Abstract
According to the NHS, it is estimated that over 50% of the adult population are, to some extent, affected by gum disease and approximately 15% of UK population have been diagnosed with severe periodontitis. Periodontitis, a chronic polymicrobial disease of the gums, causes inflammation in its milder form, whereas in its severe form affects the surrounding tissues and can result in tooth loss. During periodontitis, plaque accumulates and sits between the junctional epithelium and the tooth itself, resulting in inflammation and the formation of a periodontal pocket. An interface is formed directly between the subgingival bacteria and the junctional epithelial cells. Bacterial pathogens commonly associated with periodontal disease are, among others, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, together known as the “red complex.” This review will mostly concentrate on the role of P. gingivalis, a Gram-negative anaerobic bacterium and one of the major and most studied contributors of this disease. Because periodontal disease is associated with the development of atherosclerosis, it is important to understand the local immune response to P. gingivalis. Innate immune players, in particular, complement and antimicrobial peptides and their effects with regard to P. gingivalis during periodontitis and in the development of atherosclerosis will be presented.
Keywords: P. gingivalis, complement system, antimicrobial peptides, periodontitis, atherosclerosis
Mode of Action of P. gingivalis during Periodontitis
Porphyromonas gingivalis is an anaerobic Gram-negative bacterium involved in the onset of inflammation and tissue destruction during periodontal disease. It can be found in small numbers in the oral cavity of healthy individuals (1, 2). Pathology occurs when P. gingivalis binds to and accumulates on the tooth surface, leading to the development of a mixed biofilm, the expansion of the bacteria into the gingival sulcus, and the formation of a periodontal pocket (3). Inside this periodontal pocket lies the gingival crevicular fluid, an inflammatory exudate – source of essential nutrients for P. gingivalis growth – present in low abundance in healthy individuals, but drastically increased during gum inflammation. In this milieu, P. gingivalis invades gingival epithelial cells via the binding of its fimbriae to β1 integrin on the host cell surface followed by a rearrangement of the host actin cytoskeleton (4, 5). It then blocks apoptosis through the PI3K/Akt and JAK/Stat pathways, allowing intracellular bacterial proliferation (6, 7). In addition, it inhibits IL-8 expression by epithelial cells, creating what is known as the “local chemokine paralysis” (8). This mechanism induces a delay in neutrophil recruitment, which allows the proliferation of bacteria in this new niche, leading to an alteration of the subgingival microbiome with respect to its composition and total bacterial count (9, 10). The emergence of this dysbiotic assembly of microorganisms is believed to be partly responsible for the pathology observed. This is supported by findings in a murine model of P. gingivalis-induced periodontitis, where P. gingivalis was shown to contribute to periodontal bone loss by reshaping the normal commensal microbiota, while it failed to induce bone loss in germ-free animals (11). Its activity as a “keystone pathogen” may well arise directly from its atypical LPS, which does not activate TLR4 – acting either as a weak TLR4 agonist or even a TLR4 antagonist according to the local inflammatory state – and rendering it immunologically silent, potentially facilitating the initiation of the colonization (12).
Manipulation of the Complement System by P. gingivalis
Early studies documented the activation and regulation of complement components in the gingival crevicular fluid where complement is believed to be present at 70% of its serum concentration (13, 14). P. gingivalis has developed different strategies to evade killing by the complement system. First, its surface anionic polysaccharide confers P. gingivalis serum resistance (15). Moreover, two types of cysteine proteases – known as gingipains – are produced by P. gingivalis: the lysine specific Kgp and the arginine specific RgpA and RgpB (16). While these proteases take part in the destruction of the extracellular matrix, they are also able to cleave the complement components C1, C3, C4, and C5, as well as to capture C4b-binding protein (17–20). This leads to the inhibition of complement activation, but intermittently also to a local accumulation of the anaphylatoxin C5a, the only bioactive fragment present after the actions of gingipains (20). While the massive degradation of complement proteins does not directly benefit complement resistant P. gingivalis, it could allow the colonization and proliferation of other bacterial strains with a higher sensitivity toward complement killing.
