Abstract
Most in vitro and ex-vivo studies indicate a profound suppression of NK cell cytotoxicity (NKCC) by glucocorticoids; while catecholamines and prostaglandins were reported both to suppress and to enhance NKCC. However, methodological considerations hinder our ability to deduce from these findings to the impact of endogenous release of these factors on in vivo levels of NKCC and their implications to NK-dependent resistance to pathologies in living humans or animals. Here we used an in vivo approach that sensitively and specifically reflects NKCC in living F344 rats, based on lung clearance of NK-sensitive tumor cells (MADB106), and based on comparing effects between NK-intact and NK-depleted rats. To study the role of corticosterone, epinephrine, and prostaglandins, we administered these factors to rats, or antagonized their endogenous release following different stress paradigms or surgery. The results indicated that endogenous or exogenous elevated corticosterone levels can suppress in vivo NKCC levels, but only under some conditions, and mostly secondarily to the NK-suppressing impact of epinephrine. Specifically, corticosterone-induced NKCC suppression occurred (i) only under prolonged, but not short exposure to stress, and mainly in males; (ii) was smaller than the prominent impact of epinephrine; (iii) was mostly ascribed to corticosterone-induced potentiation of the effects of epinephrine or/and prostaglandins; and (iv) was completely abolished through antagonizing epinephrine or/and prostaglandins. Overall, these findings markedly limit the significance of stress/surgery-induced corticosterone release in the in vivo suppression of NKCC, and highlight the blockade of epinephrine or/and prostaglandins as effective and clinically feasible approaches to overcome such immuno-suppressive effects.
Keywords: glucocorticoids, catecholamines, prostaglandins, NK cell cytotoxicity, in vivo
1. INTRODUCTION
Prominent among stress responses are the systemic release of glucocorticoids and catecholamines, which were repeatedly shown to modulate various aspects of the immune system, including natural killer (NK) cell activity [1, 2], which is an essential aspect of innate immunity, controlling virally infected cells and malignant cells [3]. Specifically, surgery, swim stress, wet-cage exposure, anxiety, or social confrontation were all shown to suppress NK cytotoxicity [4-7] or NK cell capacity to release IFN-gamma [8, 9], in humans or in animals. Furthermore, suppression of NKCC by stress or surgery was shown to promote cancer metastasis in animal models [5, 10, 11], and human studies indicated associations between perioperative suppression of NKCC and increased long-term cancer recurrence rates [12].
Glucocorticoids are considered the major mediators of stress-induced suppression of NKCC, as numerous in vitro studies, employing rodent or human leukocytes, reported profound suppression of NKCC by synthetic glucocorticoid analogs or by physiological concentrations (3×10-6 to 3×10-7 M) of corticosterone (CORT) or cortisol (for example [13-15]). Additionally, others [16] and us [17] observed ex-vivo suppressive effects of exogenous or stress-induced elevated CORT levels on NKCC (measured in vitro). It was therefore concluded that under stressful conditions, endogenously elevated levels of glucocorticoids are the major or the sole mediators of in vivo suppression of NKCC [17, 18]. However, some ex-vivo and in vivo evidence challenge this prevalent notion. Specifically, interventions that apparently do not affect CORT levels, such as beta-adrenergic blockade, were shown to abolish stress- and surgery-induced suppression of NKCC and NK-dependent resistance to metastasis [10, 19, 20]. Additionally, increased CORT levels following corticotropin-releasing factor (CRF) administration were dissociated from the consequent NK-suppression [21], and some human studies deduced that physiologically-relevant changes in plasma cortisol alone have no significant effect on NKCC [22].
Similar to CORT, catecholamines and prostaglandins were repeatedly shown in vitro to suppress NKCC [23-26], but ex-vivo studies are inconclusive, suggesting increased, unchanged, or decreased NKCC following in vivo exposure to catecholamines or prostaglandins [19, 27-29].
The inconsistency between the reliable and robust in vitro suppression of NKCC by CORT, catecholamines, and prostaglandins, on the one hand, and the lack of consistent ex-vivo findings, on the other hand, yields uncertainty regarding the true in vivo effects of each of these factors on NKCC in the context of stress and surgery. For obvious reasons, in vitro studies alone are insufficient to conclude about the in vivo effects of these stress factors, and the ex-vivo approach may distort previous in vivo effects, as cytotoxicity is tested in vitro following the removal of all endogenous factors and in artificial conditions that do not simulate the in vivo milieu and its complex processes [30, 31].
Because in vivo suppression of NKCC may have detrimental clinical outcomes in the context of cancer metastasis or infectious diseases, it is critical to understand whether it occurs and what are its specific humoral mediators. Such knowledge could allow the use of specific prophylactic measures of clinical applicability. Thus, in this study in F344 rats we aimed at determining the relative impact of CORT, catecholamines, and prostaglandins, in mediating potential in vivo suppression of NKCC. We assumed that all three factors are involved [32], but hypothesized that catecholamines and prostaglandins are the most prominent mediators of stress-induced in vivo suppression of NKCC, whereas CORT has a secondary role.
To test this hypothesis we used an in vivo model-system that is highly sensitive to changes in NKCC levels in the living animal (also see Methods). Shortly, this approach is based on quantifying lung tumor retention (LTR) of a tumor cell line (the syngeneic MADB106) following its intravenous inoculation. This index of LTR is highly sensitive to alterations in in vivo levels of NKCC. Specifically, NK cells were shown to create immunological synapses with MADB106 cells in the lungs [33], marginating pulmonary NK cells were shown to efficiently kill MADB106 cells [31, 34, 35], and selective in vivo depletion of NK cells decreased in vivo killing of MADB106 tumor cells, and increased MADB106 LTR and lung metastases by 20- to 200-fold [27, 36-40]. Most importantly, comparing effects of stress or of other in vivo manipulations between normal rats and rats that are temporarily depleted of NK cells in vivo (see Methods) distinguishes between effects that are mediated through alterations in NKCC and those that involve other or additional mechanisms. The use of these in vivo approaches overcomes most of the shortcomings of the in vitro and ex-vivo approaches, and enables the assessment of the cumulative effects of stress exposure on in vivo NKCC levels along the course of quantifying LTR.
Employing these in vivo approaches, we subjected male and female F344 rats to the administration of CORT, other compounds, or to different stress paradigms or surgery, and assessed in vivo levels of NKCC and the mediating role of elevated levels of CORT, epinephrine, and prostaglandins. Most experiments were conducted in both sexes, as sexual dimorphism was reported with respect to the effects of stress and surgery on hypothalamic-pituitary-adrenal (HPA) axis reactivity [41], NKCC, other aspects of immunity, and cancer resistance [42, 43].
2. MATERIALS AND METHODS
2.1 Animals
Male and female F344 rats (Harlan Laboratories, Jerusalem), were housed 4 per cage, with saw-dust bedding, under a 12:12 hour light/dark cycle at 22±1°C, with free access to standard food and fresh water. Animals were acclimatized to our vivarium for at least 4 weeks, and were 12 to 20 weeks old at the beginning of experimentation. In any given experiment, all animals were of the same age. All experiments were approved by the Institutional Animal Care and Use Committee of Tel Aviv University.
2.2 Drugs
2.2.1 Nadolol
(Sigma, Israel), a hydrophilic nonselective β-adrenergic antagonist, which does not cross the blood-brain-barrier, was dissolved in PBS and injected subcutaneously at 0.2mg/kg.
2.2.2 RU-486
(mifepristone) (Sigma, Israel), a hydrophobic glucocorticoid and progesterone receptor antagonist, was ground and dissolved overnight in corn oil and injected subcutaneously at 25 mg/kg.
2.2.3 Etodolac, a hydrophobic semiselective COX2 inhibitor was dissolved in corn oil and administered subcutaneously at 12.5 mg/kg. Etodolac was kindly donated by Taro, Israel.
2.2.4 Epinephrine
(Sigma, Israel), was first dissolved in PBS. Given the short half-life time of this hormone, and in order to achieve an extended duration of elevated plasma levels, epinephrine was administered subcutaneously (1 mg/kg) in a slowly absorbed emulsion (consisting of 4 parts of the PBS containing the active ingredient, 3 parts of mineral oil, and 1 part of mannide-monooleate – a non specific surface active emulsifier – all purchased from Sigma, Israel).
2.2.5 Corticosterone (CORT)
(Sigma, Israel), the active glucocorticoid in rats, was ground and dissolved overnight in corn oil and injected subcutaneously at doses of 1-9 mg/kg.
