Abstract
The social defeat paradigm involves aggressive encounters between Long-Evans (LE) (resident) and Sprague-Dawley (SD) (intruder) rats. Successful application of chronic social defeat stress in SD rats is dependent upon selection of highly aggressive LE rats. Half of the LE rats screened for aggression did not meet the criterion for aggression (LE rats performing a defeat, characterized by the intruder surrendering or acquiring a supine position for at least 3 sec). The observation of the differences in the level of aggression between age and weight matched LE rats was quite compelling which led us to the present study. Herein, we measured behavioral differences between aggressor and non-aggressor LE rats. We analyzed their anxiety-like behavior using open-field and elevated plus maze tests. We also measured aggression/violence-like behavior using two tests. In one, time taken to defeat the intruder SD rat was recorded. In the second test, time taken to attack a novel object was compared between the two groups. We observed a significant increase in anxiety-like behavior in aggressor rats when compared to the non-aggressive group. Furthermore, time taken to defeat the intruder rat and to attack a novel object was significantly lower in aggressive LE rats. Biochemical data suggests that heightened anxiety-like behavior and aggression is associated with increased plasma levels of corticosterones and elevated oxidative stress. Significant alterations in dopamine (DA), norepinephrine (NE) and epinephrine (EPI) were observed within the hippocampus, amygdala and the prefrontal cortex, suggesting potential involvement of dopaminergic and noradrenergic systems in regulation of aggressive behaviors.
Keywords: Aggression, dopamine, norepinephrine, stress and anxiety
Introduction
Rats display differential response to stress. In an outbred population of SD (Sprague Dawley) rats, Wood et al. (2010) reported that rats exhibit a significant difference in coping behavior when subjected to social defeat stress, with one group showing defeated (depression-like) behaviors while the other exhibiting resilience [1]. During screening for aggressor rats in order to conduct social defeat test, which involves aggressive encounters by a large Long Evans (LE) male resident rat toward a smaller SD male intruder rat, we observed that almost half of all screened LE rats (6 out of 13) did not meet the criterion for aggression. Two groups of rat populations were identified, one exhibiting extreme aggression, and the other displaying docile behavior. It seems reasonable to suggest that just as not all rats within the same strain will be defeated [1], all rats within the same strain may not be aggressive.
While socially defeated rats are reported to have elevated stress levels and heightened anxiety-like and depression-like behaviors [2], stress parameters or anxiety-like behaviors have not been evaluated in LE rats. The question of whether aggression is associated with stress and anxiety is important. Anxiety is believed to be associated with fear [3]. It is not typically associated with anger. While this may be true but the fact that stress-induced anxiety can provoke an aggressive, violent response, is also valid. Although, aggression is a highly complex behavior [4], it is generally believed to be of two types, reactive aggression and instrumental aggression. Reactive aggression is defined as a violent behavior arising from intimidating events, while instrumental aggression is defined as a means of achieving personal objectives without the intension of inflicting harm [5]. Interestingly, reactive aggression in humans is commonly linked to anxiety and impulsivity [6], and an association between stress and aggressive behavior is also reported [7]. In fact, it is postulated that there exists a positive feedback loop between stress hormones and a brain-based aggression-control center in rats [8]. Relevant to this, studies have shown that serotonin hypofunction may predispose individuals to impulsive aggression with selective serotonin reuptake inhibitors proven to be useful in managing aggression and the accompanying anxiety and panic attacks [9]. Involvement of dopaminergic (DA) system in aggression is also known [10], and corticotropin-releasing factor (CRF) by regulation of central NE system [11], are also postulated to be involved. The neural circuit comprising of the prefrontal cortex, amygdala, hippocampus, hypothalamus, striatum is considered critical in emotional regulation. Therefore, functional abnormalities in any one or more of these regions are likely to increase the susceptibility for impulsive aggression and violence [12].
