Abstract
Purpose of review
Tumor growth elicits antigen-specific cytotoxic as well as immune suppressive responses. Interleukin-10 (IL-10) is a key immune-suppressive cytokine produced by regulatory T-cells (Tregs) and by helper T-cells (TH). Here we review pleiotropic functions of IL-10 that impact the immune pathology of cancer.
Recent findings
The role of IL-10 in cancer has become less certain with knowledge of its immune stimulatory functions. IL-10 is needed for T-helper cell functions, T-cell immune surveillance, and suppression of cancer-associated inflammation. By promoting tumor specific immune surveillance and hindering pathogenic inflammation IL-10 is emerging as a key cytokine in the battle of the host against cancer.
Summary
IL-10 functions at the cross roads of immune stimulation and immune suppression in cancer. Immunological mechanisms of action of IL-10 can be ultimately exploited to develop novel and effective cancer therapies.
Keywords: IL-10, Tregs, immune surveillance, inflammation, cancer
Introduction: IL-10 and popular view of tumor immune surveillance
With the discovery of tumor antigens [1-4] cancer immunology entered a new era where efforts to control cancer could be focused on the mechanistic principles of improving tumor specific T-cell responses. The concept of T-cell help [5] and natural tolerance induced by regulatory T-cells [6] were both established after the discovery of tumor antigens, and provided additional knowledge of mechanisms that promote or hinder cancer immune surveillance. Interleukin-10 (IL-10) was first recognized as a T-Helper-2 (TH2) cytokine that modulates the growth and/or differentiation of innate immune cells, keratinocytes, and endothelial cells and suppresses the activation and effector functions of T cells [7]. Simplistically, protective cytotoxic responses require CD4+ T-cell help [8-11] and are driven by the T-helper (TH)-1 cytokines INF-γ and IL-12. By contrast, inflammatory responses produced by the TH2 committed CD4+ T-cells are a diversion if not an obstacle to effective transplant and tumor rejection by cytotoxic T-cells [12-14]. IL-10 and TGFβ that are produced by regulatory T-cells (Tregs) are expected to be major obstacles to CD8+ T-cell mediated tumor lysis [15] and cancer immune therapy [16]. Defying this expectation, protective roles for IL-10 in cancer are emerging that stimulate a fresh look at the functions of this cytokine and of its cellular sources [17]. IL-10 is now recognized not only as the most potent anti-inflammatory cytokine but also for enabling cancer immune surveillance and tumor rejection. This review addresses the controversial functions of IL-10 in cancer and their potential value for cancer immune therapy.
Text of Review
1. Cellular sources of IL-10
Many cell types have the ability to produce IL-10, including CD25+Foxp3+ [18,19], Tr1 CD4+ T cells [19-23], B cells [24-28], macrophages, mast cells, eosinophils, and dendritic cells (DCs) [29-31], as well as CD8+ T cells [32]. Interestingly, while IL-10 inhibits expression of TH1 cytokines, IL-10-producing TH1 cells have been described in both mice and humans [33-42]. The balance between IL-10 and IFN-γ secreted by TH1 cells is thought to achieve a healthy equilibrium that permits effector responses while preventing autoimmunity [43-45]. The source as well as the spatial and temporal availability of IL-10 may differ between tissues and depend on the immune status of the organism. Macrophages are a major source of IL-10 in the intestine/colon of immune deficient Rag1−/− mice [46]. In immune competent mice T-cells are the major source of IL-10 in the intestine and colon [19], and are solely responsible for the increase in levels of IL-10 during polyposis [47]. IL-10 deficient mice without exception develop microbial dependent colitis [48]. Ablating IL-10 expression in T-cells alone is sufficient to cause colitis, revealing the importance of T-cells as a source of IL-10 and in controlling microbial induced inflammation in the colon [47]. The fact that the small intestine is not affected by IL-10 deficiency shows that the impact of IL-10 on immune homeostasis is tissue environment specific (Dennis, et al 2013, manuscript submitted).
2. IL-10 and suppression of immune response
Interleukin-10 (IL-10) was initially heralded as a major immune suppressive factor that was critical for induction of tolerance through inhibition of TH1 immune response and T-cell cytotoxic activity [49-52]. IL-10 was shown to impair the proliferation, cytokine production and migratory capacities of effector T cells [50]. Elevated levels of IL-10 obstructed cytolytic activity in grafted tumors [53-56], and conversely the blockade of IL-10 in animal models facilitated rejection of transplanted tumors [57-60]. The suppressive activity of IL-10 was reported to be direct, based primarily on in vitro experiments [49,61-66]. However, there is evidence suggesting that much of the suppression attributed to IL-10 is indirect and cell mediated [67].