2.3 General experimental procedures and counterbalancing
Rats were handled daily for 4 days prior to each experiment in order to reduce unwanted procedural stress, and were randomly allocated to groups. Exposure to stress and adrenalectomy/sham procedures were counterbalanced across cages (i.e., conducted in different cages) in order to prevent unwanted stress in controls and allow maintenance of adrenalectomized animals (see below). Drug administration was counterbalanced within cages. Blood samples for serum corticosterone level measurements (see below) were collected by cardiac puncture or by tail vein draw within less than 3 minutes of approaching a cage. Sera were separated and frozen at -80°C for later analysis of CORT levels.
2.4 Wet-cage stress paradigm
Rats were placed for one or five hours in standard transparent Plexiglas cages filled with 2 cm deep water, at room temperature, with free access to food and drinking water.
2.5 Adrenalectomy (ADX) and sham operation, and maintenance of rats following ADX
Rats were anesthetized with 2.5% isoflurane and a 4cm midline abdominal incision was performed. Both adrenal glands were exposed and removed completely using standard surgical techniques. Sham-operated rats underwent the exact same procedure without removal of the adrenal glands. In order to expedite recovery from surgery, ADX rats were subsequently injected s.c. three times, 3 hours apart, with 3 mg/kg cort/injection. Following surgery, and to ensure complete recovery, rats were kept in their home cages undisturbed for 4 weeks before entering experiments. To overcome the lack of mineralocorticoids, and to approximate normal levels of CORT, adrenocorticotropic hormone (ACTH), and their circadian rhythms, the regular drinking water of ADX rats was replaced with 0.9% NaCl saline containing 15 mg cort/liter. Previous studies showed that this method approximates normal circadian CORT levels (~20-100ng/ml), as rats drink mostly during their first activity hours [44]. Two hours before experimentation (during the light phase), and to ensure low physiological CORT levels, ADX rats were given only normal 0.9% saline without CORT as their drinking water.
2.6 Experimental laparotomy
To simulate stress caused by surgery rats underwent laparotomy. This procedure has been described elsewhere [45]. Briefly, rats were anesthetized with 2.5% isoflurane and a 4 cm midline abdominal incision was performed. The small intestine was externalized, rubbed with a PBS-soaked gauze pad and left hydrated with a PBS-soaked gauze pad for 40 minutes. Finally, the intestine was internalized and the abdomen sutured and cleaned.
2.7 Selective in vivo temporary functional depletion of NK cells
Approximately 1.5 mg/kg of mouse anti-rat NKR-P1 monoclonal antibody (mAb) (CD161) was injected i.v. under isoflurane anesthesia. Previous studies using the abovementioned dose of the anti-NKR-P1 mAb showed immediate, selective, and complete abolition of blood and splenic NK cytotoxicity [46, 47] and up to 200-fold increase in the lung retention and metastatic colonization of MADB106 tumor cells [27, 38, 39]. Our previous studies using control mAbs (R73, W3/25, and ED2), mouse serum, or saline as controls for anti-NKR-P1 administration, have shown that neither had an effect on NK-cell function and metastatic dissemination, and therefore saline was used as the control [38].
2.8 CORT radioimmunoassay (RIA) and blood withdrawal for its assessment
A standard 125I competitive RIA kit (MP Biomedicals, Solon, OH, USA) was used to measure CORT levels according to the manufacturer’s instructions. Blood was drawn using a heparinized syringe (30 units/ml) within less than 3 minutes from disturbing the animals. Cardiac puncture was used in Exp. 1a-c, and tail vein withdrawal in Exp. 9 & 10. To overcome the impact of CORT circadian rhythm while comparing different groups or conditions, blood withdrawal was always conducted at the same time of the day, and the order of blood withdrawal was counter-balanced between the different groups.
2.9 Maintenance and radiolabeling of MADB106 tumor cells, and assessment of lung tumor retention (LTR)
The MADB106 cell line was maintained in monolayer cultures in complete media (RPMI-1640 media supplemented with 10% heat-inactivated FCS, 50μg/mL of gentamicin, 2mM of L-glutamine, 0.1mM of non-essential amino-acids, and 1mM of sodium pyruvate, Biological Industries, Kibbutz Biet Haemek, Israel) in 100% humidity, 5% CO2 at 37°C. Cells were removed from the culture flask with trypsin solution (0.25% in PBS), and were washed with complete media. Tumor cell DNA radiolabeling for assessment of LTR was accomplished by adding 0.5 μCi/ml of 125Iododeoxyuridine (125IDUR, Danyel Biotech, Rehovot, Israel) to the cell culture for 24 hrs. For tumor cell injection, rats were lightly anesthetized with isoflurane, and 4×105/kg MADB106 tumor cells in 2ml/kg PBS containing 0.1% BSA were injected into their tail vein. For assessment of LTR, animals were sacrificed with CO2 21 hrs after inoculation with 125IDUR-labeled tumor cells, and their lungs were removed and placed in a γ-counter to assess percent radioactivity retained in this organ. LTR was calculated using the following formula: (radioactivity count of lung – background radioactivity) ×100/(radioactivity count of the total injected cell suspension – background radioactivity).
2.10 LTR index - its sensitivity to NKCC, its use to distinguish between NK-dependent and NK-independent effects, and other characteristics of this model
The LTR index is highly sensitive to in vivo levels of NKCC. Specifically, NK cells were shown to create immunological synapses with MADB106 cells in the lungs [33], marginating pulmonary NK cells were shown to efficiently kill MADB106 cells [31, 34, 35], and selective in vivo depletion of NK cells increased LTR and lung metastases by more than a hundred fold [27, 36-40]. Furthermore, we have shown that several stress paradigms, or the administration of stress-related factors (catecholamines and their agonists, and prostaglandins), can increase LTR in normal F344 rats, but not in NK-depleted rats, indicating that increased LTR in normal rats under these conditions reflects an in vivo suppression of NK-mediated activity [26, 27]. On the other hand, other manipulations, including surgery or LPS administration [40, 48], increased LTR in both normal and NK-depleted rats, indicating the involvement of non-NK mechanisms in modulating LTR. Thus, the use of this LTR index, in conjunction with an NK-depletion paradigm, can indicate the specific in vivo involvement of NK cells in various manipulations that affect LTR.
While developing this tumor models, others and us have shown that immediately after intravenous tumor administration, more than 95% of tumor cells can be found in the lungs. This percentage decreases dramatically within the first 24 hrs, along with excretion of radioactive molecules in the urine, and both of these processes are markedly inhibited by the aforementioned selective in vivo depletion of NK cells. Other organs present markedly lower (or undetectable) levels of radioactivity, and metastases eventually develop only in the lungs [36-39]. Last, the index of LTR predicts the number of metastases that will be evident weeks later [26, 27, 35].
2.11 Enumerating MADB106 lung metastases
Rats were lightly anesthetized with isoflurane, and 105 MADB106 tumor cells were injected into their tail vein in 0.5 ml of PBS (supplemented with 0.1% BSA). Three weeks later, rats were killed, and their lungs removed and placed for 24 h in Bouin’s solution (72% saturated picric acid solution, 23% formaldehyde (37% solution) and 5% glacial acetic acid). After being washed in ethanol, visible surface metastases were counted separately by two researchers uninformed of the origin of each lung.
2.12 Statistical analysis
One- or two-way analyses of variance (ANOVA) were used in all studies to analyze LTR or CORT levels. When significant group differences were indicated by ANOVA, Fisher’s Protected Least Significant Differences (PLSD) post-hoc contrasts were used to test specific planned pair-wise differences. For unplanned pair-wise differences, Tukey post-hoc contrasts were used. For all analyses, p values of less than 0.05 were considered significant.
3. Experimental Procedures and Results
3.1 Exp. 1. The kinetics of plasma CORT levels following s.c. administration of CORT: Studies of dose, time, and sexual dimorphism
The following three experiments (1.a, 1.b, & 1.c) were conducted to determine what doses should be administered to male and female F344 rats in order to induce physiological levels of CORT (approximately 100-1000 ng/ml), and to identify potential sex-related differences in their kinetics. This information is critical to simulate physiological levels in the following studies and to interpret potential sex-related differences.
1.a. Procedure
Ninety-six F344 male rats were subjected to administration of CORT (s.c., 1, 3, or 9 mg/kg) or vehicle (oil), and plasma CORT levels were assessed, once in each rat, at 1, 2, 4, or 6 hrs following administration. To control for diurnal changes in CORT levels, all samples were collected at the same time of day, about 4 hrs before the end of the light phase, while counterbalancing the order of blood draw between the different groups.
