Abstract
Purpose.
To investigate the pupillary light reflex (PLR) of patients with severe loss of vision due to Leber's Hereditary Optic Neuropathy (LHON) in the context of a proposed preservation of melanopsin-expressing retinal ganglion cells (mRGCs).
Methods.
Ten LHON patients (7 males; 51.6 ± 14.1 years), with visual acuities ranging from 20/400 to hand motion perception and severe visual field losses, were tested and compared with 16 healthy subjects (7 males; 42.15 ± 15.4 years) tested as controls. PLR was measured with an eye tracker and the stimuli were controlled with a Ganzfeld system. Pupil responses were measured monocularly, to 1 second of blue (470 nm) and red (640 nm) flashes with 1, 10, 100, and 250 cd/m2 luminances. The normalized amplitude of peak of the transient PLR and the amplitude of the sustained PLR at 6 seconds after the flash offset were measured. In addition, optical coherence topography (OCT) scans of the peripapillary retinal nerve fiber layer were obtained.
Results.
The patient's peak PLR responses were on average 15% smaller than controls (P < 0.05), but 5 out of 10 patients had amplitudes within the range of controls. The patients' sustained PLRs were comparable with controls at lower flash intensities, but on average, 27% smaller to the 250 cd/m2 blue light, although there was considerable overlap with the PLR amplitudes of control. All patients had severe visual field losses and the retinal nerve fiber layer thickness was reduced to a minimum around the optic disc in 8 of the 10 patients.
Conclusions.
The PLR is maintained overall in LHON patients despite the severity of optic atrophy. These results are consistent with previous evidence of selective preservation of mRGCs.
Keywords: pupillometry, melanopsin, ganglion cells, LHON, OCT
Preserved pupillary light reflex in patients with Leber's hereditary optic neuropathy is demonstrated here and it can be explained by the relative sparing of the melanopsin-expressing retinal ganglion cells system.
Introduction
Pupillary light reflex (PLR) evaluation is a valuable tool for the objective evaluation of the optic nerve function. In most cases, there is a strong correlation between pupil response to light and the severity of optic nerve disease.1–5
Optic neuropathies are frequently characterized by abnormalities of the PLR. However, in patients with mitochondrial optic neuropathies, there can be visual-pupillary dissociation such that even with severe visual loss, there is some pupillary sparing.6–9 In Leber's hereditary optic neuropathy (LHON), a maternally inherited form of optic neuropathy due to mitochondrial DNA point mutations10,11 some have found a reduced PLR,12,13 while others have found a relatively preserved response.6,7
LHON is characterized by a severe loss of RGCs that begins with those that constitute the papillomacular bundle, but eventually produces generalized retinal ganglion cells (RGC) loss. The relative preservation of the PLR observed in some patients with LHON has been hypothesized to be due to the relative sparing of the melanopsin-expressing (mRGCs) cells, which are mediators of the PLR.14–18 In particular, Bose et al. described a preservation of retinofugal pathway to the olivary nuclei of the pretectum,19 the pathway of the mRGC projections. Moreover, a relative sparing of these mRGCs has been recently demonstrated by La Morgia et al., in a postmortem evaluation of RGCs and their axons in the retina and the optic nerves from affected patients with mitochondrial optic neuropathies, including two with LHON and one with dominant optic atrophy (DOA).20
The PLR to brief light exposures shows a transient phase of pupillary constriction attributed to the rod and cone input to the mRGCs, followed by a sustained pupil constriction, mainly driven by the intrinsic melanopsin response of the mRGCs.21,22 We measured the transient and sustained response of the PLR in a group of LHON patients with profound loss of vision due to severe optic atrophy. In addition, optical coherence tomography (OCT) was employed to measure the extent of retinal nerve fiber damage. The results are interpreted in the context of the hypothesized preservation of the mRGCs.
Methods
Subjects
Ten affected patients, carrying the 11778/ND4 mtDNA mutation (7 males; mean age 51.6 ± 14.1 years), were included in this study. Nine of these patients belonged to the previously published LHON Brazilian pedigree.23 The age of disease onset ranged from 7 to 35 years. All patients were submitted to a complete ophthalmological exam and no additional ophthalmological or neurological diseases were found. Patients had visual acuity ranging from 20/400 to hand motion perception (Table). Sixteen healthy age-matched control subjects (7 males; mean age 42.15 ± 15.4 years) were included for comparison.
