Abstract
Background
Previous studies suggest that mitral valve replacement is comparable to repair in the elderly, and a national trend exists toward tissue valves. However, few direct comparison data are available, and this study evaluated the effects of patient age on risk-adjusted survival after mitral procedures.
Methods
From 1986 to 2006, 2,064 patients underwent isolated primary mitral operations (±CABG). Maximal follow-up was 20 years with a median of 5 years. Valve disease etiology was the following: degenerative, 864; ischemic, 450; rheumatic, 416; endocarditis, 98; and “other,” 236. Overall, 58% had repair and 39% had concomitant coronary artery bypass grafting. Survival differences were evaluated with a Cox proportional hazards model that included baseline characteristics, valve disease etiology, and choice of repair versus replacement with tissue or mechanical valves.
Results
Baseline risk profiles generally were better for mechanical valves, and age was the most significant multivariable predictor of late mortality [hazard ratio = 1.4 per 10-year increment, Wald χ2 = 32.7, p < 0.0001]. As compared with repair, risk-adjusted survival was inferior with either tissue valves [1.8, 27.6, <0.0001] or mechanical valves [1.3, 8.1, 0.0044], and no treatment interaction was observed with age (p = 0.18). At no patient age did tissue valves achieve equivalent survival to either repair or mechanical valves.
Conclusions
Mitral repair is associated with better survival than valve replacement across the spectrum of patient age. If replacement is required, mechanical valves achieve better outcomes, even in the elderly. These data suggest that tissue valves should be reserved only for patients with absolute contraindications to anticoagulation who are not amenable to repair.
Improvements in mitral repair have increased the number of valves amenable to autologous reconstruction, as compared with prosthetic valve replacement [1–22]. Nationally, repair rates for isolated mitral procedures have increased to almost 70% in the most recent National sample [23]. While newer analyses suggest that patients with ischemic or degenerative mitral regurgitation experience better survival after valve repair [24, 25], techniques and applicability of mitral repair, as well as the most effective approach for older patients, are controversial [5, 6, 24–35]. National data indicate that elderly patients more frequently receive tissue mitral valve replacement, and this trend seems to be increasing [23]. Unfortunately, few direct multivariable comparisons are available to document outcomes for mitral repair versus replacement in the elderly, as well as for contemporary bioprosthetic versus mechanical valves. The purpose of this study was to examine the influence of patient age on survival after mitral valve repair, and to compare repair survival with that observed with both mechanical and tissue valves.
Material and Methods
This study was performed with approval from the Duke Institutional Review Board and under a waiver of informed consent, but new late patient contact was not allowed. In the Duke Databank for Cardiovascular Disease, 2,064 consecutive patients with isolated mitral disease who underwent cardiac surgery from January 1, 1986 through December 31, 2006 were reviewed. Patients having concomitant coronary artery bypass grafting (CABG) or electrophysiologic procedures were included, but other major cardiac procedures were excluded (eg, aortic valves, tricuspid valves, postinfarct ventricular septal defects, ventricular aneurysm repair). While patients with previous CABG were included, those with previous mitral replacement were excluded, because they were not candidates for either procedure.
Preoperative baseline and intraoperative characteristics for all patients were recorded prospectively over the entire 20 years, with consistent variables throughout. Late outcome data were collected prospectively on patients with significant concomitant coronary disease per Duke Databank protocols. A National Death Index search was conducted through 2006 to acquire mortality results for remaining patients. Patients were divided into two groups; the first was patients having mitral repair (n = 1,188), and the second was patients having prosthetic valve replacement (n = 876) with mechanical valves (n = 680 [78%]; predominantly St. Jude valves [St. Jude Medical, Inc, St. Paul, MN] or tissue valves (n = 196 [22%]; predominantly Carpentier Edwards [Edwards Life-sciences, Irvine, CA] porcine or pericardial bioprostheses). Operative notes of all 2,064 patients were audited to ensure proper categorization. Most repairs had full ring annuloplasty (usually Edwards Physio, Carpentier classic, or Séguin [St Jude Medical] rings) along with appropriate leaflet or chordal procedures. Innumerable different repair combinations were used, depending on surgeon preference, anatomy encountered, and evolution of techniques over time, and 18 different surgeons contributed patients. Partial or total chordal sparing valve replacement was performed frequently, but this variable was not documented well and was not assessed in the analysis. Follow-up for survival was 92% complete and only all-cause mortality was available consistently for analysis.