Results
Plasma CORT levels were significantly elevated in a dose-dependent and time dependent manner as evident in Fig. 1A. ANOVA indicated significant group differences (F(13,82)=104.729, p<0.0001), and Fischer’s PLSD indicated significant pair-wise higher CORT levels compared to the control vehicle group (p<0.05), as indicated by *. Post-hoc Tukey test did not indicate significant decrease in any of the groups.
Figure 1. The kinetics of plasma CORT levels following s.c. administration of CORT: Studies of dose, time, and sexual dimorphism.
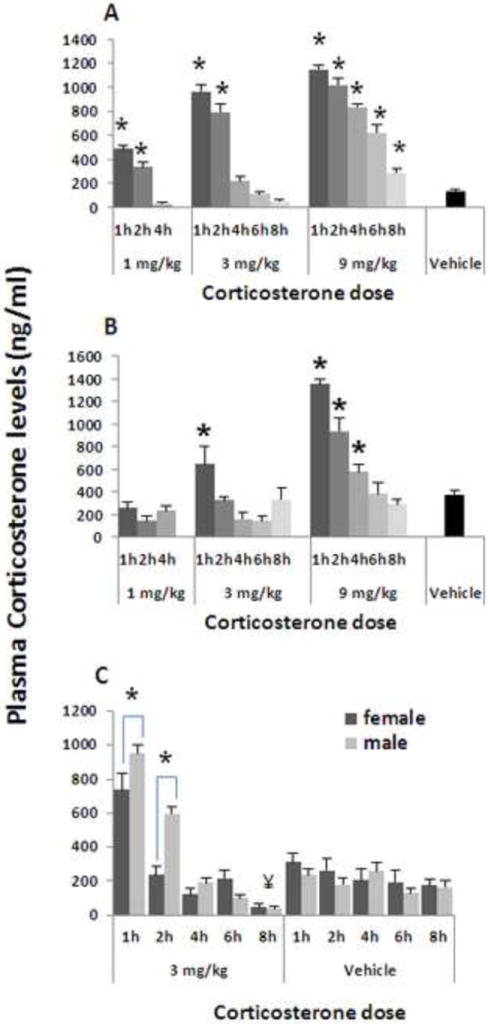
Plasma CORT levels increased in a dose-dependent manner, and decreased in a time-dependent manner, both in male (n=6-7/group) (A), and in female (n=6-7/group) (B) F344 rats. * indicates significant higher levels than Vehicle administration. Females exhibited greater CORT clearance rates than males, at 1h and 2h after 3mg/kg CORT injection (indicated by *) (n=5- 6/group) (C). Eight hours after injection, CORT plasma levels decreased beyond baseline levels compared to the matched Vehicle group (indicated by ¥). Experiments 1.a.,1.b., and 1.c. were each performed once, on different independent occasions. Data are presented as means + SEM.
1.b. Procedure
The exact same procedure as in 1.a was conducted, employing 91 females instead of males.
Results
Plasma CORT levels were significantly elevated in a dose-dependent and time dependent manner, as evident in Fig. 1B. ANOVA indicated significant group differences (F(13,77)=18.18, p<0.0001), and Fischer’s PLSD indicated significant pair-wise differences from the control vehicle group only when the higher doses of 3 and 9 mg/kg were used, as indicated in Fig. 1B by * for increased CORT levels. Post-hoc Tukey test did not indicate significant decrease in any of the groups.
1.c. Procedure
Here we compared the kinetics of CORT levels between males and females within the same experiment. We used a single dose of CORT injection that induces moderate to high physiological levels in both sexes (s.c., 3 mg/kg). Additionally, both the increase and the decrease in CORT levels were studied in reference to the time-interval-matched vehicle group. Fifty-six male and 56 female rats were subjected to receive CORT (s.c., 3 mg/kg) or vehicle, and plasma CORT levels were assessed once in each rat at 1, 2, 4, 6, or 8 hrs following administration.
Results
A 2×6 ANOVA (sex by time point) indicated a significant main effect for sex (F(1,100)=8.387, p=0.0046), for time point (F(5,100)=74.545, p<0.0001), and an interaction (F(5,100)=8.02, p<0.0001). Irrespective of sex, Fischer’s PLSD indicated a significant increase in CORT levels at the 1 and 2 hr time points, and a significant decrease at 8 hrs compared to the matched vehicle injection group (indicated by ¥) (Fig. 1C) (p<0.05). As the interaction between sex and time point was significant, and as planned, we also compared females to males at specific time points. Fisher’s PLSD indicated that plasma CORT levels at the 1 and 2 hr time points were significantly lower in females than in males (as indicated by *), while no other time points yielded significant sex differences (p<0.05).
3.2 Exp. 2. Prolonged exposure and high levels of CORT are needed to effectively increase MADB106 LTR
Procedure
Sixty-six male rats were s.c. injected with vehicle or CORT in a dose of 3 or 6 mg/kg, at 3 hrs before tumor administration or simultaneously with it. One group was injected with 3 mg/kg twice - once at each time point (see Fig. 2 for all groups). To control for the potential stress of injection, all rats received two injections (of CORT or vehicle), one at each time point. Twenty-one hours following MADB106 tumor administration, rats were sacrificed to assess MADB106 LTR.
Figure 2. Prolonged exposure and high levels of CORT are needed to increase MADB106 LTR.
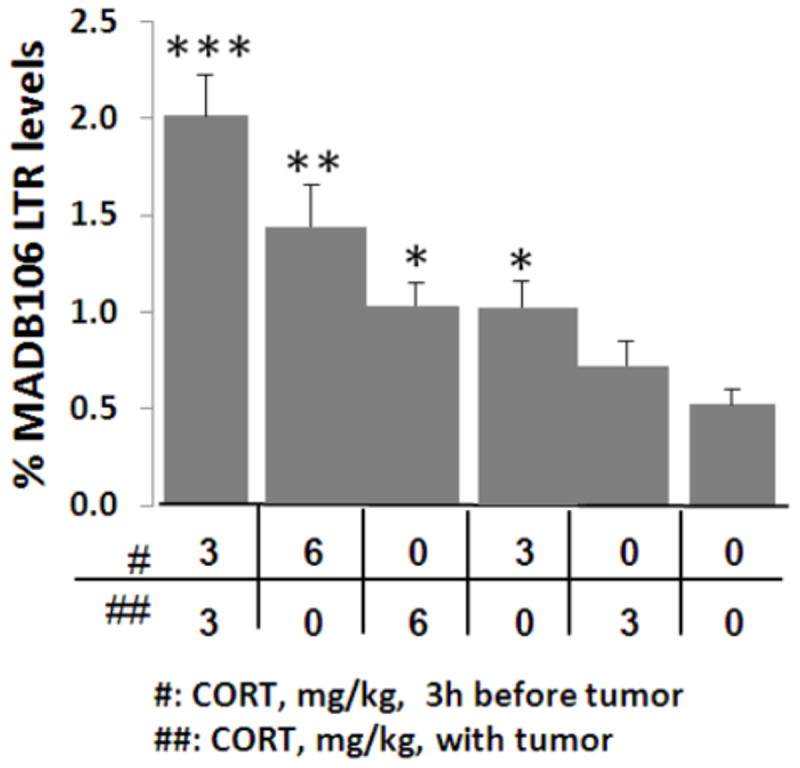
CORT administration increased MADB106 LTR compared to vehicle administration (0/0) (as indicated by 1, 2, or 3 *), and did so in a dose dependent manner. In both doses (3 or 6 mg/kg) CORT administration three hrs before tumor administration, rather than it with, was significantly more effective in increasing LTR. Dividing the 6 mg/kg dose to two 3 mg/kg doses (three hrs apart) increased LTR even further. This experiment was performed once, n=11/group. Data are presented as means + SEM.
Results
One way ANOVA indicated significant group differences (F(5,93)=12.01, p<0.001). Within each administration timing (3 hrs before tumor inoculation or simultaneously with it), the deleterious effects of CORT on LTR were dose dependent. Additionally, administration of CORT 3 hrs before tumor inoculation (irrespective of the dose) increased LTR more than the administration simultaneously with the tumor (Fisher’s PLSD, p<0.05). The administration of 3 mg/kg twice (at both time points) caused the largest increase, significantly more than the 6 mg/kg dose administered with MADB106 administration (p<0.05) (see Fig. 2).