The local gingipain-induced accumulation of C5a at the site of infection then activates C5aR. C5aR−/− mice have been shown to be resistant to age dependent as well as P. gingivalis-induced experimental periodontitis (11, 21). Similarly, periodontal inflammation and subsequent bone loss could nearly be abrogated by treating conventional wild-type animals with a C5aR antagonist, underlining the important role played by this anaphylatoxin during periodontitis (22). In neutrophils, P. gingivalis has been shown to inhibit bacterial killing in a Mal/PI3K, C5aR-, and TLR2-dependent manner (23). This could explain the increase in anaerobic oral bacteria and the change in microbiota observed after infection with P. gingivalis in conventional, but not C5aR−/− mice (11). In macrophages, a C5aR-TLR2 crosstalk has been demonstrated to activate the cAMP-dependent PKA pathway, leading to a reduction of intracellular nitric oxide, which permits intracellular bacterial survival (19). The presence of CXCR4 activation further accentuated this synergism (19, 24). This C5aR-TLR2 crosstalk seems particularly important in understanding how P. gingivalis can directly dampen the immune response in an already immunologically tolerant tissue such as the mucosa. In addition, C5aR activation in macrophages inhibits the TLR2-induced IL-12p70 production (21).
The interaction of P. gingivalis fimbriae with TLR2 leads to the inside-out activation of the β2 integrin CR3 (CD11b/CD18) via PI3K (25). Direct interaction of P. gingivalis fimbriae with the chemokine receptor CXCR4 similarly results in CR3 activation (26). In macrophages, P. gingivalis uses this TLR2-activated CR3 as a port of entry as well as to survive intracellularly (25). In fact, inside-out activation of CR3 has been shown to suppress IL-12p70 production in macrophages (21, 25, 26). Also, the pro-inflammatory cytokines IL-1β, IL-6, and TNFα, known to induce bone resorption, are up-regulated in a C5aR/TLR2- and CR3-dependent manner by P. gingivalis (21, 23, 27). The resulting inflammatory breakdown products may then further strengthen the dysbiosis as recently suggested by a study underlining the inflammophilic character of the periodontitis-associated microbiota (28). Taken together, these results highlight the role played by the complement system during periodontitis: P. gingivalis manipulates the host complement components to escape immune clearance, colonize its new niche, and reshape the local microbiota.
Antimicrobial Peptides of the Oral Cavity
The oral cavity is home to various peptides with antimicrobial activity, secreted by epithelial cells, neutrophils, and salivary glands. Their expression often increases during periodontitis [reviewed in Ref. (29)]. One of these molecules, the cathelicidin LL-37, plays a major role in oral health, as illustrated by the severe periodontitis observed in patients suffering from either Kostmann or Papillon–Lefèvre syndromes, two rare conditions characterized by the absence of mature bioactive forms of LL-37 (30, 31). Various studies have nevertheless suggested that cathelicidins only possess a very limited direct microbicidal activity in vivo and instead exert a plethora of immunomodulatory effects [reviewed in Ref. (32)]. More recently, LL-37 has been shown to promote phagocytic uptake by macrophages, which could be used at its advantage by P. gingivalis (33). Alpha (HNP1-3) and beta (hBD1-3) defensins are another class of antimicrobial peptides present in the gingival crevicular fluid (29). During periodontitis, the expression of cathelicidins, α, and β defensins is increased in the gingival crevicular fluid, most particularly in the presence of P. gingivalis (34–36). However, P. gingivalis has been shown to be highly resistant to killing by LL-37 in vitro. Similar observations were made for defensins, suggesting that the higher antimicrobial activity observed during periodontitis may have very little direct effect on P. gingivalis, but most probably has a major impact on other more susceptible bacteria (36–38). This could represent another way by which P. gingivalis shapes the local microbiota thereby selecting for periodontopathic strains, non-periodontopathic strains having been shown to be more susceptible to the activity of antimicrobial peptides (36, 38). Importantly, LL-37 can act as a pro-inflammatory trigger during periodontitis. In fact, as well as being a chemoattractant for neutrophils expressing FPRL1 receptor, LL-37 was demonstrated to induce the production of leukotriene B4 (LTB4), a potent chemotactic agent, in human neutrophils via binding to the cathelicidin receptor FPR2/ALX (39, 40). LTB4 can then trigger LL-37 release by neutrophils in an autocrine manner, thus creating a pro-inflammatory loop eventually leading to bone tissue destruction. This inflammatory response is eventually dampened by lipoxin A4 – a ligand for the FPR2/ALX receptor produced during the resolution phase of inflammation (39, 41, 42). Determining copy number variation in antimicrobial peptides and screening for relevant SNP may help to stratify those at risk of developing aggressive periodontitis who would benefit from early periodontal management (43–45).