Baseline characteristics and clinical event rates were described using medians with 25th and 75th percentiles for continuous variables and frequencies and proportions for categoric variables. Descriptive data were compared using the Wilcoxon rank-sum test for continuous and ordinal variables, and a Pearson χ2 or Fisher’s exact test for categoric variables. Three propensity models were created to determine the propensity for repair versus mechanical replacement, repair versus tissue replacement, and mechanical versus tissue replacement [36]. A multivariable Cox proportional hazards regression model was employed with an analysis strategy that adjusted for the impact of baseline characteristics on survival [37]. To develop the risk-adjustment model, a pool of all known clinical covariates that have been shown to be important in previous analyses was developed [25]. Variables proving significant by stepwise univariable-multivariable procedures were included in the final Cox model and also used for risk adjustment. Propensity scores also were included in the Cox model, as were the valve repair-replacement variables of interest. Continuous and ordinal variables were tested for linearity over the log hazard and transformed as necessary. Adjusted survival estimates for each group were calculated by applying their baseline hazard functions, along with parameter estimates, to all patients in the entire cohort and then averaging over all patients at each time point. Statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC), and a p value of 0.05 or less was considered significant.
Results
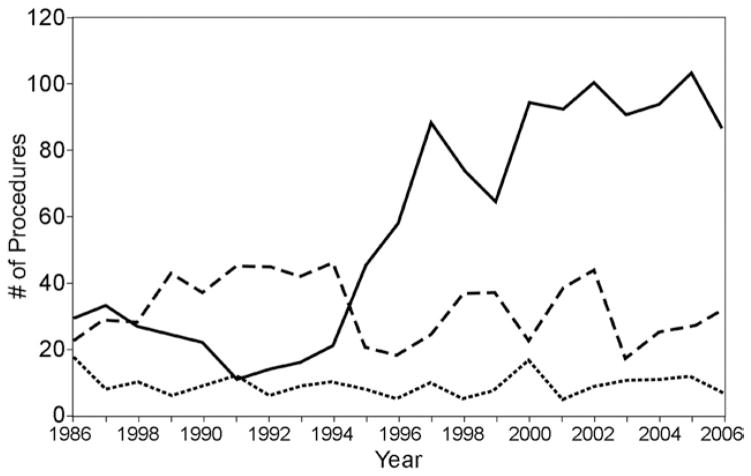
Baseline characteristics of the entire population are detailed in Table 1. Among the groups, tissue replacement patients were significantly older with less elective surgery. Mechanical replacement patients were younger, and repair patients were more predominantly male, had a higher incidence of concurrent 3-vessel disease and CABG, and lower ejection fractions. Procedural incidence over time is shown in Figure 1.
Table 1.
Baseline Characteristics of Overall Population
| Total (n = 2,064) | Mitral Valve Repair (n = 1,188) | Tissue Mitral Valve Replacement (n = 196) | Mechanical Mitral Valve Replacement (n = 680) | Overall p Value | |
|---|---|---|---|---|---|
| Age | 64 (53, 72) | 64 (53, 72)a,b | 72 (63, 77)c,b | 62 (52, 70)c,a | <0.0001 |
| Gender | |||||
| % Male | 46% | 54%a,b | 33.7%c | 36%c | <0.0001 |
| % Female | 54% | 46%a,b | 66%c | 64% | |
| Caucasian race | 76% | 76% | 74% | 77% | 0.6581 |
| History of diabetes | 17% | 19%b | 16% | 13%c | 0.0020 |
| Hypertension | 50% | 55%b | 49% | 44%c | <0.0001 |
| Hyperlipidemia | 34% | 39%a,b | 28%c | 28%c | <0.0001 |
| BMI | 26 (23, 30) | 26 (23, 30)a | 25 (22, 28)c,b | 26 (23, 30)a | 0.0024 |
| History of renal failure | 4% | 4%a,b | 10%c,b | 2%c,a | <0.0001 |
| NYHA class | |||||
| I | 32% | 33% | 29% | 29% | 0.0428 |
| II | 15% | 16% | 16% | 14% | |
| III | 31% | 30% | 26% | 35% | |
| IV | 22% | 21% | 29% | 22% | |
| Chronic lung disease | 10% | 10% | 9% | 10% | 0.8542 |
| Infectious endocarditis | 3% | 2%a | 7%c,b | 3%a | <0.0001 |
| History of CVA | 10% | 9% | 9% | 11% | 0.1893 |
| History of MI | 24% | 30%a,b | 21%c | 16%c | <0.0001 |
| History of tobacco abuse | 42% | 41% | 39% | 44% | 0.3539 |
| Ejection fraction | 0.50 (0.40, 0.60) | 0.50 (0.34, 0.58)a,b | 0.55 (0.45, 0.64)c | 0.55 (0.45, 0.63)c | <0.0001 |
| 3-vessel disease | 22% | 29%a,b | 19%c,b | 11%c,a | <0.0001 |
| Previous CABG | 3% | 3% | 5% | 2% | 0.1366 |
| Concomitant CABG | 39% | 46%b | 39%b | 29%c,a | <0.0001 |
| Clinical status: | |||||
| Elective | 70% | 68%a,b | 59%c,b | 75%c,a | <0.0001 |
| Nonelective | 30% | 32%a,b | 41%c,b | 25%c,a | |
p < 0.05 compared with tissue replacement;
p < 0.05 compared with mechanical replacement;
p < 0.05 compared with repair.