Professional antigen presenting cells, also known as dendritic cells (DC), are important targets of action of IL-10. In earlier studies IL-10 down-regulated expression of MHC class II and co-stimulatory molecules CD80/B7-1 and CD86/ B7.2, and Th1 cytokines including IL-12 by DCs [50,68,69] (reviewed in [70]). T cells that were activated in the presence of IL-10 or DC previously treated with IL-10 failed to respond to re-stimulation, and were described as anergic [64,71]. Tolerogenic DCs produced IL-10 [21,72,73] and autocrine activation of the IL-10 receptor (IL-10R) signaling helped to maintain DCs in an immature tolerogenic state [50,74]. IL-10 expressing DCs were shown to generate Tregs and Tr1 cells, which were also IL-10 producing cells [75-78]. Furthermore, IL-10 contributed to sustained expression of Foxp3 [46,79], TGFβ-Receptor-2 [80] and TGFβ [81,82] by recently activated Tregs, thus stabilizing Treg phenotype and functions[67,81]. IL-2 enhanced the expression of IL-10 by Tregs in a STAT5 dependent manner [83]. Tregs in turn catalyzed the generation of Tr1 cells through secretion of IL-27 [84]. IL-27, a member of the IL-12 cytokine family, induced both Th1 development and production of IL-10 by CD4+ T cells [84-86]. Tr1 cells were also generated through the direct actions of IL-10 and INF-α [87,88] or through antigen presentation by tolerogenic IL-10 producing DC [72,73,89]. These observations showed that much of the immune suppression that is attributed to IL-10 in vivo can be accounted for by the generation and the complex immune modulatory mechanisms of action of Tregs and Tr1.
The impact of IL-10 on immune homeostasis is spatially and temporally controlled. Naïve CD4 T-cells were shown to be more sensitive than memory T-cells to IL-10, explained by down-regulation of IL-10 receptor (IL-10R) upon T cell activation [50,69,90]. For example L. major vaccination produced more robust TH1 responses when IL-10 was limiting at the time of antigen priming [91]. Also, neutralization of endogenous IL-10 with anti-IL-10 mAb inhibited the development of insulin dependent diabetes mellitus when performed early in mice life (priming phase) [92], while the same treatment had no influence on the disease when given to older animals (memory phase) [93]. IL-10 could also compromise immune surveillance by changing immunogenicity of the antigen presenting cell through down-regulation of Transporter Associated with Antigen Processing (TAP1/2) and therefore antigen presentation by MHC class I / HLA class I [94,95]. In fact, both TH17 and TH2 cells express IL-10 and there is good reason to expect IL-10 to work in a negative feedback loop to control activation of T-helper cells [96].
Mechanistically, ligation of IL-10R on DC triggers phosphorylation of janus kinases (JAK) that in turn activate the signal transducer and activator of transcription 3 (Stat3) [97-99]. STAT3 is critical for the expression of IL-10 but is also known to activate the expression of pro-inflammatory cytokines including IL-6. Interestingly, the IL-10 mediated activation of STAT3 is anti-inflammatory. This is achieved through IL10R signaling through Lymphocytic Activation Molecule (SLAM), Src Homology 2 Domain-containing Protein tyrosine phosphatase-1 (SHP-1), and Suppressor of Cytokine Signaling 3 (SOCS3) [100]. SLAM activates SHIP-1 that dephosphorylates and inactivates the co-stimulatory receptors CD28, ICOS, and CD2 [101,102]. Dephosphorylation inhibits the recruitment of phosphatidylinositol-3-kinase (PI3K) and blocks co-stimulatory signaling [90,103-107](for reviews see: [17,80]). Simultaneously, SOCS3 suppresses Stat-dependent signaling of inflammatory cytokines IL-6, [108] TNF-α, and IL-1β [109]. SOCS3 also suppresses signaling through the IL-23R and the expression of IL-17 in inflammatory Th17 T cells [110]. Inhibition of pro-inflammatory cytokines is critical for generating functional extrathymic Tregs, since exposure of Tregs to IL-6 alone can compromise their lineage commitment and ability to suppress inflammation functions [111-113]. Thus, IL-10R signaling utilizes STAT3 but avoids the inflammatory consequences of action STAT3.