Table.
Demographic and Ophthalmologic Data of LHON Affected Patients
|
Patient No. |
Code |
Age |
Sex |
Eye |
VA |
TOD |
RNFL AV |
S |
T |
I |
N |
| 1 | C10005 | 65 | M | OD | 20/400 | 25 | 61 | 82 | 26 | 81 | 57 |
| 2 | C10008 | 74 | F | OD | HM | 42 | 27 | 31 | 21 | 29 | 27 |
| 3 | C10012 | 68 | F | OD | HM | 36 | 27 | 33 | 23 | 26 | 24 |
| 4 | C10027 | 41 | M | OD | HM | 22 | 16 | 1 | 24 | 31 | 9 |
| 5 | C10091 | 30 | M | OD | HM | 12 | 29 | 35 | 21 | 35 | 23 |
| 6 | S10020 | 54 | M | OD | 20/500 | 13 | 64 | 88 | 25 | 82 | 59 |
| 7 | V10013 | 51 | M | OS | CF 20 cm | 6 | 27 | 32 | 20 | 34 | 22 |
| 8 | V10097 | 49 | M | OD | CF 10 cm | 29 | 34 | 38 | 22 | 39 | 37 |
| 9 | V10105 | 48 | F | OD | 20/600 | 5 | 37 | 40 | 29 | 46 | 32 |
| 10 | PVE611 | 36 | M | OS | 20/400 | 10 | 23 | 39 | 26 | 40 | 30 |
| Mean | 51.6 | 20.0 | 34.5 | 41.9 | 23.7 | 44.3 | 32.0 | ||||
| SD | 14.1 | 12.8 | 15.8 | 25.3 | 2.8 | 20.4 | 15.6 |
M, male; F, female; OD, right eye; OS, left eye; VA, visual acuity; HM, hand motion; CF, counting fingers; TOD, time of disease (y); RNFL AV, retinal nerve fiber layer average (μm); RNFL thickness (μm) according to quadrants as S (superior), T (temporal), I (inferior), and N (nasal).
Patients' visual function was also evaluated with the standard automated perimetry (Humphrey Field Analyzer program 30-2 full threshold, white stimulus, size V; Carl Zeiss Meditec, Dublin, CA), and all patients showed a severe reduction of sensitivity along the 30° central area. Due to the poor visual acuity of the group, a size V stimulus was used. Visual field data served to document the extent of the visual function damage in our patients, but were not included for further analyses because of the depth of the defect and low reliability in some of the exams.
The procedures used in this study complied with the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants and the study protocol was approved by the Committee on Ethics in Research from Federal University of São Paulo–UNIFESP (# 0974-01).
OCT
Structural evaluation of the retina nerve fiber layer was determined with the optic disc cube protocol on HD-OCT software (Cirrus HD-OCT, version 6.0; Carl Zeiss Meditec). This commercial software places a circle, 3.46 mm in diameter, around the optic nerve head, extracts 256 A-scan samples from the data cube along this circle, and calculates retinal nerve fiber layer (RNFL) thickness based upon a segmentation algorithm.
Stimuli
Stimuli consisted of a 1-second flash of either blue (470 nm) or red (640 nm) light, generated by the corresponding LED in a Ganzfeld, controlled by the A-pattern simulation system (RETI-port; Roland Consult, Brandenburg, Germany) They were presented at the following photopic range of luminances: 1, 10, 100, and 250 cd/m2. The choice of stimulus duration, wavelengths, and luminances was based on a previous study, which concluded that cone and melanopsin-driven mRGC contributions to the PLR could be identified based upon these parameters.22 We did not use the lower intensities suggested by Park et al.22 to assess rod contributions, as the nature of the testing situation made extensive dark-adaptation impractical.
Procedure
All subjects were tested monocularly in the eye with the worst visual acuity. The other eye was occluded with a patch.
After 10 minutes of dark adaptation, alternating red and blue flashes were presented starting with the 1 cd/m2 luminance and proceeding to the 250 cd/m2 stimuli. Following each flash, the PLR was recorded continuously during a period of time determined by the luminance of the flash. In particular, after the 1 and 10 cd/m2 flashes, PLR was recorded for 20 seconds and for 60 seconds after the 100 and 250 cd/m2 flashes.