BMI = body mass index; CABG = coronary artery bypass grafting; CVA = cerebrovascular accident; MI = myocardial infarction; NYHA = New York Heart Association.
Fig 1.
Incidence of mitral procedures over time. (— = repair; ··· = replacement-tissue; --- = replacement-mechanical.)
In an analysis subset 65 years of age or greater (n = 998 [data table available at jsrmd.com/table_elderly.pdf and jsrmd.com/90_day_coefficients.pdf]), baseline characteristics were more similar, but mitral repair patients (n = 563) still had more 3-vessel disease, CABG, nonelective presentation, and lower ejection fractions. Mitral replacement patients (mechanical, n = 293; tissue, n = 142) were more predominantly female. Regardless of age and operative procedure, the most common etiology of mitral valve disease was degenerative followed by ischemic (Table 2). Rheumatic patients comprised 20% of the population and more frequently underwent mitral replacement (88%), while ischemic and degenerative usually had repair.
Table 2.
Distribution of Valve Disease Etiology
| Variable | Total (n = 2064) | Mitral Valve Repair (n = 1,188) | Tissue Mitral Valve Replacement (n = 196) | Mechanical Mitral Valve Replacement (n = 680) |
|---|---|---|---|---|
| Degenerative | 42% | 51% | 32% | 28% |
| Ischemic | 22% | 31% | 12% | 9% |
| Rheumatic | 20% | 4% | 26% | 47% |
| Other | 11% | 11% | 12% | 11% |
| Infectious | 5% | 3% | 18% | 5% |
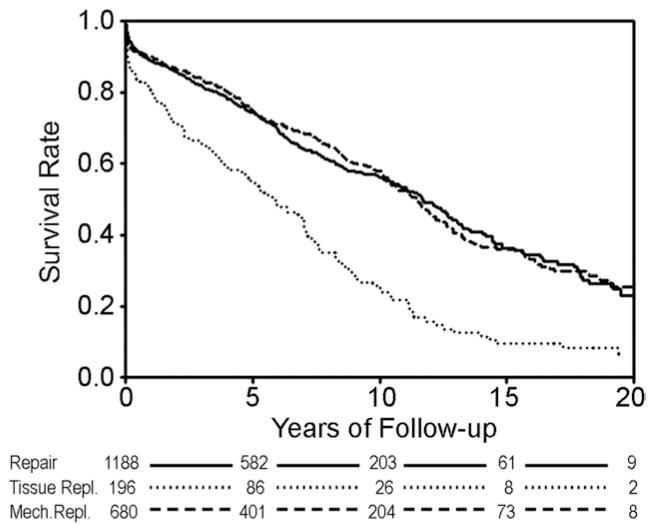
Raw unadjusted 30-day mortality was 3.5% for mitral repair, 5.9% for mechanical replacement, and 8.2% for tissue replacement. Long-term unadjusted Kaplan-Meier survival was not significantly different between mitral valve repair and mechanical mitral valve replacement (Fig 2), and both groups had significantly better raw survival as compared with tissue valve replacement. This finding was preserved in the unadjusted Kaplan-Meier survival comparison of patients 65 years or greater (Fig 3).
Fig 2.
Unadjusted Kaplan-Meier survival analysis. Log-rank p value less than 0.0001 for tissue valves versus either mechanical valves or repair.
Fig 3.
Unadjusted Kaplan-Meier survival analysis for patients greater than 65 years of age. Log-rank p value = 0.001 for tissue valves versus either mechanical valves or repair.