3.3 Exp. 3. Sex differences in the influence of CORT administration on LTR and on numbers of metastases
3.a. Procedure
Employing a 2×4 factorial design, 48 male and 48 female rats that were inoculated intravenously with radio-labeled MADB106 tumor cells, were subjected to receive 2 s.c. injections of 0 (vehicle), 1.5, 3, or 6 mg/kg of CORT. The first injection was given 3 hrs before tumor administration, and the second simultaneously with it. Twenty-one hrs later, the rats were sacrificed to assess LTR.
Results
A 2×4 ANOVA indicated significant main effects for sex (F(1,68)=30.213) and for CORT (F(3,68)=18.524, p<0.0001 for both), and a significant interaction between the effects of CORT and sex (F(3,68)=8.569, p<0.0001) (Fig. 3A). This indicates that the deleterious effect of CORT was greater in males than in females. When conducting ANOVA separately for males and females, Fisher’s PLSD indicated significantly higher LTR levels for 1.5, 3, and 6 mg/kg CORT compared to control levels in males (p<0.05 for each), whereas in females only the highest dose reached statistical significance (p<0.05).
Figure 3. Sex differences in the influence of CORT on LTR and on numbers of metastases.
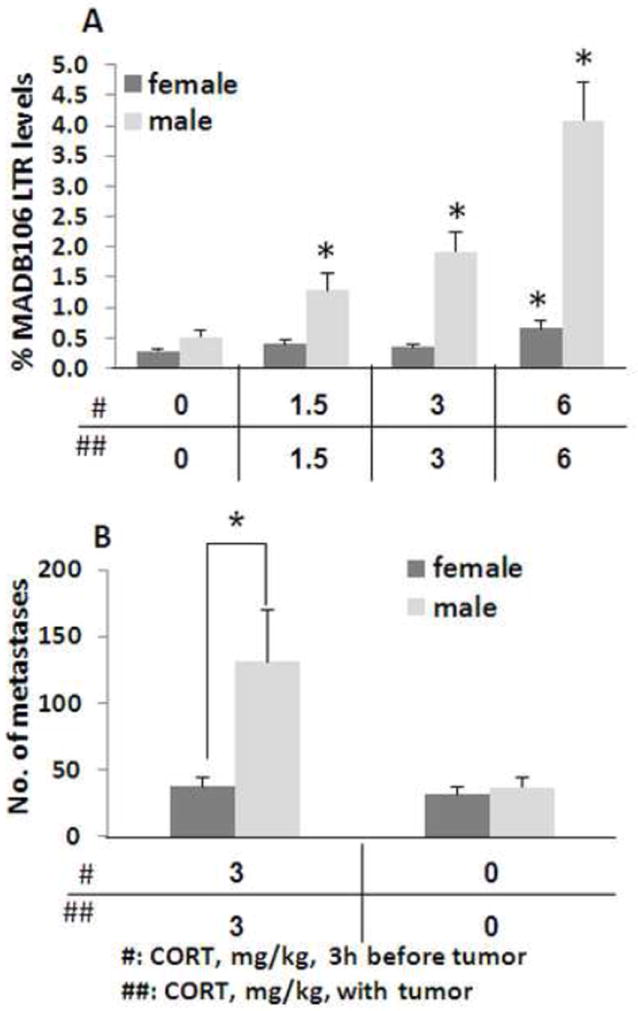
CORT administration increased MADB106 LTR in males in a dose-dependent manner, beginning at 2 × 1.5 mg/kg, while affecting female LTR only at the maximal dose of 2 × 6 mg/kg, and to a much lesser extent that in males (n=12/group) (A). * indicates significant difference from the respective control (0/0) group. Number of actual lung metastases formed 21 days after CORT injection was increased only in males (n=13/group) (B). * indicates significant sex difference. Experiments 3.a., and 3.b. were each performed once, on different independent occasions. Data are presented as means + SEM.
3.b. Procedure
In this study, assessing lung metastases, we examined whether the deleterious effect of our CORT paradigm has long term significance, affecting the actual development of macro metastases. To this end we counted the actual numbers of surface lung metastases 21 days following MADB106 tumor administration. In this experiment, 26 males and 26 females were injected with either two injections of CORT (3 mg/kg each, 3 hrs apart) or vehicle. Simultaneously with the second CORT/vehicle injection, all rats were administered with non-labeled MADB106 tumor cells, and 21 days later sacrificed and lung metastases were enumerated.
Results
A 2×2 ANOVA indicated significant main effects for sex (F(1,48)=5.041, p=0.0294) and for CORT (F(1,48)=5.138, p=0.0280), and a significant interaction (F(1,48)=4.045, p<0.005), indicating that CORT elevated the number of lung metastases in males more than in females (Fig. 3B). Fischer’s PLSD indicated a significant effect of CORT only in males (P<0.05).
3.4 Exp. 4. NK cells are the primary mediators of the in vivo effects of CORT on MADB106 LTR in both female and male rats
As reviewed above, the LTR index is highly sensitive to NK cell activity, but can also be affected by other factors. To understand the in vivo role of NK cells in mediating the impact of CORT on MADB106 LTR, we conducted studies that also utilized selective functional depletion of NK cells. Two experiments were conducted, one in males and one in females.
4.a. Procedure
Seventy-five male rats were randomly assigned to undergo NK depletion or to serve as controls (see Methods), and were simultaneously administered with radio-labeled MADB106 tumor cells. Three hrs before, and simultaneously with tumor administration, rats received 2 injections of CORT (s.c. 1.5 or 3 mg/kg) or vehicle (oil). Our previous studies [40] had shown that laparotomy stress increases LTR both through NK-dependent and through NK-independent mechanisms. Therefore, as a positive control, to demonstrate NK-independent effects, additional groups of rats were subjected to laparotomy 2 hrs before tumor administration, and were randomized to undergo NK-depletion or vehicle administration simultaneously with tumor administration.
Results
In vehicle-treated animals, NK depletion elevated LTR levels more than 70-fold (p<0.001), confirming the sensitivity of the LTR index to NKCC. Given this marked differences in baseline levels between NK-intact and NK-depleted animals (0.4% vs. 35%), and the consequent marked differences in variance (violating the ANOVA assumption of homogeneity of variance), ANOVA was conducted separately in each condition. In NK-intact rats, ANOVA indicated significant group differences (F(3,34)=21.2, p<0.001), and surgery, and 1.5 and 3 mg/kg of CORT, each significantly elevated LTR levels compared to the control (vehicle) group (Fisher’s PLSD, p<0.01 for each comparison) (Fig. 4A). On the other hand, in the NK depletion groups, ANOVA indicated significant group differences (F(3,33)=5.1, p<0.01), but neither doses of CORT had an effect. In contrast, surgery, which had a smaller effect than that of 3 mg/kg of CORT in NK-intact animals, significantly elevated LTR levels in NK-depleted animals (Fisher’s PLSD, p<0.05). These findings indicate that the effects of 1.5 and 3 mg/kg of CORT on LTR are primarily mediated through NK cells, while the effects of surgery involve non-NK dependent mechanisms.
Figure 4. NK cells are the primary mediators of the in vivo effects of CORT on MADB106 LTR in both female and male rats.