Pupil Response Recording
A binocular eye-tracking camera system with infrared light emitting diodes (Arrington Research, Scottsdale, AZ) was used for real-time pupil recording with a sample rate of 60 Hz. The video camera system was attached to a plastic eye frame in order to avoid any physical contact between the camera and the eye. The equipment is the same as used by previous studies.22,24,25
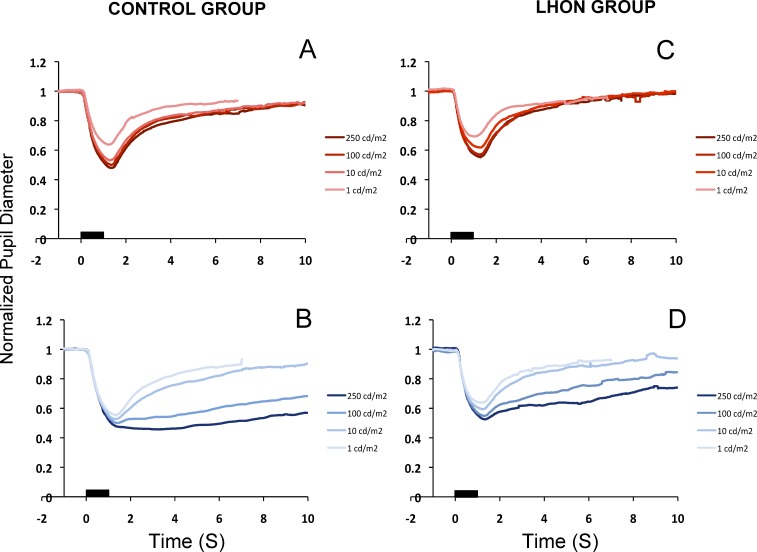
As the stimulator system was separate from the eye tracker, we used the scene camera of the eye tracker to synchronize the stimuli and the recordings. The scene camera recorded a video of the stimulus and its frame timing was synchronized to the eye tracker data. Time 0 in Figure 1 corresponds to the time of onset of light emitting diodes (LED).
Figure 1.
Normalized pupillary diameter for different flash luminances. Upper- and lower-left panels show the averages of the control group (n = 16), for the red flashes (A) and for the blue flashes (B). Upper- and lower-right panels show the averages of the LHON patients (n = 10) for red flashes (C) and blue flashes (D). Black bars represent the 1-second stimulus flash.
Data Analysis
Data were analyzed using commercial spreadsheet software (Microsoft Excel; Microsoft Corporation, Redmond, WA) using automated macros written in commercial programming language (Microsoft Visual Basic for Applications 7.0; Microsoft Corporation). Noise and blinking artifacts were removed from recordings with median filtering using a 200-ms window and derivative filtering.
The average pupil diameter during 3 seconds before the flash onset was used as a baseline value. The filtered response values were divided by the baseline pupil diameter in order to normalize the PLR parameters and compare the results of different subjects. The peak amplitude (PA) was calculated as the maximum pupil constriction and expressed relative to the baseline value (PA = max constriction diameter/baseline diameter). (Note: An analysis of the incremental change from baseline, not shown, produced similar results.) The sustained response (SR) was expressed as the pupil diameter at 6 seconds after the flash offset, relative to the baseline.22
As the results did not fit a normal distribution, as shown by the Shapiro-Wilk test, the nonparametric Mann-Whitney test was applied for comparisons. A two-tailed Spearman correlation test was used to assess correlation between variables. To correct for possible errors in multiple comparisons, the Holm-Bonferroni method was applied.
Results
Pupil Data
Figure 1 presents the average of normalized PLR of the control (Figs. 1A, 1B) and LHON groups (Figs. 1C, 1D) for the set of red (Figs. 1A, 1C) and blue (Figs. 1B, 1D) stimuli. Each line represents the average normalized pupillary size in response to a particular luminance of the 1-second duration flash. For the control group, the peak amplitude (Fig. 1A) of the pupillary constriction increases with the increasing intensity of the red stimulus. At each of the luminances presented, there is a return to the baseline after reaching the peak constriction. A similar pattern can be observed for the blue flash (Fig. 1B). However, the return to the baseline takes longer. This sustained response increases with the luminance of the stimulus. Thus, two distinct components in the PLR were identified: a transient waveform in response to both red and blue flashes and a sustained waveform in response to the blue flash, as previously reported.22,24,25 The LHON group (Figs. 1C, 1D) showed qualitatively similar responses to those of the control group for both red and blue stimuli.