3. IL-10 and immune stimulation
The immune suppressive action of IL-10 was so attractive that it overshadowed the almost concomitant discovery of its stimulatory effects on thymocytes, B cells, and mast cells [24,114,115], and the interesting fact that the human IL-10 gene was first cloned from a T-cell line that also secreted IFN-γ [116]. A growing literature is revealing pleiotropic functions of IL-10 that include T-cell activation and tumor rejection. It has been even argued that many of the attributes of IL-10 that were associated with immune suppression are equally likely to enhance immune activation [67].
Stimulatory effects of IL-10 on T-cells and NK are known. IL-10 in combination with IL-2 substantially increased the frequency of CD8+ T cells and augmented their cytolytic activity [117]. In the OVA specific OT1 model, exposure of CD8 T-cells to IL-10 during priming increased T-cell survival and numbers but not proliferation [118]. IL-10 in combination with IL-18 increased the frequency and cytolytic activity of natural killer (NK) cells [119]. These immune stimulatory functions of IL-10 had biologically meaningful consequences. Expression of IL-10 by tumor cells or antigen-presenting cells was protective in tumor transplant models [120,121]. Ectopic expression of IL-10 in mouse B16 melanoma cells induced NK cell mediated shrinkage of the transplanted tumors [122]. Similarly, expression of IL-10 reduced growth of the mouse TSA mammary adenocarcinoma cells upon transplantation [123]. Polyposis prone APCΔ468 mice that were rendered IL-10 deficient had a significant delay in the onset of small intestine polyposis that coincided with increased intrapolyp cytotoxic activity at the onset of polyposis, suggesting improved priming of polyp-specific T-cells (Dennis et. al. 2013, manuscript submitted). There are also reports of protective benefits of systemic delivery of IL-10 [124,125]. A comprehensive study of the anti-tumor properties of IL-10 used multiple mouse tumor models including both chemically induced tumors and transplanted tumor cells [126]. In this study IL-10 induced several essential mechanisms of antitumor immune surveillance: infiltration and activation of intra-tumoral tumor-specific cytotoxic CD8+ T cells, expression of IFNγ and granzymes by CD8+ T cells, and enhancement of intratumoral antigen presentation. Consequently, IL-10 deficiency weakened tumor immune surveillance whereas transgenic overexpression of IL-10 or treatment with pegylated IL-10 protected mice from carcinogenesis[126]. Thus, IL-10 stimulated immune responses by modulating the quality of antigen presentation and influencing lymphocyte differentiation and lineage commitment. The need or IL-10 in TH1 lineage commitment suggests that it may have a role in establishing CD8 T-cell memory. Our observations in a mouse model of hereditary polyposis support these findings. In contrast to IL-10 competent mice that have prolonged intra-polyp cytotoxicity and never developed invasive lesions, IL-10 deficient mice rapidly lost their intra-polyp cytotoxicity and their lesions progressed into large invasive cancers, suggesting an abortive immune response that did not lead to establishment of T-cell memory (Dennis et. al. 2013, manuscript submitted).
The immune stimulatory functions of IL-10 were corroborated by studies of human T-cells, in which IL-10 enhanced IL-2-stimulated proliferation of both human CD4+ and CD8+ T cells by increasing cell division after activation, and augmented IL-2 but not IL-15-induced cytotoxicity of intestinal lymphocytes against colon cancer [127]. IL-10 in combination with IL-2 consistently increased the cytotoxic activity of human papillomavirus E7-specific CD8+ cytolytic T-cells, while combinations of IL-2 and IFNγ or IL-12 or TGF-β failed to do the same [128]. In addition, while IL-10 inhibited the ability of DCs to generate allospecific cytotoxic activity, IL-10 in combination with IL-2 and anti-CD3 activated and promoted growth of human CD8+ T-cells [129]. Finally, IL-10 effector CD4+ T-cells with no immune regulatory functions have been described that are generated by treatment of naïve CD4+ T-cells with TGF-β and IL-4 [130].
Altogether these observations support the provocative view that IL-10 is essential for TH1 and CD8 cytotoxic T-cell responses. They also raise questions as to the mechanisms by which IL-10 stimulates immunity, how these differ from mechanisms by which IL-10 suppresses immunity, which of these mechanisms of action is direct and which indirect result of IL10R signaling in effector T-cells, and to what extent the tissue environment determines the immunologic outcome of the action of IL-10.