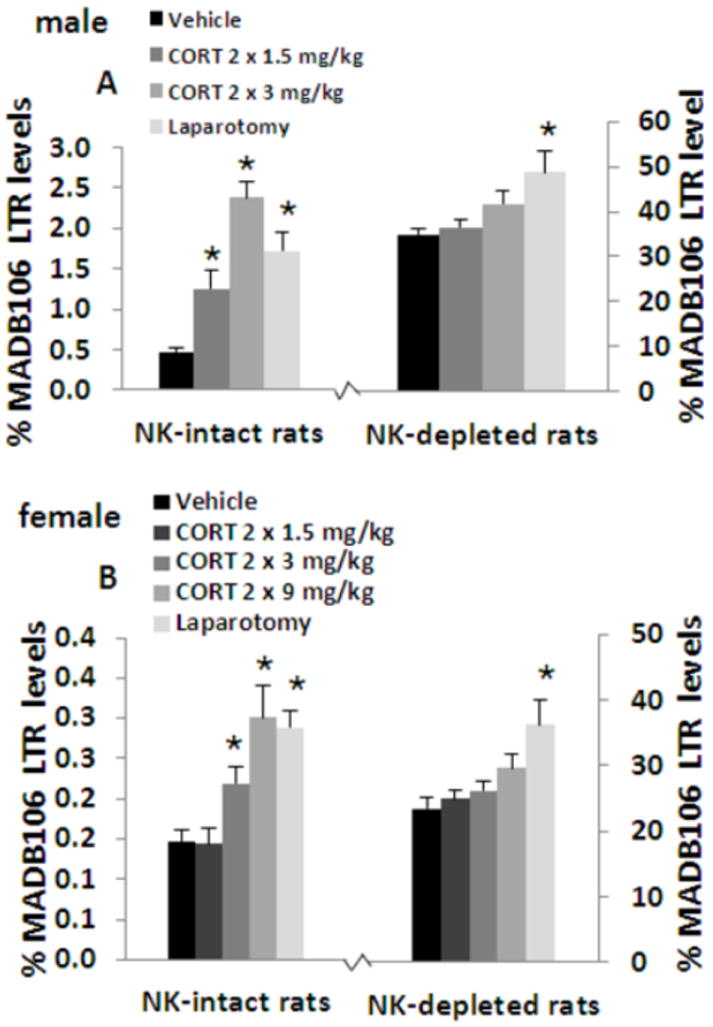
NK-depletion increased MADB106 LTR by more than 70 folds in males (n=7-8/group) (A) and females (n=9-10/group) (B). In NK-intact rats, LTR was increased by administration of 1.5 or 3 mg/kg CORT in males, and by 9 mg/kg in females. In NK-depleted male and female rats CORT did not affect MADB106 LTR in any of the doses used, suggesting that the effects of CORT on LTR are primarily mediated through NK cells. Laparotomy, on the other hand, increased LTR to a similar degree in both NK-intact and NK-depleted male and female rats, validating the ability of this depletion approach to distinguish between NK-dependent and NK-independent effects. Experiments 4.a., and 4.b. were each performed once, on different independent occasions. Data are presented as means + SEM. * indicates a significant difference from the respective control (Vehicle) group.
4.b. Procedure (female)
A similar experiment was conducted employing 99 female rats and an additional higher dose of CORT (9 mg/kg), as our experiments above show that females are less reactive to the effects of CORT administration.
Results
The same pattern of results as in males was evident (F(4,45)=9.3, p<0.01) (F(4,44)=5.1, p<0.01), with the exception that only the highest two doses of 3 and 9 mg/kg of CORT reached statistical significance in the NK-intact condition (Fisher’s PLSD, p<0.05 for each comparison) (Fig. 4B). Thus, in both males and females, NK cells are the prominent mediator of the effects of CORT on LTR levels, indicating in vivo suppression of NK function by CORT.
3.5 Exp. 5. The effects of CORT on LTR are mediated by glucocorticoid receptors (GRs), and not by mineralocorticoid or beta-adrenergic receptors
CORT can exert its effects through either mineralocorticoid or glucocorticoid receptors (GRs). Additionally, CORT and catecholamines were reported to modulate each other’s levels and activity [49, 50]. Therefore, in the following studies we addressed the specific role of GRs and the specific role of beta-adrenergic receptors in mediating or modulating the effects of CORT on MADB106 LTR.
5.a. Procedure
35 male rats were used in a 2×2 design, and were subjected to receive 2 vehicle (oil) or 2 CORT injections (s.c. 3 mg/kg, 3 hrs apart). Both groups were subdivided to receive a single vehicle (oil) or RU486 (s.c. 25 mg/kg) (a GR, but not MR, antagonist) injection 15 minutes before the first CORT injection. MADB106 tumor cells were administered simultaneously with the second injection, and rats were sacrificed 21 hrs later to assess LTR.
Results
A 2×2 ANOVA indicated significant main effects for CORT and for RU486 on LTR levels (F(1,31)=40.194, p<0.0001, F(1,31)=17.229, p=0.0002 respectively), and a significant interaction (F(1,31)=17.25, p=0.0002), indicating a significant blockade of the effects of CORT by RU486 (Fig. 5A). Thus, the GR seems to be a prominent mediator of the effects of CORT on MADB106 LTR. RU486 also blocks the progesterone receptor (PR). However, a role for PRs is unlikely in this experimental setting, as CORT does not bind to the PR, and as RU486 did not have an effect in vehicle treated animals, ruling out a modulatory impact of endogenous progesterone.
Figure 5. The effects of CORT on LTR are mediated by glucocorticoid receptors (GR), but not by β-adrenergic receptors.
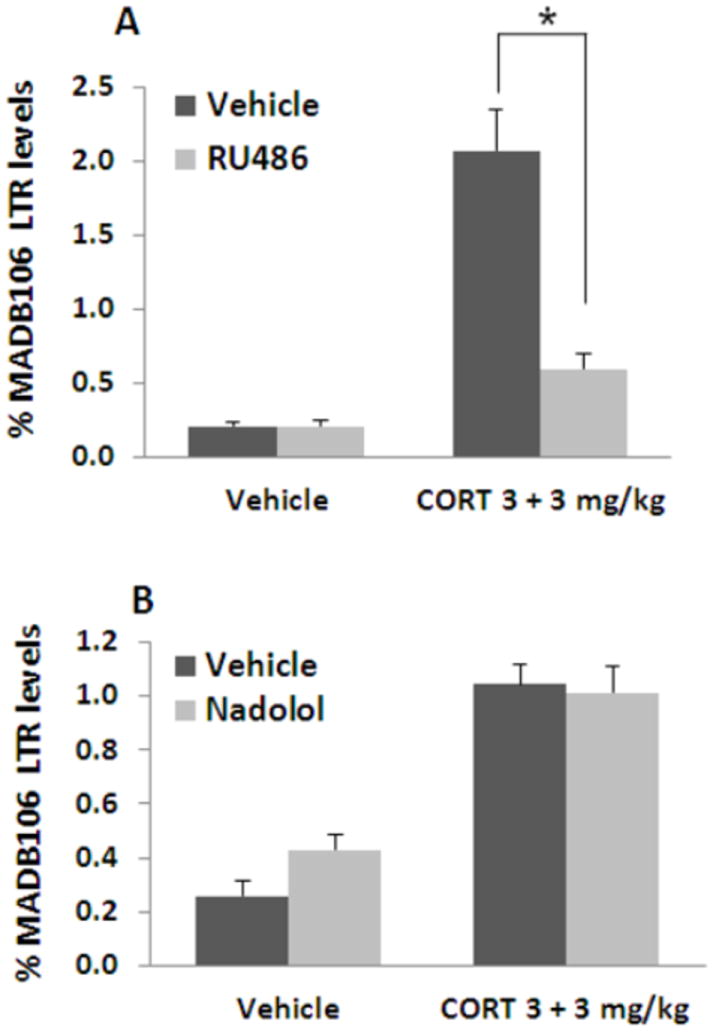
CORT administration increased MADB106 LTR. Administration of the GR blocker RU-486 abolished ~80% of the effects of CORT (indicated by *) (n=8-9/group) (A), suggesting that CORT exerts its effects through the glucocorticoid, rather than the mineralocorticoid receptor. Administration of the beta-adrenergic blocker nadolol did not counteract the increase in LTR caused by CORT (n=8-9/group) (B), suggesting that the effects of CORT are not mediated by a subsequent release of catecholamines. Experiments 5.a., and 5.b. were each performed once, on different independent occasions. Data are presented as means + SEM.
5.b. Procedure
34 male rats were used in the same 2×2 design, replacing RU486 with the beta-adrenergic antagonist nadolol.
Results
A 2×2 ANOVA indicated a significant main effect for CORT (F(1,30)=36.587, p<0.0001), without an effect for nadolol or an interaction (Fig. 5B), suggesting that beta-adrenergic receptors are not involved in the effects of CORT on MADB106 LTR.
3.6 Exp. 6. Endogenous adrenal CORT significantly potentiates the effects of administered epinephrine on MADB106 LTR, but not vise-versa
In the intact animals, stress commonly increases the release of both CORT and catecholamines, and these two hormones often modulate the activity of each other. Here we aimed at disentangling the effect of each hormone from their composed interactive impact by employing ADX animals.
Procedure
Seventy-seven male rats were subjected either to ADX or to a sham operation, and allowed 3-4 weeks for recovery (See Methods for conducting ADX, and for maintenance of ADX rats along the study). Sham and ADX rats were then subjected to the administration of either CORT (s.c. 3 mg/kg), epinephrine (s.c. 0.5 mg/kg in a slow-release preparation), both drugs, or vehicle. All rats received two injections of their designated drug/vehicle treatment, first 3 hrs before, and then simultaneously with radio-labeled MADB106 administration. Twenty-one hrs after MADB106 administration rats were sacrificed to assess LTR.
