammack SE. Pituitary adenylate cyclase activating peptide (PACAP) in the bed nucleus of the stria terminalis (BNST) produces anorexia and weight loss in male and female rats. Neuropsychopharmacology. 2014;39:1614–1623. doi: 10.1038/npp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Shintani N, Matsumura S, Okuda-Ashitaka E, Hashimoto H, Muratani T, Minami T, Baba A, Ito S. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci. 2004;24:7283–7291. doi: 10.1523/JNEUROSCI.0983-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- Morano TJ, Bailey NJ, Cahill CM, Dumont EC. Nuclei- and condition-specific responses to pain in the bed nucleus of the stria terminalis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:643–650. doi: 10.1016/j.pnpbp.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Katsuya A, Tanimoto S, Yamamoto J, Yamauchi Y, Minami M, Satoh M. Differential patterns of c-fos mRNA expression in the amygdaloid nuclei induced by chemical somatic and visceral noxious stimuli in rats. Neurosci Lett. 2003;344:197–200. doi: 10.1016/s0304-3940(03)00465-8. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa M, Brailoiu GC, Shiraki M, Dun NJ, Paul K, Tseng LF. Modulation of nociceptive transmission by pituitary adenylate cyclase activating polypeptide in the spinal cord of the mouse. Pain. 2002;100:27–34. doi: 10.1016/s0304-3959(02)00207-5. [DOI] [PubMed] [Google Scholar]
- Pettersson LM, Dahlin LB, Danielsen N. Changes in expression of PACAP in rat sensory neurons in response to sciatic nerve compression. Eur J Neurosci. 2004a;20:1838–1848. doi: 10.1111/j.1460-9568.2004.03644.x. [DOI] [PubMed] [Google Scholar]
- Pettersson LM, Heine T, Verge VM, Sundler F, Danielsen N. PACAP mRNA is expressed in rat spinal cord neurons. J Comp Neurol. 2004b;471:85–96. doi: 10.1002/cne.20015. [DOI] [PubMed] [Google Scholar]
- Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab. 2013;305:E1452–E1463. doi: 10.1152/ajpendo.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, J., M. A., Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Kocho-Schellenberg M, Garret MA, Braas K, May V, Hammack SE. Excitotoxic lesions of the bed nucleus of the stria terminalis (BNST) attenuate the effects of repeated stress on weight gain: evidence for the recruitment of BNST activity by repeated, but not acute, stress. Behav Brain Res. 2012;227:300–304. doi: 10.1016/j.bbr.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V. PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology. 2014;47:151–165. doi: 10.1016/j.psyneuen.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwette T, Vanelderen P, de Reus M, Loohuis NO, Giele J, van Egmond J, Scheenen W, Scheffer GJ, Roubos E, Vissers K, Kozicz T. Experimental neuropathy increases limbic forebrain CRF. Eur J Pain. 2011;16:61–71. doi: 10.1016/j.ejpain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Rouwette T, Vanelderen P, Roubos EW, Kozicz T, Vissers K. The amygdala, a relay station for switching on and off pain. Eur J Pain. 2012;16:782–792. doi: 10.1002/j.1532-2149.2011.00071.x. [DOI] [PubMed] [Google Scholar]
- Sandor K, Bolcskei K, McDougall JJ, Schuelert N, Reglodi D, Elekes K, Petho G, Pinter E, Szolcsanyi J, Helyes Z. Divergent peripheral effects of pituitary adenylate cyclase-activating polypeptide-38 on nociception in rats and mice. Pain. 2009;141:143–150. doi: 10.1016/j.pain.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Katahira M, Sugawara H, Inoue K, Miyata A. Diverse effects of intrathecal pituitary adenylate cyclase-activating polypeptide on nociceptive transmission in mice spinal cord. Regul Pept. 2004;123:117–122. doi: 10.1016/j.regpep.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: Evidence from neuroimaging. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22199. in press., doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Suwanprathes P, Ngu M, Ing A, Hunt G, Seow F. c-Fos immunoreactivity in the brain after esophageal acid stimulation. Am J Med. 115 Suppl. 2003;3A:31S–38S. doi: 10.1016/s0002-9343(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Wiskur B, Greenwood-Van Meerveld B. The role of the anteriolateral bed nucleus of the stria terminalis in stress-induced nociception. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1301–G1309. doi: 10.1152/ajpgi.00501.2011. [DOI] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Xie W, Meij JTA, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88:67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD's emotional numbing symptoms in Chinese earthquake survivors. J Affect Disord. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Xu W, Lundeberg T, Wang YT, Li Y, Yu LC. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience. 2003;118:1015–1022. doi: 10.1016/s0306-4522(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, Fahrenkrug J, Hökfelt T. Expression of pituitary adenylate cyclase -activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–158. doi: 10.1016/0006-8993(95)01150-1. [DOI] [PubMed] [Google Scholar]