4. IL-10 and suppression of Inflammation at environmental interfaces
Focal inflammation can improve antigen uptake and presentation leading to better specific CD8+ T-cell responses and effective anti-tumor immune surveillance [131-136]. However among the T-cells that infiltrate the intestines the cytolytic status of CD8 T-cells in unknown while there is evidence suggesting that the intestine infiltrating CD4+ cells can be cytolytic [137]. The majority of CD8+ T-cells that infiltrate the intestines are either anergic or immune suppressive [138]. The intestines are also the major extrathymic sites of generation of anti-inflammatory Tregs [139] as well as pro-inflammatory T-helper -17 (TH17) cells [140]. Here, a fine balance between anti- and pro-inflammatory T-cells critically maintains microbes at bay while providing growth and repair functions [141]. Deregulation of inflammation in the colon predisposes to inflammatory bowel diseases [142-145]. Inflammation is an inherent component of sporadic colon cancer progression [146,147]. TH17 cells and cytokines are elevated in human colon cancer tumors [148] and negatively correlate with patient survival [149]. Animal modeling has demonstrated that inflammatory responses in intestinal cancers start in aberrant crypts and early adenomatous polyps [150-152]. Inflammation correlated with focal accumulation of microbes [47] and with the breakdown of epithelial barrier functions [153,154]. Much of this inflammation was driven by TH17 cytokines [113,155] whose genetic ablation (of IL-6, IL-23, TNFα) in bone marrow derived cells hindered polyp growth with varying efficacies [148]. Interestingly, control of inflammation in mice with polyposis also enhanced polyp specific TH1 response, increased intra-polyp cytolytic activity, and provided long term protection[148]. TH17 inflammation in polyposis is microbial driven and we recently showed that colonization of the mouse colon with beneficial microbiota can increase Treg expression of IL-10, protect against inflammation, and hinder polyposis [156].
TH17 responses are suppressed by IL-10 producing Tregs [140,157-159]. The adoptive transfer of IL-10 proficient Tregs from healthy donor mice to recipient mice that have polyposis or are otherwise prone to intestinal carcinogenesis suppressed inflammation and protected mice from intestinal carcinogenesis [155,160]. Only IL-10 competent Tregs were effective, and IL-10-deficient Tregs failed to prevent colitis [161] and carcinogenesis [155,162]. T-cell-specific ablation of IL-10 in mice exacerbated microbe driven inflammation and polyposis in the colon [47]. Treg specific ablation of IL-10 and germ-line deletion of IL-10 produced similar colonic inflammations emphasizing the role of Tregs as a major source of IL-10 in the control of microbial inflammation [159]. These observations define a central role for IL-10 expressing Tregs in control of pathogenic inflammation at environmental interfaces. In line with these findings, in a range of inflammation driven cancers including colon [163-167], gastric [168], and head and neck [169,170] as well as HER2+ breast cancer[171], infiltration of tumors with Tregs is associated with less aggressive tumors and can be an independent predictor of longer patient survival. Altogether, these observations indicate that Treg expression of IL-10 is protective against colon cancer, by suppresing cancer-associated inflammation and improving anti-tumor immune surveillance.
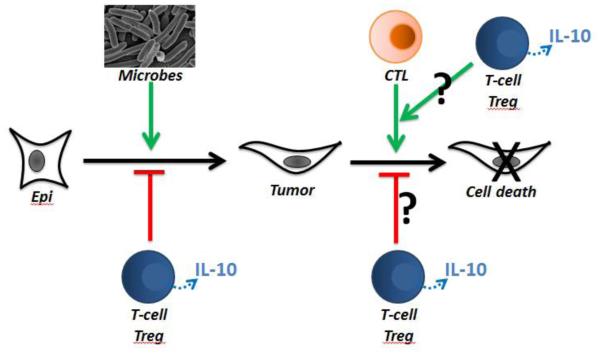
TH17 cells are responsible for orchestrating microbial induced inflammation [172,173] and are intimately linked with the control of microbiota [174]. Expression of IL-17, is contingent upon activity of the transcriptional factors RORγt [175], RORα [176] KLF4 [177]. Expression of IL-10 by Tregs is elevated in the intestines to control TH17 inflammation [19,45,178]. However, during polyposis in mice expression of IL-10 is down-regulated and Tregs acquire TH17 like characteristics including expression of the signature TH17 transcription factor RORγt as well as IL-17 [155]. Similarly, both circulating and tumor infiltrating Tregs in human colon cancer have compromised expression of IL-10 and while remaining potently T-cell suppressive are unable to control inflammation [113,148]. These characteristics are shared by Tregs derived from lung cancer and pancreatic cancer patients (our unpublished observations). Mouse modeling has demonstrated that the IL-10 deficient Treg subset significantly contributes to disease outcome [113,148,155], since ablation of RORγt in the bone marrow derived cells or even just in Tregs was sufficient to recover expression of IL-10 and protect mice against polyposis [148]. These findings provide evidence for a central role of IL-10 in control of inflammation and immune surveillance (Figure 1), and open new possibilities for improving protective immunity at environmental surfaces through manipulation of this crosstalk.