Evasion from the Oral Cavity: Link to Cardiovascular Diseases
Numerous studies have associated chronic periodontitis with various diseases, such as rheumatoid arthritis, diabetes, and cardiovascular diseases (46–48). Similarly, P. gingivalis has been observed at other sites than the oral cavity (49–51). While the exact mechanism used by P. gingivalis to reach distant anatomical locations has not yet been defined, P. gingivalis has been shown to survive intracellularly in macrophages, epithelial, endothelial, and smooth muscle cells and to be able to spread from one cell to another (4, 19, 25, 52). P. gingivalis could therefore potentially use these cells as means of transportation to travel to peripheral tissues.
Atherosclerotic disease has long been viewed as a manifestation within disease complexes such as metabolic syndrome, renal failure, and other chronic inflammatory conditions. The atherosclerotic plaque is a site of inflammation within the arterial intima, where inflammatory cells and lipids accumulate. Viable periodontic pathogens, including P. gingivalis, have been found in atherosclerotic plaques in mice and in humans (49–51). Antimicrobial peptides and complement activation products are both constituents of the plaques (53–55). The abilities of P. gingivalis to manipulate the complement and the antimicrobial systems in remote location could putatively contribute to the progression of atherosclerosis. In fact, P. gingivalis has been shown to accelerate plaque formation in an apolipoprotein E−/− mouse model (56).
C5a is present in atherosclerotic plaques and acts as a proatherogenic molecule (57). While it does not seem to play a role in the initial development of the pathology, C5a has been shown to promote apoptosis in endothelial and smooth muscle cells as well as to induce the expression of the metalloproteases MMP1 and MMP9 in macrophages in atherosclerotic plaques. This leads to the degradation of the extracellular matrix and to the rupture of the plaque (57–59). Similarly, reduced plaque size was observed after treatment of ApoE−/− mice with a C5aR antagonist (60).
Elevated expression level of LL-37 has been reported in atherosclerotic lesions, where it is thought to modulate the local immune response and induce apoptosis in vascular smooth muscle cells (54, 61). The presence of LL-37-resistant P. gingivalis in the lipid plaque could lead to an increase of the local concentration of antimicrobial peptides. Together with the gingipain-dependent local accumulation of C5a in the vicinity of P. gingivalis, this could be responsible, at least in part, for the exacerbated pathophysiology observed in the mouse model as well as in human disease.
Potential Therapeutics
The molecular actions involving complement and antimicrobial peptides (and others) in the oral cavity are now well known but the systems are not easily amenable to therapeutic targeting. Treatments against periodontitis consist mainly on reducing the formation of bacterial plaque in the oral cavity using physical and chemical forces. Antibiotics may be given as a short course but they usually only accompany periodontal treatment, as they have difficulties to penetrate periodontal biofilms. Various isolates of oral bacteria such as Lactobacilli have been shown to reduce in vitro the growth of different periodontopathogens including P. gingivalis (62, 63). Clinical trials confirmed the potential of these probiotic agents to be used as a complement to periodontal treatments (63–65). Vitamin D supplementation with its beneficial effect on bone mineralization and its anti-inflammatory potential (inhibition of IL-6, IL-8, and TNFα) may as well be an additional therapy to consider (66, 67). Another option consists on the use of proresolving mediators; in fact, topical applications of the resolvin RvE1 molecule were able to reduce and even to some extent restore periodontitis-associated bone loss in a rabbit model of experimental periodontitis (68, 69). However, the most promising therapy, to date, remains the periodontal vaccines as immunization has been shown to protect against experimental periodontitis in different animal models and could potentially prevent the overt inflammation observed in associated diseases (56, 70).