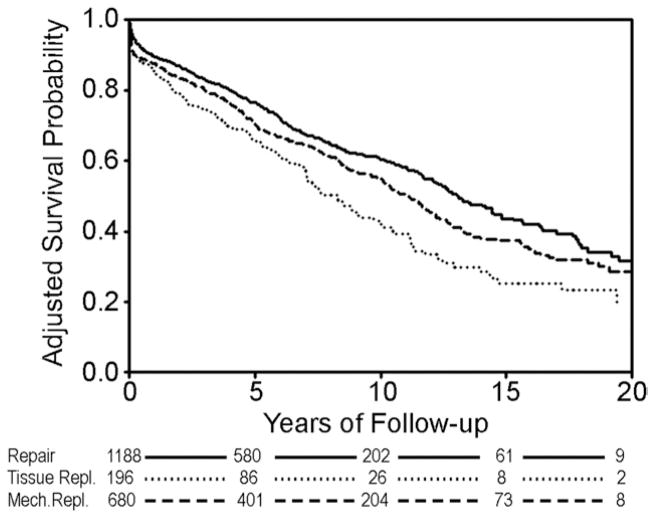
Final Cox model coefficients are shown in Table 2, and after adjusting for differences in baseline characteristics, risk-adjusted survival estimates are displayed in Figure 4. Adjusted curves demonstrated better survival with mitral repair, and even after adjustment for adverse risk profiles, tissue replacement survival was still inferior. No treatment interaction was observed between procedural choice and age in the Cox model analysis (p = 0.1781). In other words, the hazard associated with each treatment was the same across all ages.
Fig 4.
Long-term survival after adjusting for differences in baseline characteristics. Cox model p values. (Repair versus mechanical replacement = 0.0044; repair versus tissue replacement = <0.0001; mechanical versus tissue replacement = 0.0017.)
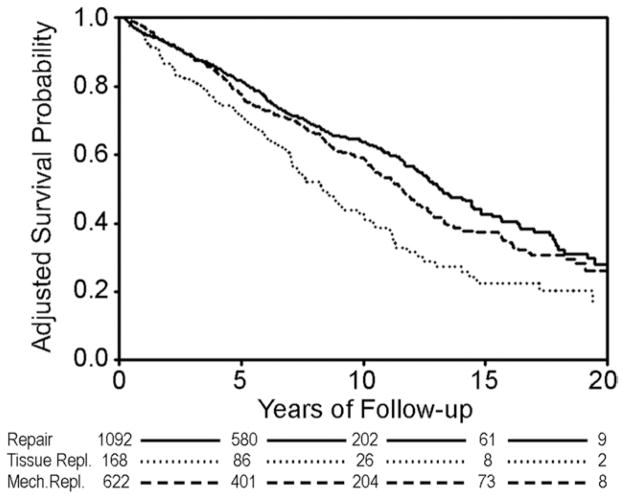
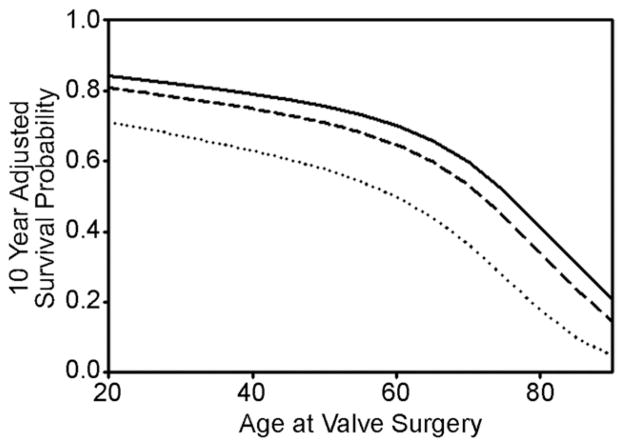
Another Cox model was generated for patients surviving 90 days after surgery (coefficients at jsrmd.com/90_day_coefficients.pdf) in order to compare relative late mortalities. Conditional adjusted survival estimates demonstrated persistent superiority of repair and mechanical replacement as compared with tissue valve replacement (Fig 5). Finally, adjusted survival probabilities at 10 years versus age at valve implant are shown in Figure 6. Regardless of patient age, mitral repair was associated with better risk-adjusted 10-year survival compared with either mechanical or tissues. At no age did tissue valve replacement achieve equivalent results to either of the other two procedures.
Fig 5.
Adjusted survival estimates conditional on 90-day survival. Cox model p values. (Repair versus mechanical replacement = 0.0493; repair versus tissue replacement <0.0001; mechanical versus tissue replacement <0.0001.
Fig 6.
On the y axis is risk-adjusted survival at 10 years after valve surgery, and on the x axis is patient age at the original surgical procedure. Adjusted 10-year survival probability was best for patients receiving mitral valve repair, followed by mechanical valve replacement for all ages. Tissue mitral valve replacement was associated with decreased adjusted 10-year survival at all ages, even in the elderly. Thus, outcome differences for the 3 procedures were fairly constant across all patient ages. (— = repair; ··· = tissue replacement; --- = mechanical replacement.)