Figure 1. Overarching hypothesis.
Colon cancer is driven by microbial associated. Typically this is a TH17 inflammation that is controlled by IL-10-expressing T-cells and Tregs. There is also a general consensus that cancer is controlled by immune surveillance and TH1 response. Ultimately, disease outcome is the product of both control of inflammation and effective immune surveillance. IL-10 is essential for both processes.
Protective anti-inflammatory roles of IL-10 in cancer are gleaned from studies with pre-clinical models of cancer immune therapy. Therapies aimed at blocking the CTLA-4 inhibitory checkpoints, have promoted antitumor immunity in mouse models of cancer and objective clinical benefits in cancer patients [179]. In mouse models CTLA4 blockade expanded IL-10 producing CD4+FoxP3+ Tregs in a dose-dependent fashion [180]. In a mouse model of colitis anti-CTLA-4 treatment induced IL-10+ Tregs that expressed the cell surface receptor inducible co-stimulator ligand (ICOS) and had potent IDO-dependent anti-inflammatory properties [181]. These observations are particularly interesting as expression of ICOS is an immunologic marker that correlates with clinical responses in prostate cancer patients who receive anti-CTLA-4 therapy [182]. Mouse modeling has revealed that expression of ICOS is intimately related to production of IL-10 by Tregs [96,183] and Tr1 cells [184]. Expression of ICOS and IL-10 were in turn related to protective anti-tumor immunity. Furthermore, Foxp3+ precursors isolated from the human thymus that co-expressed ICOS preferentially upregulated IL-10 expression in response to stimulation with DCs that express ICOS-L [185]. Together these observations suggest that protective mechanisms of CTLA4 blockade involve induction of IL-10 and suppression of inflammation by Tregs.
Concluding paragraph
Until recently, IL-10 was regarded as an immune suppressive cytokine that hindered anti-tumor immunity. It is becoming evident that IL-10 is essential for T-helper-1 cell function and anti-tumor cytolytic activity. The potent anti-inflammatory functions of IL-10, which are mainly indirect and cell mediated, synergize with its immune stimulatory functions to improve tumor specific immune surveillance. Knowledge gained thus far implicates IL-10 in the protective arm of immune response to at least those cancers that appear at environmental interfaces and are driven by microbial instigated inflammation (Figure 1). This knowledge provides new opportunities or immune intervention in cancer.
Key Bullet Point Summary.
Regulatory T-cells (Tregs) and helper T-cells (TH) are major cellular sources of interleukin-10.
IL-10 is needed for T-helper cell functions, T-cell immune surveillance, and suppression of inflammation instigated by microbes and by transformed cells.
Mechanism of action of IL-10 is mostly indirect and mediated by T-cells.
IL-10 is a protective in cancers that occur at environmental surfaces.