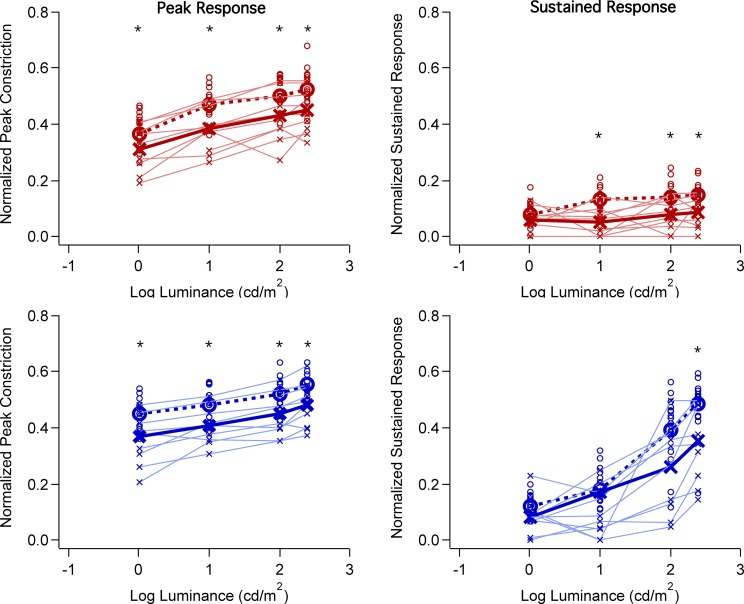
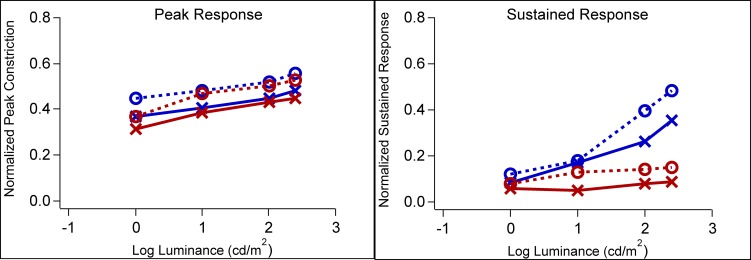
The normalized peak amplitude of the transient phase and the normalized amplitude of the sustained response (measured at 6 seconds after the flash offset) are plotted as a function of luminance in Figure 2. In all panels, the open circles and “x” symbols indicate the data for controls and patients, respectively, with the small symbols representing the values for individuals and the large symbols the averages. A thin solid line connects the data points for each patient. As expected from previous work,22 the peak amplitudes (left column) increase slightly with stimulus intensity as the luminance was increased from 0 to 2.4 log cd/m2. Further, the peak amplitudes for the photopically equated red and blue stimuli were similar for both controls and LHON patients, as shown in Figure 3 (left panel) where the mean data for the blue and red stimuli are superimposed. On the other hand, for the sustained responses, the amplitudes were small and relatively constant in response to the range of red stimuli (Fig. 3, right panel), but they markedly increased with increased luminance of the blue stimuli. This is consistent with previous work that has shown the increase we see in the sustained response at these higher intensities of blue light is driven by melanopsin. This conclusion was based on the finding that pupil responses obtained during pharmacological blockade of rod and cone photoreceptors exhibit the same spectral tuning and slow kinetics of mRGC responses.15,16
Figure 2.
Pupil constriction amplitude as a function of flash luminance (log cd/m2). Red symbols represent red flash and blue symbols, blue flash. Left panels show normalized constriction amplitude of the peak of the pupillary response and right panels show the normalized constriction amplitude of the pupillary sustained response measured at 6 seconds after flash offset. LHON patients are represented by the x symbols and thin lines; controls are represented by the circles. Average of each group is represented by the thick line and symbols. Asterisks indicate significant differences (P < 0.01) between patient and control groups.