In this study, the separation is based on animal’s innate trait towards reactive aggression, as the retired (is the age at which reproductive performance declines below acceptable levels) LE rat considers the intruder SD as a threat, as it trespasses its home cage. This research suggests the prescence of neurochemical differences between inbred rats when exposed to a hostile situation. Behavioral (anxiety-like behavior and aggression/violence-like behavior) and biochemical conseqeunces (plasma corticosterone and oxidative stress parameters, NE, EPI and DA in selected brain tissues) of intruder stress on LE rats were examined and correlated with high aggression. This study offers new insights into the variations in functional response observed in age and weight matched retired breeder LE rats. It also sheds light on how stressful stimuli activate the central sympathetic system and the pituitary-adrenal axis.
Materials and Methods
Animals
Male SD rats (225–250g) were used as intruders, and male LE retired breeders rats (400–500g) served as residents (Charles River, Wilmington,MA). Rats were housed with a 12-h light/dark cycle in a climate-controlled room with ad libitum food and water. All experiments were conducted in accordance with the NIH guidelines using protocols approved from the University of Houston Animal Care and Use Committee.
Experimental Scheme
Male SD and retired breeder LE rats were acclimatized for one week and then subjected to the social defeat protocol by the retired breeder LE rats as previously published by us [13] this method was used to separate the aggressors. Behavior assessments was performed including tests for aggression and anxiety-like behavior [Open-field (OFT) and elevated plus maze tests (EPM)], as previously published by our group [13] and others [14]. Rats were killed after the conclusion of all behavioral tests and blood was collected for corticosterone (CORT) and 8-isoprostane analysis and brains harvested for future analysis (Scheme 1).
Scheme 1. A schematic representation of the experimental regimen.
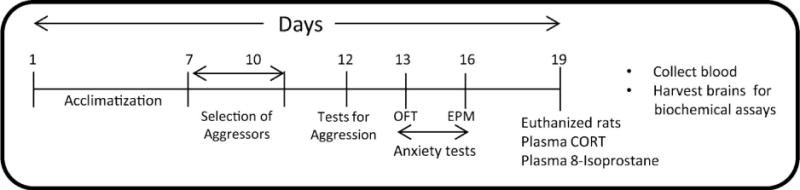
After acclimatization rats were subjected to the social defeat protocol as published by us [13] this method was used to separate the aggressors. Behavior assessments were performed including tests for aggression and anxiety-like behaviors and plasma was isolated for corticosterone and 8-isoprostane analysis.
Selection of aggressors
The social defeat paradigm involves aggressive encounters by a large retired breeder LE male rat (resident) toward a smaller SD male rat (intruder). During screening for the aggressor LE rats, we observed that half of all screened LE rats did not reach the criterion for aggression [LE rats performing a defeat, characterized by the intruder surrendering(acquiring a supine position for at least 3sec) at least 3 times]. LE rats exhibiting consistent levels of aggressive behaviors were identified by a 3-d screening process previously published by our group [15].
Tests for Aggression-like behavior
Number of attacks and time taken to defeat
The number of attacks (10 min) and the time required by a large LE male rat (resident) to defeat a smaller SD male rat (intruder) was noted. This was characterized by the intruder surrendering or acquiring a supine position for at least 3 sec. Ten minute cut-off time was kept to observe a defeat for 3 times.
Novel object attack time
The time spent by the LE male rat attacking a novel object in 10 min (fur toy) was recorded. Each session lasted 5min and started by hanging the fur toy (Target) using a metal wire in the central area of the home cage of the LE rat. The fur toys used were of different colors and similar in size to the SD rats. In between each test animal, an identical new fur toy was placed.
Anxiety-like behavior tests
First, OFT was conducted followed by EPM test as previously published by our group [16, 17]. Briefly, The open field task was carried out in an open field apparatus surrounded by high walls. The rats were placed at the center and were left free to explore the arena for 15 min and analyzed by a computer-based system; (Optovarimax, Columbus Instruments) total and ambulatory activities and distance travelled were recorded and data analyzed using the software. For EPM, a standard rat elevated plus-maze apparatus (Med associates, Vermont) was used and the rat’s movements were tracked manually. Each session lasted for 5 min and the amount of time the rat spent in the open arms was noted.
Plasma corticosterone and 8-Isoprostane
Corticosterone is a systemic marker for stress and isoprostanes are a family of eicosanoids of non-enzymatic origin produced by the random oxidation of tissue phospholipids by oxygen radicals [18]. Both corticosterone and 8-isoprostane levels in plasma were measured 9 days after completion of the test for aggression using enzyme immunoassay (EIA) based kit (Cayman chemical company, AnnArbor,MI).