Comment
An important issue in this analysis is the validity of comparing procedures that may not have been equally applicable to all patients or all mitral disease pathologies. This is an appropriate criticism, especially for the years included in this study. However, in the more recent era, repair techniques have evolved so that reconstruction can be performed in most patient categories [3, 38], and late outcome comparisons become useful to guide future patient management. Another concern regards possible undefined treatment selection biases or confounding variables, such that some patients might have been selected for one treatment or another who were at higher risk than defined by baseline variables. The potential for these types of problems exists with all observational studies. However, after 25 years of work with this data set, the determinants of mortality in mitral surgery are pretty well understood, and although minor factors may have been omitted, the major determinants are likely accounted for. With a large sample size, long follow-up, a comprehensive and consistent variable set, and meticulous multivariable modeling, this type of observational analysis has been shown to be quite accurate [39]. However, possibilities for confounders always exist, and the results need to be qualified and interpreted in this regard.
Institutional selection biases also could exist. In the Duke practice, an early bias is evident against using tissue valves in the mitral position because of higher failure rates with systolic closure stress. This factor probably accounts for the preponderance of mechanical versus tissue valves implanted over the entire experience. However, 18 different surgeons contributed patients over 20 years, so that significant variability in procedural selection philosophies existed. In studies of patient subgroups from this series [24, 25], propensity regressions showed that surgeon of record accounted for most of the procedural selection decisions, rather than any sort of systematic bias based on patient characteristics. Thus, most surgeons “believed” in one approach or the other, and practiced accordingly and in a consistent way. It is also likely that individual surgeon philosophies changed over time, and as stability of repair with autologous tissues became more apparent, the proportion of repair procedures increased dramatically (Fig 1) [25, 40]. While a general selection bias existed toward employing bioprostheses in the elderly, larger numbers of sick elderly patients received mechanical valves and repair in the greater than 65-year subgroup. In fact, the very sickest cohort, ischemic mitral regurgitation, was managed predominantly with repair [24]. Thus, a spectrum of procedural selection philosophies existed among the 18 surgeons, supporting the appropriateness of this comparison.
Several advantages existed with the approach used in this analysis. It was performed at a single institution with a relatively consistent technical and perioperative care philosophy. The sample size was good, and all patients had prospective recording of a consistent and complete set of baseline variables. Maximal follow-up was 20 years, and finally, the multivariable statistical approaches were state-of-the-art, adjusting for all known important baseline characteristics and propensity for procedural selection. The authors had no preconception of how the analysis would turn out, but after the National Death Index search, it became evident that unadjusted survival was best for mitral repair, followed by mechanical replacement, and then tissue valve replacement (Fig 2). This result with unadjusted data was not surprising as the tissue valve population was older on average, and age is a prominent predictor of survival. However, after adjustment for differences in preoperative baseline characteristics, mitral repair still had the best predicted survival, followed by mechanical valve replacement (Fig 4), and tissue valves seemed inferior to both of the other options. This relationship was maintained in the older mitral disease population and far into the advanced age group (Figs 3 and 6).
In order to minimize bias of operative mortality against tissue valve survival, adjusted survival conditional on 90-day survival was examined (Fig 5). Even in this cohort, mitral repair had better risk-adjusted outcomes, followed by mechanical then tissue valve replacement. Because nonfatal events were not available in this study the cause of this finding is unclear. However, it is likely related to worse valve-related complications, including valve degeneration which occurs at a higher rate for tissue valves in the mitral position. Somewhat surprising was the finding in the Cox model (Table 3) that tissue valve replacement had an associated hazard ratio of 1.8 (1.5, 2.3), second only to preoperative hemodialysis dependence. While the superiority of mitral repair relative to mechanical mitral replacement was definite but subtle, it seemed clear that tissue valve replacement was associated with inferior outcomes, independent of patient age (Fig 6).
Table 3.