Figure 3.
Average constriction amplitude of the pupillary light responses as a function of luminance, for red and blue stimuli. LHON patients are represented by the solid lines and x symbols. Controls are represented by the dashed lines and circles. Left panel shows the normalized pupil constriction amplitude for the peak response and right panel shows the normalized pupil constriction amplitude for the sustained response.
Of primary interest here, is the size of the patients' PLR as compared with the controls. Consider the peak response data (Fig. 2, left column) first. For both red and blue stimuli, the mean peak amplitude is, on average, smaller in the case of the patients, by approximately 15% across all intensities. These differences were small and yet statistically significant (P ≤ 0.01 level, Mann-Whitney test). There were five patients within the normal range of peak amplitudes for all intensities of both red and blue flashes. However, there was considerable variability and other patients' values fell below control values. Similarly, the sustained responses (Fig. 2, right column) on average are smaller in the case of the patients, with the largest difference seen for the most intense blue stimuli. For the blue 2.4 log cd/m2 stimuli, the average sustained response of the LHON patient group was 27% smaller than that of the controls (P < 0.016, Mann-Whitney test). However, the range of control values was large and most patient values fell within it.
Pupil size at baseline was larger in the control group (6.29 ± 1.14 mm) compared to LHON patients (5.04 ± 0.68 mm), although there was considerable overlap in the baseline values for the two groups.
We also examined the correlation between the sustained response and clinical parameters. There was no significant correlation between the sustained response and age, duration of disease, or visual acuity (P > 0.05; Spearman correlation).
OCT Data
The average peripapillary RNFL thickness for the patients was 34.5 μm as compared with 94.0 μm for controls, in accordance with data previously reported.26 The Table (“RNFL AV” column) shows the individual values, as well as the RNFL thicknesses by disc quadrants. It is important to note that complete loss of RGCs will not result in a complete loss of the RNFL as measured with OCT,27 unless there is an algorithm error, as blood vessels and glial cells remain and contribute to the thickness measure.28 This nonneural residual varies between approximately 20 and 60 μm depending upon individual differences, algorithm errors, and disc location.28 Only patients 1 (P1) and 6 (P6) had average RNFL thickness greater than 60 μm; the other eight patients had average thickness values ≤37 μm. The Table (four rightmost columns) shows the RNFL thickness values for the four optic disc quadrants. Except for the superior, nasal, and inferior quadrants of P1 and P6, all other quadrants had RNFL thickness values less than 47 μm. These values are within the range seen in ischemic optic neuropathy (ION) patients with macular field losses greater than −20 dB.27
Figure 4 (left column) shows the RNFL thickness profiles for the two patients with the thickest RNFL. Although the RNFL profiles (solid black lines) are markedly thinned (25 and 26 μm) in the temporal section of the disc, these two patients show measureable RNFL thickness in the rest of the disc, falling largely within the normal confidence limits. On the other hand, the other eight patients showed markedly thinned RNFL around the disc. Figure 4 (right column) shows two examples. The bumps in the RNFL profiles correspond to blood vessels and the density plots (insets) indicate that the thickness remaining was largely due to blood vessels. There was no correlation between the patients' RNFL thickness and their PLR amplitudes (P > 0.05, R2 = 0.1814; Spearman correlation).
Figure 4.
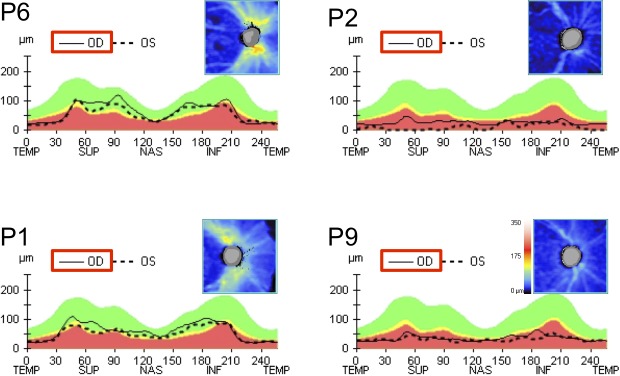
OCT RNFL representation thickness map for patients 1, 2, 6, and 9, for the right eye. Note the generalized reduced RNFL thickness among all patients. However, P1 and P6 show a small preservation of the superior, nasal, and inferior regions, compared with the temporal region.