Norepinephrine (NE), Epinephrine (EPI) and Dopamine (DA) content
Rats were killed 7 days after the test of aggression protocol. Blood was collected and plasma was isolated. The brains also were removed; the prefrontal cortex, amygdala and hippocampus were isolated and snap frozen and stored at −80°C for further studies (Scheme 1). The NE, EPI and DA contents in the prefrontal cortex, amygdala and hippocampus were determined using high performance liquid chromatography (HPLC) as described [19]. Briefly, The catecholamine levels were determined by HPLC (Model 1525, Waters corporation, Milford,MA) equipped with a C18 reverse phase, 3μ LUNA column (Phenomenex, Torrance,CA) and a coulometric electrochemical detector (Model Coulochem III, ESA, Inc., Chelmsford,MA).
Statistical Analysis
Data are expressed as mean ± SEM. Significance was determined by paired student’s t-test (Graphpad Software,Inc. SanDiego, CA). A value of P<0.05 was considered significant.
Results
Aggressive LE rats required less time to socially defeat the SD rats (AG:107±25sec, NAG:>600sec, no defeat, t=6.21, df=10, p<0.05) (Fig. 1A) and demonstrated significantly higher number of attacks (AG:6.83±1.07, NAG:3.66±0.88, t=2.72, df=10, p<0.05) (Fig. 1B). Also, aggressive LE rats spent more time (AG:170±18sec, NAG:61±17sec, t=4.21, df=10, p<0.05) attacking the novel object kept in the cage as compared to the non-aggressive LE rats (Fig. 1C). These characteristics emphasize the violent nature of this group of LE rats.
Fig 1. Examination of aggression-like behaviors.
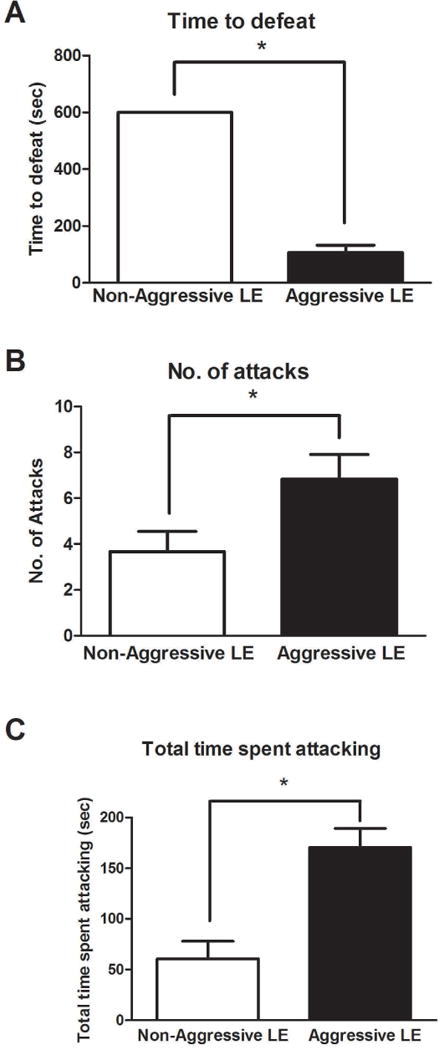
Time to defeat an intruder rat in the social defeat paradigm (A), was determined for both groups of rats. Number of attacks (B) and total time spent attacking (C) in the novel object test was examined in both populations of LE rats. (*)significantly different from non-aggressive LE rats, P<0.05. Bars represent means+SEM, n=6 rats/group.
The aggressive LE rats had lower total activity (AG:3700±230, NAG:4508±296, df=10, p<0.05) (Fig. 2A) and ambulatory activity (AG:3156±160, NAG:3833±264, df=10, p<0.05) (Fig. 2B) and covered lesser distance (AG:2779±146, NAG:3621±247, df=10, p<0.05) (Fig. 2C) than the non-aggressive LE rats. In the elevated plus maze anxiety test, non-aggressive LE rats spent significantly less time (AG:160±9 sec, NAG:114±12 sec, t=3.25, df=10, p<0.05) (Fig. 2D) in the open arms of the EPM apparatus, when compared to aggressive LE rats.