Results
Epinephrine and CORT each significantly elevated LTR in ADX and in sham-operated animals, but to different degrees, as indicated by ANOVA yielding a significant interaction between the effects of drugs and ADX (F(3,69)=3.7, p<0.05), and by specific pair-wise PLSD contrasts. Specifically, epinephrine had a significantly and markedly greater effect in sham-operated animals than in ADX animals, while CORT had a similar effect in both groups. When both hormones were applied, ADX and sham animals reached very similar levels of LTR, consistent with similarly high levels of both hormones in ADX and in sham animals (Fig. 6). These findings indicate that the presence of adrenal glands potentiates the effects of epinephrine, but not of CORT.
Figure 6. Endogenous adrenal CORT significantly potentiates the effects of administered epinephrine on MADB106 LTR, but not vise-versa.
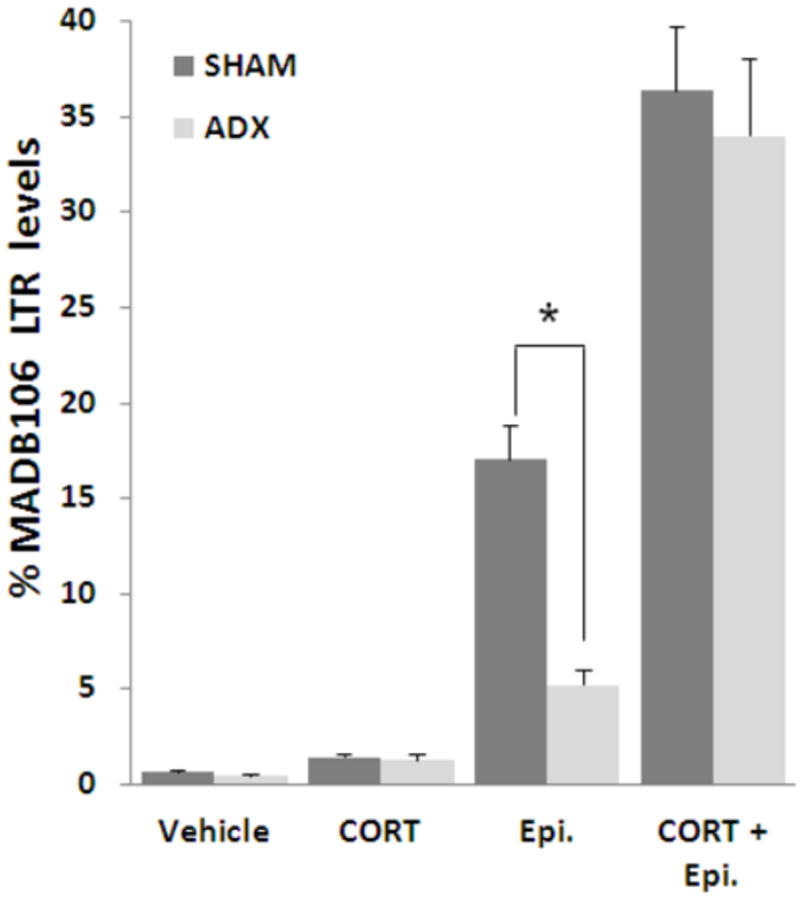
Both CORT and epinephrine (Epi.) administrations increased MADB106 LTR in both ADX and sham-operated (SHAM) rats. However, epinephrine administration resulted in a markedly greater impact in sham-operated rats than in ADX animals (indicated by *), suggesting that endogenous CORT potentiates the effects of administered epinephrine on MADB106 LTR. On the other hand, endogenous epinephrine does not seem to similarly potentiate the effects of administered CORT, as the injection of CORT resulted in the same effect in both ADX and SHAM animals. ADX did not affect LTR levels when both hormones were administered (compared to SHAM), suggesting that adrenal factors other than epinephrine and CORT do not interact with their effects on MADB106 LTR levels. This experiment was performed once, n=9-10/group. Data are presented as means + SEM.
3.7 Exp. 7. NK cells mediate the effects of one-hr and of 5-hr exposure to behavioral stress on LTR
To understand the role of endogenous CORT and catecholamines secreted in response to stressful conditions, in the following four experiments we employed two non-invasive stress paradigms – 1 or 5 hr exposure to a wet-cage – and an additional stress paradigm –laparotomy. To understand the in vivo role of NK cells in mediating the impact of these three stress paradigms on MADB106 LTR, we first conducted the following study utilizing selective functional depletion of NK cells.
Procedure
Fifty-five male rats were used. One group of rats was subjected to the wet cage paradigm for 5 hrs, and injected with tumor cells during a 30 min brake, 3 hrs following stress commencement. A second group was subjected to laparotomy (a 1 hr long procedure) 2 hrs before tumor administration, and a third group served as home cage control. A fourth group was subjected to the wet cage paradigm for one hr immediately after tumor administration. Rats from all four groups were randomly assigned to undergo NK depletion simultaneously with tumor administration, or to serve as controls (see Methods).
Results
In vehicle-treated animals, NK depletion elevated LTR levels more than 70-fold (p<0.001), reaffirming the sensitivity of the LTR index to NK-dependent activity. Given the marked differences in baseline levels between NK-intact and NK-depleted animals (0.4% vs. 30%), and the consequent marked differences in variance (violating the ANOVA assumption of homogeneity of variance), ANOVA was conducted separately in each condition. In NK-intact rats, ANOVA indicated significant group differences (F(3,25)=14.8, p<0.01), and both stress paradigms and surgery, each significantly elevated LTR levels compared to the control (vehicle) group (Fisher’s PLSD, p<0.05 for each comparison). On the other hand, in the NK depletion condition, ANOVA indicated significant group differences (F(3,22)=38.9, p<0.01), but neither stress paradigm had an effect. In contrast, surgery significantly elevated LTR levels in NK-depleted animals (Fisher’s PLSD, p<0.01) (See Fig. 7). These findings indicate that the effects of each of the stress paradigms are primarily mediated through NK cells, while the effects of surgery involve at least some non-NK dependent mechanisms.
Figure 7. NK cells mediate the effects of 1-hr and of 5-hr exposure to behavioral stress on LTR.
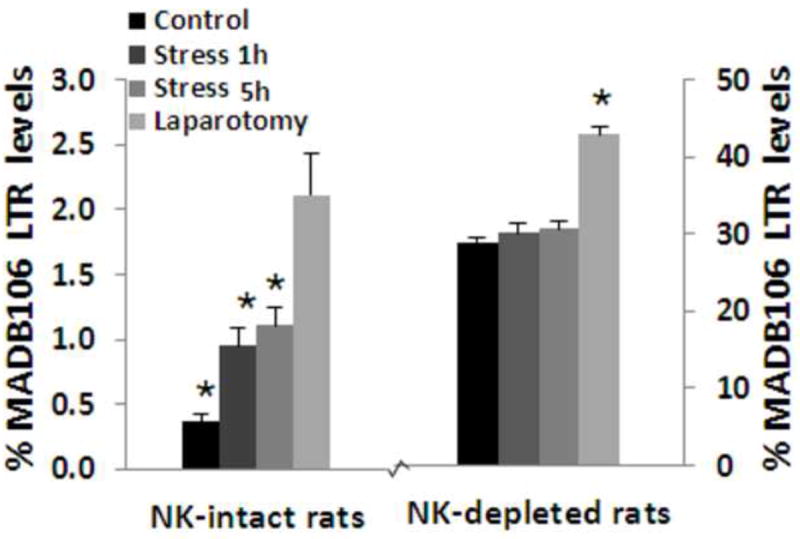
NK-depletion increased MADB106 LTR by more than 80 folds in male rats. In NK-intact rats, MADB106 LTR was increased by exposure to 1 or 5 hrs of the wet cage stress paradigm, while in NK-depleted rats stress exposure did not have an effect on LTR, suggesting that the effect of stress is primarily mediated through NK cells. Laparotomy, whose effects on MADB106 LTR are mediated through both NK-dependent and NK-independent effects, increased LTR in both NK-intact and NK-depleted rats. This experiment was performed once, n=6-7/group. * indicates a significant difference from the respective Control group. Data are presented as means + SEM.