Discussion
The PLR is relatively preserved in severely affected LHON patients with advanced optic atrophy. While many of our patients showed PLR responses that were clearly outside the normal range, five had peak PLR amplitudes within the range of controls. These results are consistent with previous studies that reported both relatively preserved and abnormal PLR responses in LHON patients.6,7,12,13,29
Overall, the difference in the PLR between LHON patients and controls was relatively modest. The mean transient response was approximately 15% smaller than controls for all four flash intensities and for both red and blue lights. The largest mean difference seen was for the sustained response to the most intense blue stimulus, which was 27% smaller than the average control amplitude. It is not clear why the differences are larger for the higher intensities. However, we do not have a good model for how mRGCs combine to produce the normal PLR, much less how fewer or less healthy cells may contribute.
In contrast to the relative preservation of mRGCs, the OCT peripapillary disc measures showed profound loss of RNFL thickness. In fact, in eight of 10 patients, the RNFL thickness was at levels consistent with those seen in patients with extreme ION or glaucoma, where this residual RNFL has been attributed to remaining blood vessels and glial cells.28 Based upon the Hood and Kardon model, the RNFL thickness falls within the noise (residual level) when 90% of the RGCs are lost. As mRGC cells are only approximately 0.2% to 0.8% of the RGCs in healthy individuals,15,30 the PLR and OCT results together are consistent with the hypothesis that mRGCs are differentially preserved in patients with LHON and consistent with the previous histological evidence in two patients with LHON.20 The lack of correlation between the patients' PLR and OCT data is not surprising given the lack of sensitivity of the OCT RNFL measure when the RGC loss exceeds 90%.
However, let's consider the alternative hypothesis that all RGCs are equally affected by LHON. Under this alternative hypothesis, we must assume that the relatively robust PLR responses from these LHON patients are supported by approximately 10% of the mRGCs. This seems unlikely based upon previous studies. Other optic neuropathies with severe RNFL loss often show an abnormal PLR.4,31–33 For example, recent studies described reduced melanopsin-driven responses in patients with glaucoma, and this reduction was proportional to the severity of the disease.32,33
In our study of LHON patients, some of them with severe optic neuropathy still showed a robust pupillary response to light while others had smaller PLRs. This is best explained by assuming that the patients might have different numbers of mRGCs remaining, consistent with published postmortem studies of LHON patients.20
In conclusion, the PLR, which is mediated largely—if not entirely—by the mRGCs, is relatively preserved in patients with LHON despite the profound vision loss, severe optic atrophy, and associated thinning of the RNFL. These findings are consistent with the histological findings described by La Morgia et al.20 and provide an explanation for the visual-pupillary dissociation in patients with LHON.
Acknowledgments
The authors thank Milton Moraes and Esther Moraes for the infrastructure and valuable help at the Instituto de Olhos de Colatina, ES, Brazil, making this study possible.
Supported by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) Projeto Temático 08/58731-2 (DFV) and the International Foundation for Optic Nerve Disease (AAS). DFV, SRS, AB, and RBJ are CNPq (National Council for Scientific and Technological Development) research fellows; ALAM and BVN are respectively CNPq and FAPESP postdoctoral fellows at DFV lab.