Fig 2. Examination of anxiety-like behaviors using open-field test and elevated plus maze.
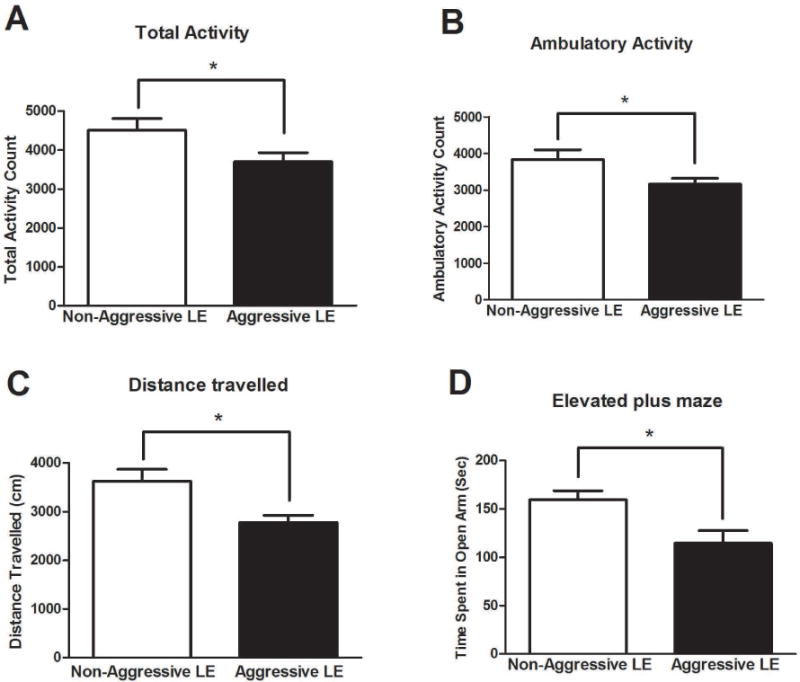
The open-field test determined total (A), ambulatory (B) activities and distance travelled (C). Elevated plus maze test determined time spent in open arms (D) of the EPM apparatus. (*)significantly different from non-aggressive LE rats, P<0.05. Bars represent means+SEM, n=6 rats/group.
Elevated stress was indicated by increased plasma corticosterone levels in both groups of LE rats. However, significantly higher corticosterone levels were observed in the aggressive LE rats when compared to the non-aggressive LE rats (AG:71.93±5.1, NAG:51.8±7.4 ng/ml, t=2.41, df=8, p<0.05) (Fig. 3A). Although not significantly different, the plasma 8-isoprostane levels in the aggressive LE rats (AG:34.8±1.7, NAG:29.8±1.7, t=2.0, df=10, p>0.05) (Fig. 3B) were slightly higher than the non-aggressor LE rats.
Fig 3. Basal plasma corticosterone and 8-isoprostane levels.
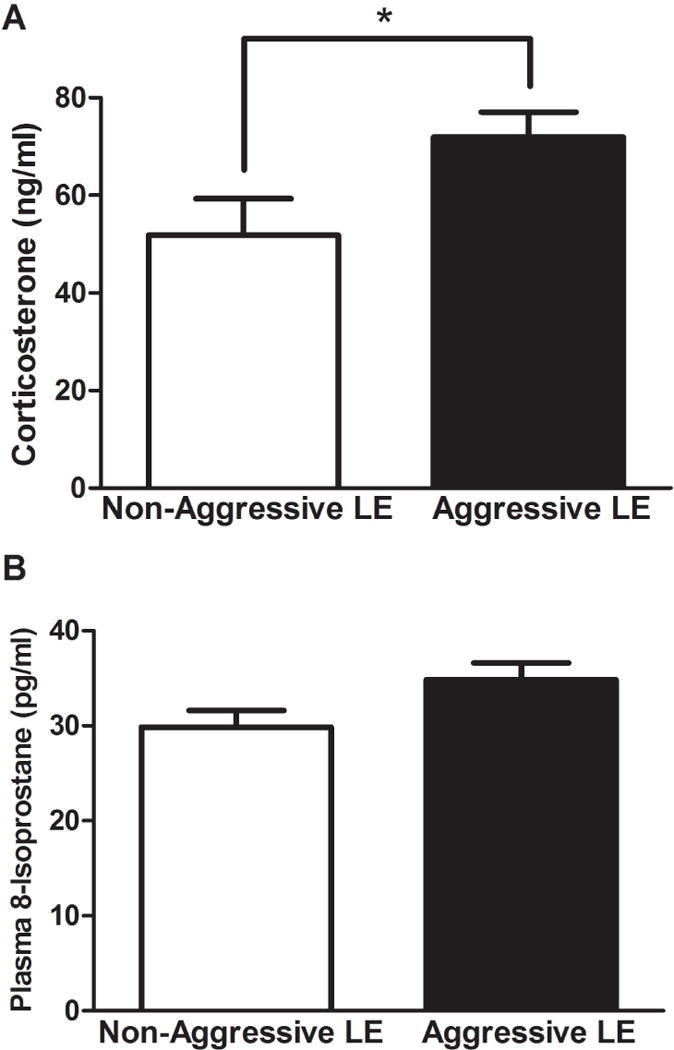
Aggressive LE rats had significantly elevated plasma corticosterone but not 8-isoprostane levels. Plasma corticosterone and 8-isoprostane levels were determined using kit based assays [2]. (*)significantly different from non-aggressive LE rats, P<0.05. Bars represent means+SEM, n=6 rats/group.