3.8 Exp. 8. Exposure to one hr of behavioral stress, 3 hrs before tumor administration or immediately after it: Beta-adrenergic receptors, but not glucocorticoid receptors, mediate the increase in LTR levels
8.a. – 1-hr stress, 3 hrs before tumor administration
Procedure
Forty-six male rats were injected with vehicle, RU486 (25 mg/kg, s.c.), nadolol (0.4 mg/kg, s.c.), or both drugs, and an hr later were subdivided to undergo 1 hr of the wet cage stress paradigm or to remain in their home cages (control). Two hrs after the end of the stress session, all rats were administered with radio-labeled MADB106 tumor cells, and 21 hrs later rats were sacrificed to assess LTR. This study was conducted only in males.
Results
A 2×4 ANOVA indicated significant main effects for stress and for drug treatment (F(1,38)=21.3, p<0.05, F(3,38)=6.1, p<0.05, respectively), and a significant interaction (F(3,38)=3.1, p<0.05), indicating a significant blockade of the effects of stress by drug treatments. Fisher’s PLSD indicated that nadolol significantly blocked the effect of stress (~95% blockade) compared to both the vehicle and RU486 treatment (p<0.05 for both comparisons). RU486 had no effect (Fig. 8A). Thus, beta-adrenergic, rather than GRs are the prominent mediators of the effects of stress on MADB106 LTR in this stress paradigm in males.
Figure 8. Exposure to 1 hr of behavioral stress, 3 hrs before tumor administration or immediately after it, increases LTR levels through beta-adrenergic receptors, but not glucocorticoid receptors.
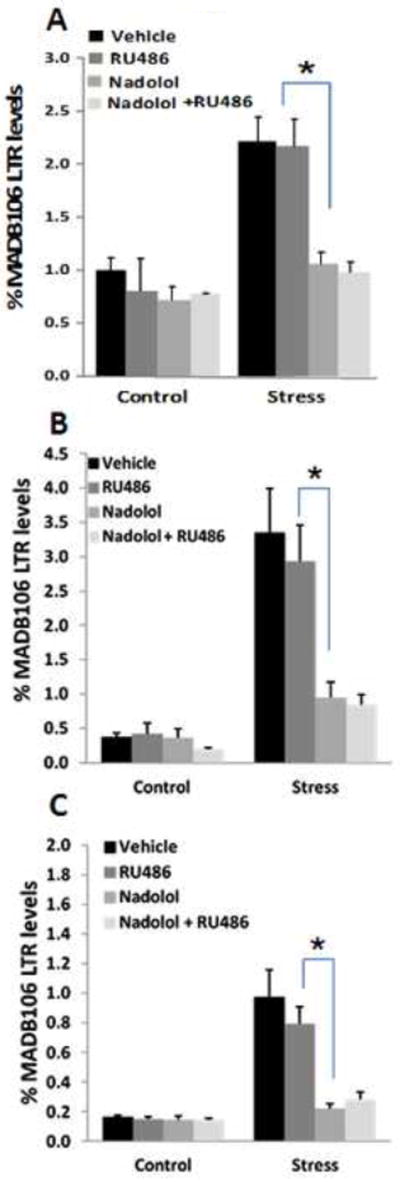
Exposure to 1 hr of the wet-cage stress paradigm increased MADB106 LTR, whether initiated 3 hrs before tumor injection (n=5-6/group) (A), or immediately after tumor injection in both male (n=8-9/group) (B) and female (n=8-9/group) (c) rats. Administration of the GR blocker RU-486 did not counteract the effects of this short stress exposure in both sexes. On the other hand, administration of the beta-adrenergic blocker nadolol abolished ~80% of the effect of stress, significantly reducing LTR compared to Vehicle or RU-486 treated rats (indicated by *). Adding RU-486 treatment to nadolol had no effect. Neither each blocker, nor their combination, affected LTR levels in non-stressed animals. Experiments 8.a., 8.b., and 8.c. were each performed once, on different independent occasions. Data are presented as means + SEM.
8.b. – 1-hr stress, immediately after tumor administration in males
Procedure
Sixty-nine male rats were administered with radio-labeled MADB106 tumor cells, and were subjected to one hr of the wet cage stress paradigm immediately after tumor administration, or remained in their home cages (control). Both groups were subdivided to receive an injection of vehicle (control), RU486 (25 mg/kg, s.c.), nadolol (0.4 mg/kg, s.c.), or both drugs, 2 hrs before stress initiation. Rats were sacrificed 21 hrs later to assess LTR.
Results
A 2×4 ANOVA indicated significant main effects for stress and for drug treatment (F(1,61)=39.6, p<0.01, F(3,61)=6.8, p=0.01, respectively), and a significant interaction (F(3,61)=5.7, p=0.01), indicating a significant blockade of the effects of stress by drug treatments. Fisher’s PLSD indicated that nadolol significantly blocked the effect of stress (~80% blockade) compared to both the vehicle and RU486 treatment (p<0.05 for both comparisons). RU486 had no significant effect (Fig. 8B). Thus, beta-adrenergic, rather than GRs are the prominent mediators of the effects of stress on MADB106 LTR in this stress paradigm in males.
8.c. – 1-hr stress, immediately after tumor administration in females
Procedure
Sixty-seven females were used in the exact 2×4 design described in 8.b.
Results
The same pattern of results as in 8.b. was observed (F(1,59)=34.2, p<0.01, F(3,59)=7.6, p=0.01 for main effects, F(3.59)=6.9, p=0.01 for the interaction, and p<0.05 for both Fisher’s contrasts) (Fig. 8C). Thus, beta-adrenergic, rather than GRs are the prominent mediators of the effects of stress on MADB106 LTR in this stress paradigm in females.
3.9 Exp. 9. Exposure to prolonged behavioral stress: CORT plays a minor role relative to catecholamines in mediating the impact on LTR
Procedure
Forty-nine males and 48 females were either subjected to a 5-hr wet cage stress paradigm (see Methods) or remained in their home cages. Fifteen minutes before the commencement of stress, control and stress rats were subdivided to be injected with RU486 (s.c. 25 mg/kg), nadolol (s.c. 0.2 mg/kg), both drugs, or vehicle. Three hrs following stress commencement (during a 30 min break in the wet cage exposure period), all rats were shortly anesthetized with isoflurane for blood withdrawal through the tail vein (300 μl) and injected with radio-labeled MADB106 tumor cells, and the nadolol-treated groups received an additional dose of nadolol (s.c. 0.2 mg/kg). Stressed rats were then returned to the wet cage for additional 2 hrs, and control rats were returned to their home cages. LTR was assessed 21 hrs after tumor administration.
Results
ANOVA indicated significant main effects for stress exposure (F(1,81)=27.945, p<0.0001), for sex (F(1,81)=4.149), p=0.0449), for drug treatment (F(3,81)=5.230, p=0.0024), and a significant interaction between stress and drugs (F(3,81)=6.371, p=0.0006).
Fisher’s PLSD indicated a significant elevation of LTR levels under stress in both males (Fisher’s PLSD, p<0.0001) and females (Fisher’s PLSD, p=0.002). Both RU486 and nadolol each significantly reduced the impact of stress on LTR in both sexes (p<0.05). In males, nadolol was significantly more effective than RU486 (indicated by #), and achieved a similar LTR reduction as did both blockers together (Fig. 9A).
Figure 9. CORT plays a minor role relative to catecholamines in mediating the impact of prolonged exposure to behavioral stress.