IL-10 can be ultimately exploited to develop novel and effective cancer therapies
Acknowledgments
Funding:
NIH1R01 1R01CA160436-01(PI: Khazaie)
Circle of Service, Robert H. Lurie Comprehensive Cancer Center (PI: Khazaie)
References
- 1.De Plaen E, Lurquin C, Van Pel A, Mariame B, Szikora JP, Wolfel T, Sibille C, Chomez P, Boon T. Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum- antigen P91A and identification of the tum- mutation. Proc Natl Acad Sci U S A. 1988;85:2274–2278. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degiovanni G, Lahaye T, Herin M, Hainaut P, Boon T. Antigenic heterogeneity of a human melanoma tumor detected by autologous CTL clones. Eur J Immunol. 1988;18:671–676. doi: 10.1002/eji.1830180503. [DOI] [PubMed] [Google Scholar]
- 3.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knuth A, Wolfel T, Klehmann E, Boon T, Meyer zum Buschenfelde KH. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci U S A. 1989;86:2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno L, Kirberg J, von Boehmer H. On the cellular basis of immunological T cell memory. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 7.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 8.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 9.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 10.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 11.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gately MK, Gubler U, Brunda MJ, Nadeau RR, Anderson TD, Lipman JM, Sarmiento U. Interleukin-12: a cytokine with therapeutic potential in oncology and infectious diseases. Ther Immunol. 1994;1:187–196. [PubMed] [Google Scholar]
- 13.van Den Broek M, Bachmann MF, Kohler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164:371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 14.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 15.Khazaie K, Bonertz A, Beckhove P. Current developments with peptide-based human tumor vaccines. Curr Opin Oncol. 2009;21:524–530. doi: 10.1097/CCO.0b013e328331a78e. [DOI] [PubMed] [Google Scholar]
- 16.Khazaie K, von Boehmer H. The impact of CD4(+)CD25(+) Treg on tumor specific CD8(+) T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Mumm JB, Oft M. Pegylated IL-10 induces cancer immunity: The surprising role of IL-10 as a potent inducer of IFN-gamma-mediated CD8(+) T cell cytotoxicity. Bioessays. 2013;35:623–631. doi: 10.1002/bies.201300004. [DOI] [PubMed] [Google Scholar]
- 18.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 20.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 21.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Garra A, Stapleton G, Dhar V, Pearce M, Schumacher J, Rugo H, Barbis D, Stall A, Cupp J, Moore K, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 25.Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods. 1997;11:98–111. doi: 10.1006/meth.1996.0393. [DOI] [PubMed] [Google Scholar]
- 26.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 27.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 29.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol. 2006;177:7551–7558. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 30.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 31.Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 32.Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, Trautmann A, Rocha B. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, Trinchieri G. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 36.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 38.Jeannin P, Delneste Y, Seveso M, Life P, Bonnefoy JY. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996;156:3159–3165. [PubMed] [Google Scholar]
- 39.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 40.Pohl-Koppe A, Balashov KE, Steere AC, Logigian EL, Hafler DA. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J Immunol. 1998;160:1804–1810. [PubMed] [Google Scholar]
- 41.Shaw MH, Freeman GJ, Scott MF, Fox BA, Bzik DJ, Belkaid Y, Yap GS. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J Immunol. 2006;176:7263–7271. doi: 10.4049/jimmunol.176.12.7263. [DOI] [PubMed] [Google Scholar]
- 42.Windhagen A, Anderson DE, Carrizosa A, Williams RE, Hafler DA. IL-12 induces human T cells secreting IL-10 with IFN-gamma. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- 43.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dennis KL, Wang YW, Blatner NB, Wang S, Saadalla A, Trudeau E, Roers A, Weaver CT, Lee JJ, Gilbert JA, et al. Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10 producing T-cells. Cancer Research. 2013 doi: 10.1158/0008-5472.CAN-13-1511. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Annacker O, Asseman C, Read S, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun. 2003;20:277–279. doi: 10.1016/s0896-8411(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 51.Trinchieri G. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J Exp Med. 2001;194:F53–57. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol Rev. 2004;201:225–238. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 53.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nature immunology. 2010;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. Journal of immunology. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 55.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 56.Urosevic M, Dummer R. HLA-G and IL-10 expression in human cancer--different stories with the same message. Seminars in cancer biology. 2003;13:337–342. doi: 10.1016/s1044-579x(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 57.Kim BG, Joo HG, Chung IS, Chung HY, Woo HJ, Yun YS. Inhibition of interleukin-10 (IL-10) production from MOPC 315 tumor cells by IL-10 antisense oligodeoxynucleotides enhances cell-mediated immune responses. Cancer immunology, immunotherapy : CII. 2000;49:433–440. doi: 10.1007/s002620000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matar P, Rozados VR, Gervasoni SI, Scharovsky OG. Down regulation of T-cell-derived IL-10 production by low-dose cyclophosphamide treatment in tumor-bearing rats restores in vitro normal lymphoproliferative response. International immunopharmacology. 2001;1:307–319. doi: 10.1016/s1567-5769(00)00028-x. [DOI] [PubMed] [Google Scholar]