Conclusion
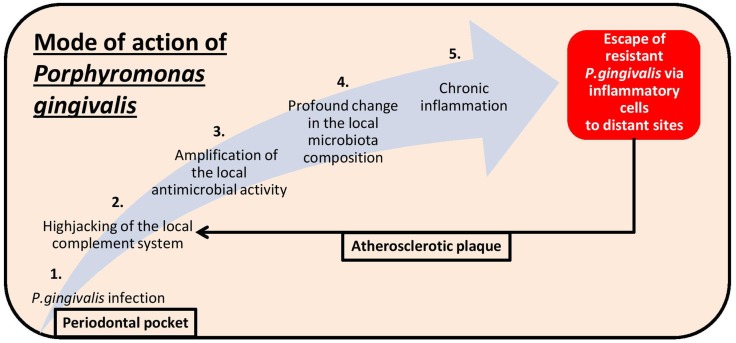
Porphyromonas gingivalis is a good example of a bacterium able to shape the composition of its microbial environment and to subvert the immune system toward chronic inflammation (Figure 1). Evidence of periodontopathogens in atherosclerotic plaques implies a direct role – which might have justified the recent broad population health advice of increasing oral hygiene – but the concomitant presence of oral and gut commensals in biopsies of atherosclerotic arteries begs as well the question of how leaky our mucosal tolerance barrier is.
Figure 1.
Pathomechanistic sequence of events leading to periodontitis following Porphyromonas gingivalis infection (light blue arrow) as well as to the exacerbated pathophysiology observed in atherosclerosis plaques after evasion of the bacteria from the oral cavity (black arrow).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Aline Dupont was supported by the DFG priority program SPP 1656 from the German Research Foundation.
References
- 1.Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol (2000) 27:722–32. 10.1034/j.1600-051x.2000.027010722.x [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Liu X, Li J, Aprecio RM, Zhang W, Li Y. Real-time PCR quantification of six periodontal pathogens in saliva samples from healthy young adults. Clin Oral Investig (2014). 10.1007/s00784-014-1316-0 [DOI] [PubMed] [Google Scholar]
- 3.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (2005) 366:1809–20. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed] [Google Scholar]
- 4.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun (1995) 63:3878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol (2002) 4:305–14. 10.1046/j.1462-5822.2002.00192.x [DOI] [PubMed] [Google Scholar]
- 6.Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol (2007) 9:1997–2007. 10.1111/j.1462-5822.2007.00931.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun (2004) 72:3743–51. 10.1128/IAI.72.7.3743-3751.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun (1998) 66:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J (2013) 7:1016–25. 10.1038/ismej.2012.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J (2012) 6:1176–85. 10.1038/ismej.2011.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe (2011) 10:497–506. 10.1016/j.chom.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, et al. Human toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4’-phosphatase activities. Cell Microbiol (2009) 11:1587–99. 10.1111/j.1462-5822.2009.01349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boackle RJ. The interaction of salivary secretions with the human complement system – a model for the study of host defense systems on inflamed mucosal surfaces. Crit Rev Oral Biol Med (1991) 2:355–67. [DOI] [PubMed] [Google Scholar]
- 14.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. I. quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol (1977) 48:772–7. 10.1902/jop.1977.48.12.772 [DOI] [PubMed] [Google Scholar]
- 15.Slaney JM, Gallagher A, Aduse-Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun (2006) 74:5352–61. 10.1128/IAI.00304-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 (2010) 54:15–44 10.1111/j.1600-0757.2010.00377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol (2007) 178:7242–50. 10.4049/jimmunol.178.11.7242 [DOI] [PubMed] [Google Scholar]
- 18.Potempa M, Potempa J, Okroj M, Popadiak K, Eick S, Nguyen KA, et al. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonas gingivalis. J Immunol (2008) 181:5537–44. 10.4049/jimmunol.181.8.5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal (2010) 3:ra11. 10.1126/scisignal.2000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem (1992) 267:18902–7. [PubMed] [Google Scholar]
- 21.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol (2011) 186:869–77. 10.4049/jimmunol.1003252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol (2012) 189:5442–8. 10.4049/jimmunol.1202339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe (2014) 15:768–78. 10.1016/j.chom.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A (2008) 105:13532–7. 10.1073/pnas.0803852105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Shakhatreh MA, James D, Liang S, Nishiyama S, Yoshimura F, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol (2007) 179:2349–58. 10.4049/jimmunol.179.4.2349 [DOI] [PubMed] [Google Scholar]