We further evaluated contents of DA, NE and EPI in the prefrontal cortex, amygdala and hippocampus. The DA levels were significantly lower in the prefrontal cortex (AG:752.8±150, NAG:2570±590 pg/mg of tissue, t=2.98, df=10, p<0.05) (Fig. 4A) and in the hippocampus (AG:608±86, NAG:944.1±86 pg/mg of tissue, t=2.63, df=10, p<0.05) (Fig. 4C) of the aggressive LE rats when compared to the non-aggressive LE rats. No change was seen in the amygdala (AG:1841±423, NAG:2566±1208 pg/mg of tissue, t=0.72, df=10, p>0.05) (Fig. 4B) between these groups. The NE levels were considerably increased in the prefrontal cortex (AG:1184±337, NAG:413±73 pg/mg of tissue, t=2.61, df=10, p<0.05) (Fig. 4D) and amygdala (AG:1900±408, NAG:808±162 pg/mg of tissue, t=2.7, df=10, p<0.05) (Fig. 4E) while NE levels were unchanged in the hippocampus (AG:521.8±150, NAG:600±76 pg/mg of tissue, t=0.46, df=10, p>0.05) (Fig. 4F). Interestingly, EPI levels were significantly increased in all three brain regions of the aggressive LE rats including prefrontal cortex (AG:831±312, NAG:235±88 pg/mg of tissue, t=2.46, df=10, p<0.05) (Fig. 4G), amygdala (AG:2539±693, NAG:196±34 pg/mg of tissue, t=3.74, df=10, p<0.05) (Fig. 4H) and hippocampus (AG:355±104, NAG:197±44 pg/mg of tissue, t=2.3, df=10, p<0.05) (Fig. 4I) when compared to the non aggressive LE rats.
Fig. 4. Assessment of catecholamine content in prefrontal cortex, amygdala and hippocampus.
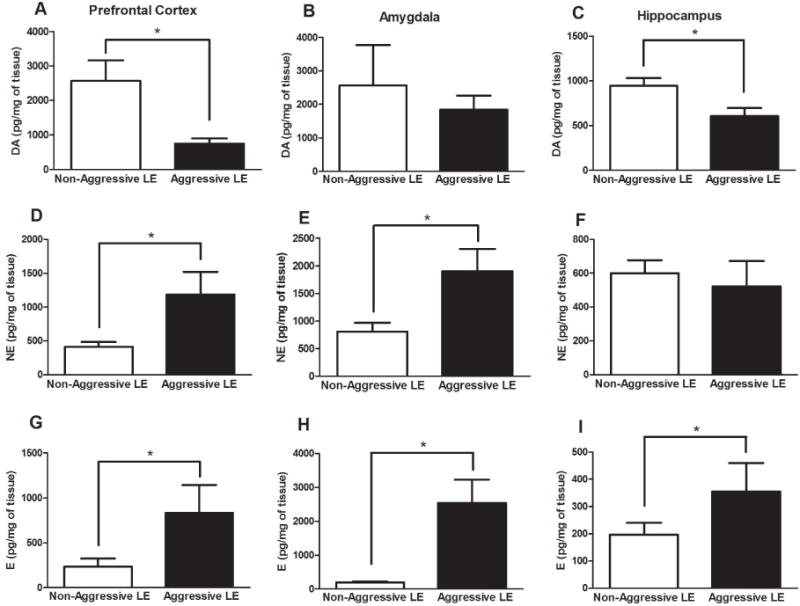
Dopamine (A–C), norepinephrine (D–F) and epinephrine (G–I) levels were determined using HPLC. (*)significantly different from non-aggressive LE rats, P<0.05. Bars represent means+SEM, n=6 rats/group.