- 59.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. The Journal of experimental medicine. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. Journal of immunology. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 61.Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu YJ, Banchereau J. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6:1177–1185. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]
- 62.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 63.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 64.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jinquan T, Quan S, Jacobi HH, Madsen HO, Glue C, Skov PS, Malling HJ, Poulsen LK. CXC chemokine receptor 4 expression and stromal cell-derived factor-1alpha-induced chemotaxis in CD4+ T lymphocytes are regulated by interleukin-4 and interleukin-10. Immunology. 2000;99:402–410. doi: 10.1046/j.1365-2567.2000.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–2971. [PubMed] [Google Scholar]
- 67.Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61–76. doi: 10.1016/j.cytogfr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J Exp Med. 1998;187:177–183. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 70.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. Journal of leukocyte biology. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 71.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 72.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 73.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 74.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 75.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–1070. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Stern JN, Strominger JL. T cell receptors in an IL-10-secreting amino acid copolymer-specific regulatory T cell line that mediates bystander immunosuppression. Proc Natl Acad Sci U S A. 2009;106:3336–3341. doi: 10.1073/pnas.0813197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443–449. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–276. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 82.Seder RA, Marth T, Sieve MC, Strober W, Letterio JJ, Roberts AB, Kelsall B. Factors involved in the differentiation of TGF-beta-producing cells from naive CD4+ T cells: IL-4 and IFN-gamma have opposing effects, while TGF-beta positively regulates its own production. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- 83.Tsuji-Takayama K, Suzuki M, Yamamoto M, Harashima A, Okochi A, Otani T, Inoue T, Sugimoto A, Toraya T, Takeuchi M, et al. The production of IL-10 by human regulatory T cells is enhanced by IL-2 through a STAT5-responsive intronic enhancer in the IL-10 locus. Journal of immunology. 2008;181:3897–3905. doi: 10.4049/jimmunol.181.6.3897. [DOI] [PubMed] [Google Scholar]
- 84.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 86.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 87.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 88.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 89.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 90.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207:1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MS, Mueller R, Wicker LS, Peterson LB, Sarvetnick N. IL-10 is necessary and sufficient for autoimmune diabetes in conjunction with NOD MHC homozygosity. J Exp Med. 1996;183:2663–2668. doi: 10.1084/jem.183.6.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 94.Kurte M, Lopez M, Aguirre A, Escobar A, Aguillon JC, Charo J, Larsen CG, Kiessling R, Salazar-Onfray F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and Transporter associated with Antigen Processing 1/2 in human melanoma cells. J Immunol. 2004;173:1731–1737. doi: 10.4049/jimmunol.173.3.1731. [DOI] [PubMed] [Google Scholar]
- 95.Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099–2105. [PubMed] [Google Scholar]
- 96.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 99.Moore A, Josephson L, Bhorade RM, Basilion JP, Weissleder R. Human transferrin receptor gene as a marker gene for MR imaging. Radiology. 2001;221:244–250. doi: 10.1148/radiol.2211001784. [DOI] [PubMed] [Google Scholar]
- 100.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 101.Akdis CA, Joss A, Akdis M, Blaser K. Mechanism of IL-10-induced T cell inactivation in allergic inflammation and normal response to allergens. Int Arch Allergy Immunol. 2001;124:180–182. doi: 10.1159/000053704. [DOI] [PubMed] [Google Scholar]
- 102.Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15:277–285. doi: 10.1016/s0952-7915(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 103.Akdis CA, Blaser K. Mechanisms of allergen-specific immunotherapy. Allergy. 2000;55:522–530. doi: 10.1034/j.1398-9995.2000.00120.x. [DOI] [PubMed] [Google Scholar]
- 104.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–1668. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 105.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 106.Taylor A, Akdis M, Joss A, Akkoc T, Wenig R, Colonna M, Daigle I, Flory E, Blaser K, Akdis CA. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Taylor A, Verhagen J, Akkoc T, Wenig R, Flory E, Blaser K, Akdis M, Akdis CA. IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol Immunol. 2009;46:622–629. doi: 10.1016/j.molimm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 108.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 109.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. Journal of immunology. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 110.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 112.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 113.Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6430–6435. doi: 10.1073/pnas.0913683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Go NF, Castle BE, Barrett R, Kastelein R, Dang W, Mosmann TR, Moore KW, Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
- 116.Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. Journal of immunology. 1991;147:528–534. [PubMed] [Google Scholar]