Overall Cox Model Parameters
| Risk Factor | Wald χ2 | HR | 95% CI | p Value | |
|---|---|---|---|---|---|
| Dialysis | 6.9 | 2.2 | 1.2 | 3.9 | 0.0088 |
| Tissue valve replacement | 28.9 | 1.8 | 1.5 | 2.3 | <0.00001 |
| History of peripheral vascular disease | 24.5 | 1.7 | 1.4 | 2.1 | <0.00001 |
| History of CABG | 10.2 | 1.7 | 1.2 | 2.3 | 0.0014 |
| Full sternotomy | 7.3 | 1.5 | 1.1 | 1.9 | 0.0069 |
| History of cerebrovascular disease | 12.4 | 1.4 | 1.2 | 1.8 | 0.0004 |
| Age (HR per 10 years; truncated low end at 50) | 32.7 | 1.4 | 1.3 | 1.6 | <0.0001 |
| History of diabetes | 12.7 | 1.4 | 1.2 | 1.7 | 0.0004 |
| Nonelective surgery | 10.8 | 1.4 | 1.1 | 1.7 | 0.0010 |
| Chronic lung disease | 5.6 | 1.3 | 1.0 | 1.6 | 0.0180 |
| Mechanical valve replacement | 8.1 | 1.3 | 1.1 | 1.5 | 0.0044 |
| Ischemic valve etiology | 4.8 | 1.3 | 1.0 | 1.5 | 0.0287 |
| GFR (HR per 5 unit decrease; truncated high end at 100) | 27.6 | 1.2 | 1.1 | 1.4 | <0.00001 |
| Number of diseased vessels (HR per increase of 1) | 2.3 | 1.1 | 1.0 | 1.1 | 0.1323 |
| Ejection fraction (HR per 5% decrease) | 0.112 | 1.0 | 1.0 | 1.1 | 0.0008 |
| Year of surgery (HR per 1 year increase) | 11.3 | 1.0 | 1.0 | 1.0 | 0.0008 |
| Caucasian race | 7.8 | 0.8 | 0.7 | 0.9 | 0.0052 |
| Mechanical vs tissue replacement propensity | 8.8 | 0.0031 | |||
| Repair vs tissue replacement propensity | 7.3 | 0.0070 | |||
| Repair vs mechanical replacement propensity | 7.2 | 0.0072 | |||
CABG = coronary artery bypass grafting; CI = confidence interval; GFR = glomerular filtration rates; HR = hazard ratio.
How can these findings be reconciled with the currently accepted philosophy of adequate performance and broad application of tissue valves in the elderly? Perhaps some of the accepted concepts suffer from artifacts caused by using univariable “freedom from event” curves, an approach that is fraught with statistical inaccuracies due to the multivariable nature of outcomes and the competing risk of death in the elderly. The observed inferiority of tissue replacement is particularly concerning in light of the recent increased utilization of bioprostheses for elderly patients [23]. In the present analysis, however, it was clear that adjusted 10-year survival was inferior for tissue replacement patients of all ages (Fig 6), and the findings of this study suggest that valve repair should be the procedure of choice for most mitral valve disease.
The result of this analysis is dependent on the quality of the mitral repairs. While valve replacement was fairly standardized during this period, repair techniques evolved significantly, enhancing both the applicability and stability of repair procedures. Repair results steadily improved, with “year of surgery” yielding a χ2 value of 11.3 in the Cox model (Table 3; p = 0.0008). Repair methods that have been shown to be less effective, such as pericardial bands, were avoided in the Duke practice, and utilization of inadequate repair techniques may account for some of the variability in the literature. In this series, full rings were used consistently, and management of chordal and leaflet abnormalities improved over time. Finally, it is probable that newer repair methods, such as artificial chordal replacement and autologous pericardial leaflet augmentation, will further enhance applicability and stability, and that repair outcomes will continue to improve into the future [1, 3, 4, 15, 35, 38].
Portions of this series have been analyzed in previous publications [24, 25, 33, 40]. Interestingly, the benefit of mitral repair on operative mortality seemed greater in acutely ill ischemic mitral regurgitation patients with adverse baseline characteristics [24]. In contrast, differences in 30-day outcome with repair versus replacement in patients with degenerative disease were smaller, perhaps because of the more elective nature of the population [25]. However, the long-term inferiority of valve replacement to repair was evident in both groups. Again, the reason for this difference will require further analysis of specific events, but it is now perhaps established that use of the body’s own tissues to reconstruct heart valve function has significant long-term advantages. The tissue valve sample size in this study was marginal, and because of small numbers, comparison of early tissue valves with more recent designs was not possible. Therefore, further testing of the concluding hypothesis of this paper is suggested in other single institutional databases and potentially in the Society of Thoracic Surgeons data set.
In summary, the results of this study support the concept that diseased mitral valves should be repaired regardless of patient age. Based on these data, valves that are not amenable to repair should receive primarily mechanical mitral valve replacement. Utilization of tissue valves perhaps should be limited to irreparable patients who have contraindications to long-term systemic anticoagulation. From this analysis, advanced age seems neither to be an indicator for mitral valve replacement nor utilization of a bioprosthesis, although confirmation of these findings in other data sets is indicated.
Acknowledgments
This statistical work was funded in part by National Institutes of Health Grant No. 5U01-HL088953-03, and by grants from Edwards Lifesciences, St. Jude Medical, and Sorin Group to Duke University Department of Surgery. All authors had full control of design of study, methods used, outcome parameters, analysis of data, and production of the written report.