Disclosure: A.L.A. Moura, None; B.V. Nagy, None; C. La Morgia, None; P. Barboni, None; A.G.F. Oliveira, None; S.R. Salomão, None; A. Berezovsky, None; M.N. de Moraes-Filho, None; C.F. Chicani, None; R. Belfort Jr, None; V. Carelli, None; A.A. Sadun, None; D.C. Hood, None; D.F. Ventura, None
References
- 1. Thompson HS, Montague P, Cox TA, Corbett JJ. The relationship between visual acuity, pupillary defect, and visual field loss. Am J Ophthalmol. 1982; 93: 681–688. [DOI] [PubMed] [Google Scholar]
- 2. Kardon RH, Haupert CL, Thompson HS. The relationship between static perimetry and the relative afferent pupillary defect. Am J Ophthalmol. 1993; 115: 351–356. [DOI] [PubMed] [Google Scholar]
- 3. Lagrèze WD, Kardon RH. Correlation of relative afferent pupillary defect and estimated retinal ganglion cell loss. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 401–404. [DOI] [PubMed] [Google Scholar]
- 4. Kerrison JB, Buchanan K, Rosenberg ML, et al. Quantification of optic nerve axon loss associated with a relative afferent pupillary defect in the monkey. Arch Ophthalmol. 2001; 119: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 5. Bergamin O, Kardon RH. Greater pupillary escape differentiates central from peripheral visual field loss. Ophthalmology. 2002; 109: 771–780. [DOI] [PubMed] [Google Scholar]
- 6. Wakakura M, Yokoe J. Evidence for preserved direct pupil light response in Leber's hereditary optic neuropathy. Br J Ophthalmol. 1995; 79: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bremner FD, Shallo-Hoffmann J, Riordan-Eva P, Smith SE. Comparing pupil function with visual function in patients with Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 1999; 40: 2528–2534. [PubMed] [Google Scholar]
- 8. Bremner FD, Tomlin EA, Shallo-Hoffmann J, Votruba M, Smith SE. The pupil in dominant optic atrophy. Invest Ophthalmol Vis Sci. 2001; 42: 675–678. [PubMed] [Google Scholar]
- 9. Perganta G, Barnard AR, Katti C, et al. Non-image-forming light driven functions are preserved in a mouse model of autosomal dominant optic atrophy. PLoS One. 2013; 8: e56350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988; 9; 242: 1427–1430. [DOI] [PubMed] [Google Scholar]
- 11. Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004; 23: 53–89. [DOI] [PubMed] [Google Scholar]
- 12. Jacobson DM, Stone EM, Miller NR, et al. Relative afferent pupillary defects in patients with Leber hereditary optic neuropathy and unilateral visual loss. Am J Ophthalmol. 1998; 126: 291–295. [DOI] [PubMed] [Google Scholar]
- 13. Lüdke H, Constantin K, Leo-Kottler B, Wilhelm H. Pupillary light reflexes in patients with Leber's hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 1999; 237: 207–11. [DOI] [PubMed] [Google Scholar]
- 14. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000; 15; 20: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dacey DM, Liao H, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005; 17; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 16. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections and intrinsic photosensitivity. Science. 2002; 8; 295: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bose S, Dhillon N, Ross-Cisneros FN, Carelli V. Relative post-mortem sparing of afferent pupil fibers in a patient with 3460 Leber's hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 20. La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010; 133: 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007; 27: 195–204. [DOI] [PubMed] [Google Scholar]
- 22. Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadun AA, Carelli V, Salomao SR, et al. A very large Brazilian pedigree with 11778 Leber's hereditary optic neuropathy. Trans Am Ophthalmol Soc. 2002; 100: 169–178. [PMC free article] [PubMed] [Google Scholar]
- 24. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009; 116: 1564–1573. [DOI] [PubMed] [Google Scholar]
- 25. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011; 118: 376–381. [DOI] [PubMed] [Google Scholar]
- 26. Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology. 2005; 112: 120–126. [DOI] [PubMed] [Google Scholar]
- 27. Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006; 47: 2006–2010. [DOI] [PubMed] [Google Scholar]
- 28. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007; 26: 688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawasaki A, Herbst K, Sander B, Milea D. Selective wavelength pupillometry in Leber hereditary optic neuropathy. Clin Experiment Ophthalmol. 2010; 38: 322–324. [DOI] [PubMed] [Google Scholar]
- 30. Hannibal J, Hindersson P, Ostergaard J, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004; 45: 4202–4209. [DOI] [PubMed] [Google Scholar]
- 31. Nakanishi Y, Nakamura M, Tatsumi Y, Nagai-Kusuhara A, Negi A. Quantification of retinal nerve fiber layer thickness reduction associated with a relative afferent pupillary defect. Graefes Arch Clin Exp Ophthalmol. 2006; 244: 1480–1484. [DOI] [PubMed] [Google Scholar]
- 32. Feigl B, Mattes D, Thomas R, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 4362–4367. [DOI] [PubMed] [Google Scholar]
- 33. Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011; 52: 2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]