Discussion
In this study we have demonstrated that age and body weight matched retired breeder LE rats exhibit different levels of aggressive behavior when encountered with a stressful stimulus in a resident-intruder paradigm. Two groups of rats within the same strain were identified. One group exhibited extreme aggression characterized by defeating the intruder (SD) in seconds, and the other group demonstrated docile behavior making no attempts of defeating the intruder rat. Wood et. al have shown that SD rats display two distinct phenotypic responses to repeated social defeat [1], i.e defeat and resilience. Our study shows that there also exists an inherent difference in aggressive behavior in LE rats as a result of which they react differently to intruder entry. It is interesting that similar to humans, rats also display individual dissimilarities in their reactivity and consequences to social stress (social defeat paradigm). It is tempting to correlate this to human behaviors as it is commonly observed that while some humans are aggressive, others are inherently submissive.
Anxiety is generally believed to be associated with aggressive behavior [9]. Therefore, we evaluated whether there is an association between aggression and anxiety-like behavior in LE rats. Interestingly, aggressive LE rats exhibited heightened anxiety-like behavior, as indicated by reduced time spent in the open arms of the EPM test as well as decreased total and ambulatory activity and distance traveled in the OF test. Increased level of aggression was observed in the aggressive group characterized by significantly higher number of attacks and shorter defeating time of the intruder rat. Additionally, increased number of aggressive attacks were observed in the aggressive group in the novel object test. This test was used to assess the level of aggression/violence-like behavior in rats.
The question of, whether differences in aggressive behaviors within the same strain of LE rats, are a result of individual variation in response to stress, is important. The fact that not all rats within the same strain are equally aggressive or submissive, suggest that perhaps the two behaviors originate from a common source, i.e stress. Perhaps the un-defeated and the non-aggressive population have better stress-coping mechanisms, while the defeated and the aggressive groups have a disrupted circuitry unable to provide an effective coping mechanism. Therefore, the levels of stress were evaluated using plasma corticosterone assay. Elevated stress in aggressive LE rats was indicated from increased plasma corticosterone levels when compared to the non aggressive LE rats. Next, we evaluated the level of oxidative stress in these rats. Earlier, we have reported a close correlation between stress, anxiety-like behavior, and oxidative stress [17], and have found causal role of oxidative stress in anxiety-like behvaior in rats [20]. It was postulated that psychological stress increases oxidative stress which by activating glycation and inflammatory processes lead to anxiety- and depression-like behaviors in rats [2]. Furthermore, a close link exists between changes in the pituitary-adrenal axis, activation of the central NE and DA system, stress responses and aggression [21]. Aggressive behaviors have been induced by administering precursors of NE [22]. However the mechanism by which NE enhances aggression are not fully understood, it is thought that it inhibits neurons in the brain stem which suppress aggression [23]. Using HPLC, we observed increased levels of NE in prefrontal cortex and amygdala and increased levels of EPI in prefrontal cortex, amygdala and hippocampus regions of the brain. This reinforces an involvement of noradrenergic system in the development of aggressive behavior. Furthermore, dopaminergic transmission impacts impulsivity, which can also affect aggression. In a study by Schluter et. al significant negative correlations between aggressive responses and the DA-synthesis capacity were observed especially in the midbrain [21]. Interestingly, we also observed a decrease in the DA levels in the prefrontal cortex and hippocampus of the aggressive LE rats signifying higher propensity towards aggressive behavior. Due to higher stress response in the aggressor rats, the reduction in DA could be due to the high demand for the synthesis of NE and EPI, since DA serves as the precursor for the synthesis of NE and then EPI.