- 26.Hajishengallis G, McIntosh ML, Nishiyama SI, Yoshimura F. Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis. Mol Oral Microbiol (2013) 28:239–49. 10.1111/omi.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita A, Murakami Y, Yamashita Y, Ishida M, Fujisawa S, Kitano S, et al. Porphyromonas gingivalis fimbriae use beta2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 beta chain plays a functional role in fimbrial signaling. Infect Immun (1998) 66:4056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol (2014) 29:248–57. 10.1111/omi.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000 (2009) 51:152–80 10.1111/j.1600-0757.2009.00310.x [DOI] [PubMed] [Google Scholar]
- 30.Eick S, Puklo M, Adamowicz K, Kantyka T, Hiemstra P, Stennicke H, et al. Lack of cathelicidin processing in Papillon-Lefevre syndrome patients reveals essential role of LL-37 in periodontal homeostasis. Orphanet J Rare Dis (2014) 9:148. 10.1186/s13023-014-0148-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with Morbus Kostmann: an observation study. Lancet (2002) 360:1144–9. 10.1016/S0140-6736(02)11201-3 [DOI] [PubMed] [Google Scholar]
- 32.Choi KY, Mookherjee N. Multiple immune-modulatory functions of cathelicidin host defense peptides. Front Immunol (2012) 3:149. 10.3389/fimmu.2012.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan M, van der Does AM, Tang X, Lindbom L, Agerberth B, Haeggstrom JZ. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol (2014) 95:971–81. 10.1189/jlb.0513304 [DOI] [PubMed] [Google Scholar]
- 34.McCrudden MT, Orr DF, Yu Y, Coulter WA, Manning G, Irwin CR, et al. LL-37 in periodontal health and disease and its susceptibility to degradation by proteinases present in gingival crevicular fluid. J Clin Periodontol (2013) 40:933–41. 10.1111/jcpe.12141 [DOI] [PubMed] [Google Scholar]
- 35.Pereira AL, Holzhausen M, Franco GC, Cortelli SC, Cortelli JR. Human beta-defensin 2 and protease activated receptor-2 expression in patients with chronic periodontitis. Arch Oral Biol (2012) 57:1609–14. 10.1016/j.archoralbio.2012.04.018 [DOI] [PubMed] [Google Scholar]
- 36.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol (2008) 23:328–35. 10.1111/j.1399-302X.2008.00433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachrach G, Altman H, Kolenbrander PE, Chalmers NI, Gabai-Gutner M, Mor A, et al. Resistance of Porphyromonas gingivalis ATCC 33277 to direct killing by antimicrobial peptides is protease independent. Antimicrob Agents Chemother (2008) 52:638–42. 10.1128/AAC.01271-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res (2007) 42:410–9. 10.1111/j.1600-0765.2006.00962.x [DOI] [PubMed] [Google Scholar]
- 39.Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolving E1. FASEB J (2011) 25:1697–705. 10.1096/fj.10-175687 [DOI] [PubMed] [Google Scholar]
- 40.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1(FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med (2000) 192:1069–74. 10.1084/jem.192.7.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Börgeson E, Lönn J, Bergström I, Brodin VP, Ramström S, Nayeri F, et al. Lipoxin A4 inhibits Porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect Immun (2011) 79:1489–97. 10.1128/IAI.00777-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry (2000) 39:4761–8. 10.1021/bi992551b [DOI] [PubMed] [Google Scholar]
- 43.Ikuta T, Inagaki Y, Tanaka K, Saito T, Nakajima Y, Bando M, et al. Gene polymorphism of beta-defensin-1 is associated with susceptibility to periodontitis in Japanese. Odontology (2013) 103(1):66–74. 10.1007/s10266-013-0139-9 [DOI] [PubMed] [Google Scholar]
- 44.Rivas-Santiago B, Serrano CJ, Enciso-Moreno JA. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect Immun (2009) 77:4690–5. 10.1128/IAI.01515-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simiele F, Recchiuti A, Mattoscio D, De Luca A, Cianci E, Franchi S, et al. Transcriptional regulation of the human FPR2/ALX gene: evidence of a heritable genetic variant that impairs promoter activity. FASEB J (2011) 26:1323–33. 10.1096/fj.11-198069 [DOI] [PubMed] [Google Scholar]
- 46.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol (1996) 67:1123–37. 10.1902/jop.1996.67.10s.1123 [DOI] [PubMed] [Google Scholar]
- 47.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J (2014) 217:433–7. 10.1038/sj.bdj.2014.907 [DOI] [PubMed] [Google Scholar]