- 118.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174:5382–5389. doi: 10.4049/jimmunol.174.9.5382. [DOI] [PubMed] [Google Scholar]
- 119.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29:2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 120.Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S, McNeil T, Bigler M, Roncarolo MG, Coffman RL. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 121.Kundu N, Beaty TL, Jackson MJ, Fulton AM. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J Natl Cancer Inst. 1996;88:536–541. doi: 10.1093/jnci/88.8.536. [DOI] [PubMed] [Google Scholar]
- 122.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang XY, Catino JJ, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, De Giovanni C, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 124.Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT. Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J Immunol. 1996;157:231–238. [PubMed] [Google Scholar]
- 125.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98:2143–2151. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 126.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Ebert EC. IL-10 enhances IL-2-induced proliferation and cytotoxicity by human intestinal lymphocytes. Clin Exp Immunol. 2000;119:426–432. doi: 10.1046/j.1365-2249.2000.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Santin AD, Hermonat PL, Ravaggi A, Bellone S, Pecorelli S, Roman JJ, Parham GP, Cannon MJ. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2000;74:4729–4737. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 130.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T Helper 17 Cells Promote Cytotoxic T Cell Activation in Tumor Immunity. Immunity. 2009 doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G, Zitvogel L. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer metastasis reviews. 2011;30:71–82. doi: 10.1007/s10555-011-9283-2. [DOI] [PubMed] [Google Scholar]
- 136.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 137.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–289. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 139.Coombes JL, Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19:116–126. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O'Connor W, Rongvaux A, Van Rooijen N, Haberman AM, et al. Control of T(H)17 cells occurs in the small intestine. Nature. 2011 doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 142.Maloy KJ. The Interleukin-23 / Interleukin-17 axis in intestinal inflammation. J Intern Med. 2008;263:584–590. doi: 10.1111/j.1365-2796.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 143.Dubinsky MC, Wang D, Picornell Y, Wrobel I, Katzir L, Quiros A, Dutridge D, Wahbeh G, Silber G, Bahar R, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn's disease. Inflamm Bowel Dis. 2007;13:511–515. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. e2105. [DOI] [PubMed] [Google Scholar]
- 147.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, et al. The significant role of mast cells in cancer. Cancer metastasis reviews. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 148.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. Expression of RORgammat Marks a Pathogenic Regulatory T Cell Subset in Human Colon Cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Research. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 150.Marten K, Bremer C, Khazaie K, Sameni M, Sloane B, Tung CH, Weissleder R. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002;122:406–414. doi: 10.1053/gast.2002.30990. [DOI] [PubMed] [Google Scholar]
- 151.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gounaris E, Tung CH, Restaino C, Maehr R, Kohler R, Joyce JA, Ploegh HL, Barrett TA, Weissleder R, Khazaie K. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochimica et biophysica acta. 2011;1812:1601–1606. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, Blatner NR, Owen JL, Klaenhammer TR, Mohamadzadeh M. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10462–10467. doi: 10.1073/pnas.1207230109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 160.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Research. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 163.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tougeron D, Maby P, Elie N, Fauquembergue E, Le Pessot F, Cornic M, Sabourin JC, Michel P, Frebourg T, Latouche JB. Regulatory T lymphocytes are associated with less aggressive histologic features in microsatellite-unstable colorectal cancers. PLoS One. 2013;8:e61001. doi: 10.1371/journal.pone.0061001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 166.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 168.Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 170.Loose D, Signore A, Bonanno E, Vermeersch H, Dierckx R, Deron P, Van de Wiele C. Prognostic value of CD25 expression on lymphocytes and tumor cells in squamous-cell carcinoma of the head and neck. Cancer Biother Radiopharm. 2008;23:25–33. doi: 10.1089/cbr.2007.0373. [DOI] [PubMed] [Google Scholar]
- 171.Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- 172.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 173.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ivanov, II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 176.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lebson L, Gocke A, Rosenzweig J, Alder J, Civin C, Calabresi PA, Whartenby KA. Cutting edge: The transcription factor Kruppel-like factor 4 regulates the differentiation of Th17 cells independently of RORgammat. J Immunol. 2010;185:7161–7164. doi: 10.4049/jimmunol.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 179.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 180.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coquerelle C, Oldenhove G, Acolty V, Denoeud J, Vansanten G, Verdebout JM, Mellor A, Bluestone JA, Moser M. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut. 2009;58:1363–1373. doi: 10.1136/gut.2008.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106:2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]