Footnotes
Presented at the Fifty-sixth Annual Meeting of the Southern Thoracic Surgical Association, Marco Island, FL, Nov 4–7, 2009.
Dr Glower discloses that he has financial relationships with St. Jude Medical and Edwards Lifesciences.
References
- 1.Lawrie GM, Earle EA, Earle NR. Feasibility and intermediate term outcome of repair of prolapsing anterior mitral leaflets with artificial chordal replacement in 152 patients. Ann Thorac Surg. 2006;81:849–56. doi: 10.1016/j.athoracsur.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 2.Chauvaud S, Jebara V, Chachques JC, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg. 1991;102:171–7. [PubMed] [Google Scholar]
- 3.Rankin JS. Artificial chordal replacement in complex mitral valve repair. Available at CTSNet: http://www.ctsnet.org/sections/clinicalresources/videos/vg2009_rankin_ACRinComplexMVR.html.
- 4.Rankin JS, Alfery DD, Orozco R, et al. Techniques of artificial chordal replacement for mitral valve repair: Use in multiple pathologic disorders. Op Tech Thorac Cardiovasc Surg. 2008;13:74–82. [Google Scholar]
- 5.Ailawadi G, MD, Swenson BR, Girotti ME. Is mitral valve repair superior to replacement in elderly patients? Ann Thorac Surg. 2008;86:77–86. doi: 10.1016/j.athoracsur.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 6.David T. Techniques and results of mitral valve repair for ischemic mitral regurgitation. J Card Surg. 1994;9 (2 suppl):274–7. doi: 10.1111/j.1540-8191.1994.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 7.El Oumeiri B, Boodhwani M, Glineur D, et al. Extending the scope of mitral valve repair in rheumatic disease. Ann Thorac Surg. 2009;87:1735–40. doi: 10.1016/j.athoracsur.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Rankin J, Orozco R, Addai T, et al. Several new considerations in mitral valve repair. J Heart Valve Dis. 2004;13:399–409. [PubMed] [Google Scholar]
- 9.Risteski P, Aybek T, Dzemali O, et al. Artificial chordae for mitral valve repair: mid-term clinical and echocardiographic results. Thorac Cardiovasc Surg. 2007;55:239–44. doi: 10.1055/s-2006-955947. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Sakata R, Iguro Y, Nagata T, Otsuji Y, Tei C. New surgical approach to reduce tethering in ischemic mitral regurgitation by relocation of separate heads of the posterior papillary muscle. Ann Thorac Surg. 2006;81:2324–5. doi: 10.1016/j.athoracsur.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 11.David TE, Omran A, Armstrong S, Sun Z, Ivanov J. Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg. 1998;115:1279–85. doi: 10.1016/S0022-5223(98)70210-7. [DOI] [PubMed] [Google Scholar]
- 12.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg. 2000;70:2166–8. doi: 10.1016/s0003-4975(00)02047-6. [DOI] [PubMed] [Google Scholar]
- 13.Chiappini B, Sanchez A, Noirhomme P, et al. Combined replacement of chordae tendineae with polytetrafluoroethylene (PTFE) sutures in mitral valve repair: Early and long-term results. J Heart Valve Dis. 2006;15:657–63. [PubMed] [Google Scholar]
- 14.Rankin JS, Orozco RE, Rodgers TL, Alfery DD, Glower DD. “Adjustable” artificial chordal replacement for repair of mitral valve prolapse. Ann Thorac Surg. 2006;81:1526–8. doi: 10.1016/j.athoracsur.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Salvador L, Mirone S, Bianchini R, et al. A 20-year experience with mitral valve repair with artificial chordae in 608 patients. J Thorac Cardiovasc Surg. 2008;135:1280–7. doi: 10.1016/j.jtcvs.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Seeburger J, Falk V, Borger MA, et al. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: à ègalité. Ann Thorac Surg. 2009;87:1715–20. doi: 10.1016/j.athoracsur.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ng C-K, Nesser J, Punzengruber C, et al. Valvuloplasty with glutaraldehyde-treated autologous pericardium in patients with complex mitral valve pathology. Ann Thorac Surg. 2001;71:78–85. doi: 10.1016/s0003-4975(00)02327-4. [DOI] [PubMed] [Google Scholar]