- 48.Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep (2014) 16:408. 10.1007/s11926-014-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armingohar Z, Jorgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol (2014) 6:23408. 10.3402/jom.v6.23408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol (2005) 25:e17–8 10.1161/01.ATV.0000155018.67835.1a [DOI] [PubMed] [Google Scholar]
- 51.Velsko IM, Chukkapalli SS, Rivera MF, Lee JY, Chen H, Zheng D, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One (2014) 9:e97811. 10.1371/journal.pone.0097811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Michel R, Cohen J, Decarlo A, Kozarov E. Intracellular survival and vascular cell-to-cell transmission of Porphyromonas gingivalis. BMC Microbiol (2008) 8:26. 10.1186/1471-2180-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnathan ES, Raghunath PN, Tomaszewski JE, Ganz T, Cines DB, Higazi AA. Immunohistochemical localization of defensin in human coronary vessels. Am J Pathol (1997) 150:1009–20. [PMC free article] [PubMed] [Google Scholar]
- 54.Ciornei CD, Tapper H, Bjartell A, Sternby NH, Bodelsson M. Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study. BMC Cardiovasc Disord (2006) 6:49. 10.1186/1471-2261-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francescut L, Steiner T, Byrne S, Cianflone K, Francis S, Stover C. The role of complement in the development and manifestation of murine atherogenic inflammation: novel avenues. J Innate Immun (2012) 4:260–72. 10.1159/000332435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi C, Viereck J, Hua N, Phinikaridou A, Madrigal AG, Gibson FC, III, et al. Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis (2011) 215:52–9. 10.1016/j.atherosclerosis.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, et al. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J (2011) 25:35–44. 10.1096/fj.10-156083 [DOI] [PubMed] [Google Scholar]
- 58.Patel S, Thelander EM, Hernandez M, Montenegro J, Hassing H, Burton C, et al. ApoE(-/-) mice develop atherosclerosis in the absence of complement component C5. Biochem Biophys Res Commun (2001) 286:164–70. 10.1006/bbrc.2001.5276 [DOI] [PubMed] [Google Scholar]
- 59.Wezel A, de Vries MR, Lagraauw HM, Foks AC, Kuiper J, Quax PH, et al. Complement factor C5a induces atherosclerotic plaque disruptions. J Cell Mol Med (2014) 18:2020–30. 10.1111/jcmm.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manthey HD, Thomas AC, Shiels IA, Zernecke A, Woodruff TM, Rolfe B, et al. Complement C5a inhibition reduces atherosclerosis in ApoE-/- mice. FASEB J (2011) 25:2447–55. 10.1096/fj.10-174284 [DOI] [PubMed] [Google Scholar]
- 61.Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, et al. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol (2006) 26:1551–7. 10.1161/01.ATV.0000223901.08459.57 [DOI] [PubMed] [Google Scholar]
- 62.Kõll-Klais P, Mändar R, Leibur E, Marcotte H, Hammarström L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol (2005) 20:354–61. 10.1111/j.1399-302X.2005.00239.x [DOI] [PubMed] [Google Scholar]
- 63.Tenorio EL, Klein BA, Cheung WS, Hu LT. Identification of interspecies interactions affecting Porphyromonas gingivalis virulence phenotypes. J Oral Microbiol (2011) 3:8396. 10.3402/jom.v3i0.8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol (2013) 40:1025–35. 10.1111/jcpe.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivekananda MR, Vandana KL, Bhat KG. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. J Oral Microbiol (2010) 2:5344. 10.3402/jom.v2i0.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grant WB, Boucher BJ. Are hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinol (2010) 2:30–6. 10.4161/derm.2.1.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein SH, Tipton DA. Vitamin D and its impact on oral health – an update. J Tenn Dent Assoc (2011) 91:30–3. [PubMed] [Google Scholar]
- 68.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol (2007) 179:7021–9. 10.4049/jimmunol.179.10.7021 [DOI] [PubMed] [Google Scholar]
- 69.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J (2006) 20:401–3. 10.1096/fj.05-4724fje [DOI] [PubMed] [Google Scholar]
- 70.Page RC, Lantz MS, Darveau R, Jeffcoat M, Mancl L, Houston L, et al. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral Microbiol Immunol (2007) 22:162–8. 10.1111/j.1399-302X.2007.00337.x [DOI] [PubMed] [Google Scholar]