Conclusions
In summary, differences in aggressive behavior are not only categorized by separate behavioral reactions to stress, but also a distinct neuroendocrine and physiological response mediated by distinct brain regions. Understanding the changes occurring within the brain that are related to passive and aggressive phenotype may shed light on the etiology of aggressive behavior.
Highlights.
Aggressive LE rats are more anxious and have increased corticosterone levels.
Alterations occur in the catecholamine levels in the aggressive LE rats.
Aggressive behaviors are mediated by neuroendocrine and neurochemical changes.
Acknowledgments
Funding was provided by 1R15MH093918-01A1 grant awarded to S.S. G.P. and S.S. conceived and designed the experiments and wrote the manuscript. G.P., F.A., I.A., N.S conducted research and analyzed the data.
Abbrevations
- LE
Long Evans
- SD
Sprague Dawley
- DA
dopamine
- EPI
Epinephrine
- NE
Norepinephrine
- CRF
corticotropin releasing factor
- OFT
Open field test
- EPM
Elevated plus maze
- CORT
corticosterone
- EIA
Enzyme immunoassay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors read and approved the final manuscript and declare no conflict of interest.
References
- 1.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emmelkamp PG. Anxiety and Fear. In: Bellack A, Hersen M, Kazdin A, editors. International Handbook of Behavior Modification and Therapy. Springer; US: 1982. pp. 349–95. [Google Scholar]
- 4.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Hormones and behavior. 2003;44:161–70. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz L. Physical pain and the inclination to aggression. Progress in clinical and biological research. 1984;169:27–47. [PubMed] [Google Scholar]
- 6.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 7.Verona E, Kilmer A. Stress exposure and affective modulation of aggressive behavior in men and women. Journal of abnormal psychology. 2007;116:410–21. doi: 10.1037/0021-843X.116.2.410. [DOI] [PubMed] [Google Scholar]
- 8.Kruk MR, Halasz J, Meelis W, Haller J. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behavioral neuroscience. 2004;118:1062–70. doi: 10.1037/0735-7044.118.5.1062. [DOI] [PubMed] [Google Scholar]
- 9.Rylands AJ, Hinz R, Jones M, Holmes SE, Feldmann M, Brown G, McMahon AW, Talbot PS. Pre- and postsynaptic serotonergic differences in males with extreme levels of impulsive aggression without callous unemotional traits: a positron emission tomography study using (11)C-DASB and (11)C-MDL100907. Biological psychiatry. 2012;72:1004–11. doi: 10.1016/j.biopsych.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 12.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 13.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013 doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biological psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014:1–13. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, Asghar M, Jafri F, Bohat R, Alkadhi KA, Salim S. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female wistar rats. PloS one. 2013;8:e74522. doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allam F, Dao AT, Chugh G, Bohat R, Jafri F, Patki G, Mowrey C, Asghar M, Alkadhi KA, Salim S. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. The Journal of nutrition. 2013;143:835–42. doi: 10.3945/jn.113.174649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patki G, Solanki N, Atrooz F, Ansari A, Allam F, Jannise B, Maturi J, Salim S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiology & behavior. 2014;130:135–44. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patki G, Lau YS. Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson’s disease. Pharmacology, biochemistry, and behavior. 2011;99:704–11. doi: 10.1016/j.pbb.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain research. 2010;1359:178–85. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard BE. Stress, norepinephrine and depression. Journal of psychiatry & neuroscience: JPN. 2001;26(Suppl):S11–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Lal H, Brown RM. A possible relationship between catecholamines and acute toxicity of desipramine. Psychopharmacologia. 1968;12:354–7. doi: 10.1007/BF00401414. [DOI] [PubMed] [Google Scholar]
- 23.Reis DJ. The relationship between brain norepinephrine and aggressive behavior. Research publications – Association for Research in Nervous and Mental Disease. 1972;50:266–97. [PubMed] [Google Scholar]