- 18.Jokinen JJ, Hippeläinen MJ, Pitkänen OA, Hartikainen JE. Mitral valve replacement versus repair: Propensity-adjusted survival and quality-of-life analysis. Ann Thorac Surg. 2007;84:451–8. doi: 10.1016/j.athoracsur.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Langer F, Rodriguez F, Cheng A, et al. Posterior mitral leaflet extension: An adjunctive repair option for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2006;131:868–77. doi: 10.1016/j.jtcvs.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Zegdi R, Khabbaz Z, Chauvaud S, Latremouille C, Fabiani JN, Deloche A. Posterior leaflet extension with an autologous pericardial patch in rheumatic mitral insufficiency. Ann Thorac Surg. 2007;84:1043–4. doi: 10.1016/j.athoracsur.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 21.Seeburger J, Kuntze T, Mohr FW. Gore-Tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg. 2007;19:111–5. doi: 10.1053/j.semtcvs.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Rankin JS, Sharma MK, Teague SM, McLaughlin VW, Johnston TS, McRae AT. A new method of mitral valve repair for rheumatic disease: preliminary study. J Heart Valve Dis. 2008;17:614–9. [PubMed] [Google Scholar]
- 23.Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons adult cardiac database. Ann Thorac Surg. 2009;87:1431–9. doi: 10.1016/j.athoracsur.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 24.Milano CA, Daneshmand MA, Rankin JS, et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg. 2008;86:735–44. doi: 10.1016/j.athoracsur.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Daneshmand MA, Milano CA, Rankin JS, et al. Mitral valve repair for degenerative disease: a 20-year experience. Ann Thorac Surg. 2009;88:1828–37. doi: 10.1016/j.athoracsur.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Di Mauro M, Di Giammarco G, Vitolla G, et al. Impact of no-to-moderate mitral regurgitation on late results after isolated coronary artery bypass grafting in patients with ischemic cardiomyopathy. Ann Thorac Surg. 2006;81:2128–34. doi: 10.1016/j.athoracsur.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 27.Diodato MD, Moon MR, Pasque MK, et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78:794–9. doi: 10.1016/j.athoracsur.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation: a multivariate analysis. Circulation. 1995;91:1022–8. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- 29.Gazoni LM, Kern JA, Swenson BR, et al. A change in perspective: Results for ischemic mitral valve repair are similar to mitral valve repair for degenerative disease. Ann Thorac Surg. 2007;84:750–8. doi: 10.1016/j.athoracsur.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 30.Geidel S, Lass M, Krause K, et al. Early and late results of restrictive mitral valve annuloplasty in 121 patients with cardiomyopathy and chronic mitral regurgitation. Thorac Cardiovasc Surg. 2008;56:262–8. doi: 10.1055/s-2008-1038420. [DOI] [PubMed] [Google Scholar]
- 31.Gelsomino S, Lorusso R, Capecchi I, et al. Left ventricular reverse remodeling after undersized mitral ring annuloplasty in patients with ischemic regurgitation. Ann Thorac Surg. 2008;85:1319–30. doi: 10.1016/j.athoracsur.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 32.Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2008;135:885–93. doi: 10.1016/j.jtcvs.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 33.Glower DD, Tuttle RH, Shaw LK, Orozco RE, Rankin JS. Patient survival characteristics after routine mitral valve repair for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005;129:860–8. doi: 10.1016/j.jtcvs.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Lawrie GM, Earle EA, Earle NR. Nonresectional repair of the Barlow mitral valve: Importance of dynamic annular evaluation. Ann Thorac Surg. 2009;88:1191–6. doi: 10.1016/j.athoracsur.2009.05.086. [DOI] [PubMed] [Google Scholar]
- 35.Tesler UF, Cerin G, Novelli E, Popa A, Diena M. Evolution of surgical techniques for mitral valve repair. Tex Heart Inst J. 2009;36:438–40. [PMC free article] [PubMed] [Google Scholar]
- 36.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 37.Cox D. Regression model and life tables (with discussion) J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 38.Rankin JS, Burrichter CA, Walton-Shirley MK, et al. Trends in mitral valve surgery: a single practice experience. J Heart Valve Dis. 2009;18:359–66. [PubMed] [Google Scholar]
- 39.Burfeind WR, Jr, Glower DD, Wechsler AS, et al. Single versus multiple internal mammary artery grafting for coronary artery bypass: 15-year follow-up of a clinical practice trial. Circulation. 2004;110(11 suppl 1):II27–35. doi: 10.1161/01.CIR.0000138193.51635.6f. [DOI] [PubMed] [Google Scholar]
- 40.Williams ML, Daneshmand MA, Jollis JG, et al. Mitral gradients and frequency of recurrence of mitral regurgitation after ring annuloplasty for ischemic mitral regurgitation. Ann Thorac Surg. 2009;88:1197–201. doi: 10.1016/j.athoracsur.2009.06.022. [DOI] [PubMed] [Google Scholar]