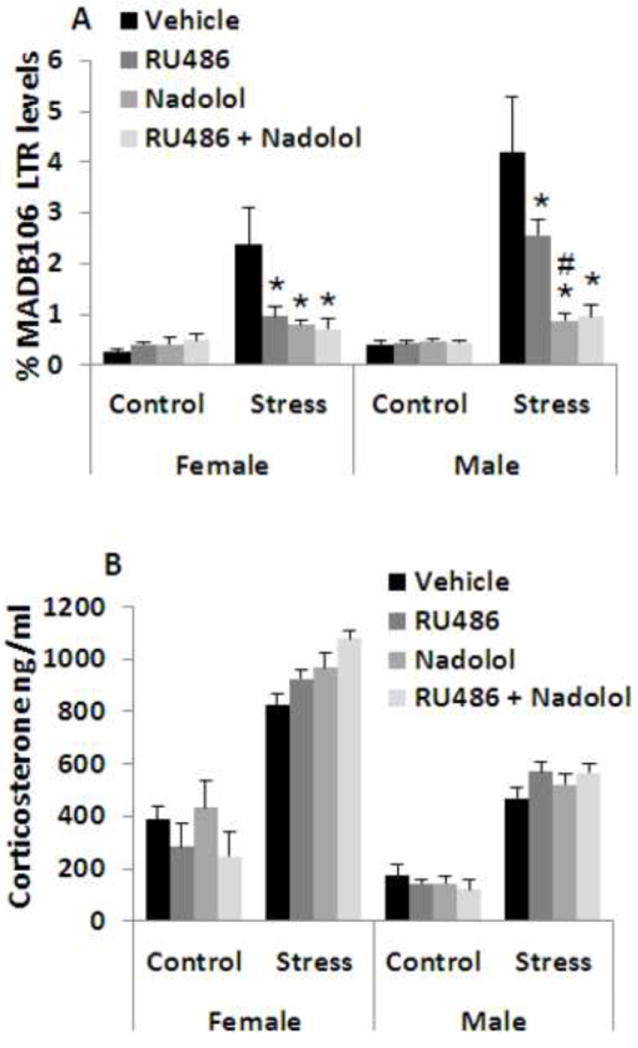
Exposure to a prolonged 5 hr wet-cage stress paradigm increased MADB106 LTR (A) in both males and females. Administration of the beta-adrenergic blocker nadolol abolished ~80% of this effect in both sexes (indicated by *). Administration of the GR blocker RU-486 reduced the effects of this prolonged stress exposure in both sexes (indicated by *), but in males to a significantly lesser extent than did nadolol (indicated by #). In both sexes, the addition of RU-486 to nadolol administration did not further reduce LTR. Neither each blocker nor their combination affected LTR levels in non-stressed animals. CORT plasma levels were increased by 2-3 folds by wet cage exposure in both sexes (assessed at 3 hrs from the initiation of stress), however, neither blocker, nor their combination affected CORT levels in either stressed or naïve rats (B). This experiment was performed once, n=6- 7/group. Data are presented as means + SEM.
CORT levels were significantly increased by stress in both sexes, and were higher in females, which also showed a greater increase than males. ANOVA indicated a significant main effect for stress (F(1,97)=358.572, p<0.0001), for sex (F(1,97)=133.672, p<0.0001), and a significant interaction between stress and sex (F(1,97)=18.041, p<0.0001). Fisher’s PLSD pair-wise comparisons indicated that none of the drug treatments (in either sex) significantly reduced CORT levels under stress conditions (Fig. 9B).
3.10 Exp. 10. The blockade of catecholamines and prostaglandins is at least as efficient as the blockade of CORT in preventing the deleterious effects of laparotomy
As indicated by the above studies, the effects of laparotomy on LTR are mediated, at least partly, through non-NK-dependent mechanisms. Nevertheless, our previous studies, employing the exact same laparotomy procedure, clearly suggested a significant role for NK cells in NK-intact animals in determining LTR levels [10, 40] (i.e. in addition to the NK-independent effects). Given the clinical significance of the perioperative period in cancer treatments and in preventing cancer metastasis, we here also studied the role of CORT, catecholamines, and prostaglandins in mediating surgery-induced alterations in LTR; alterations that are most likely both NK-independent and NK-dependent [40]. It is also worthy to note that laparotomy was shown to increase CORT levels for a prolonged period of time [17], which better allows CORT to exert its impact (based on the above experiments).
Procedure
A 4×2 experimental design was used, in which 98 male rats were subjected to receive nadolol (a total of 0.4 mg/kg, s.c.) and etodolac (12.5 mg/kg, s.c.), RU486 (25 mg/kg s.c.), all three blockers, or vehicle. Thirty minutes later, each of the 4 groups were subdivided to undergo laparotomy or to remain in their home cages. Half the dose of nadolol (0.2 mg/kg) was injected 30 minutes before surgery, simultaneously with the other drugs, and the other half was injected simultaneously with tumor administration (following our previous protocol with this blockade paradigm). Two hrs after initiation of laparotomy, all rats were shortly anesthetized with isoflurane for blood withdrawal through the tail vein (300 μl) and injected with radio labeled MADB106 tumor cells. Twelve hrs later LTR was assessed.
Results
ANOVA indicated a significant main effect for surgery and for drugs on LTR (F(1,90)=45.959, p<0.0001, F(3,90)=4.711, p=0.0042), and a significant interaction between surgery and drugs (F(3,90)=5.791, p=0.0011). Fisher’s PLSD indicated that surgery without the blockers significantly elevated LTR levels (p<0.0001). Each of the three drug treatments significantly reduced the effects of surgery (p<0.05 for each). Although nadolol and etodolac reduced LTR to a lower level than did RU486, this difference was not significant, nor did the addition of RU486 improve the effects of nadolol and etodolac (Fig 10A).
Figure 10. The blockade of catecholamines and prostaglandins is at least as efficient as the blockade of CORT in preventing the deleterious effects of laparotomy.
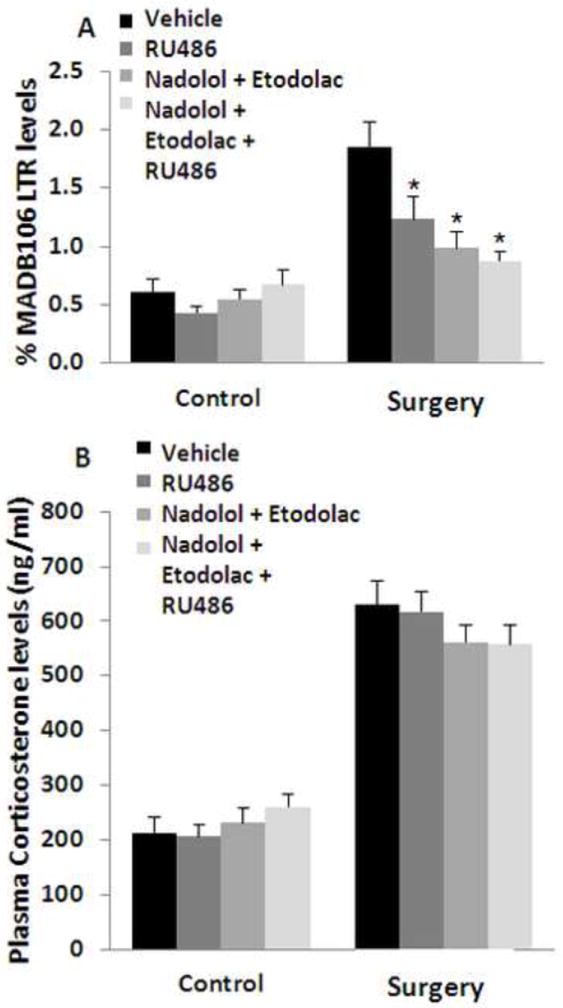
Laparotomy increased MADB106 LTR (A) and tripled plasma CORT levels (B). Blocking GRs by RU-486, or combining a beta-adrenergic blockade by nadolol and prostaglandin synthesis by etodolac similarly counteracted the laparotomy-induced increase in LTR (indicated by *). Combining the three drugs did not show an additional advantage (A). Laparotomy increased plasma CORT levels, and none of the drugs or their combinations affected CORT levels in either the Laparotomy or Control conditions. This experiment was performed once, n=12-13/group. Data are presented as means + SEM.
Plasma CORT levels were significantly increased by laparotomy, as indicated by a significant main ANOVA effect for laparotomy (F(1,90)=359, p<0.01), and no effects for drug or drug by laparotomy interaction (Fig. 10B).
4. DISCUSSION
Here we sought to elucidate the significance of stress-induced elevated CORT levels in suppressing NKCC in vivo, comparing it directly within the same stress paradigms to the impact of other stress factors. The effects of physiologically relevant doses of CORT administration (e.g., 2 × 3 mg/kg) and the effects of the two stress paradigms (1 or 5 hrs) on LTR were mediated through alterations in NKCC, as they were completely absent in NK-depleted animals. On the other hand, the effects of laparotomy (which were often smaller than the effects of CORT or stress exposure in NK-intact animals) were evident and significant in both NK-intact and NK-depleted animals. These findings indicate a mediating role for factors other than NK cells (or in addition to NK cells) in the effects of laparotomy, and refuting potential methodological confounds in NK-depleted animals (such as ceiling effects).
Thus, and as will be elaborated below, our findings clearly indicate that elevated CORT levels, either induced by stress or by the administration of CORT, can be involved in in vivo suppression of NKCC. However, the findings also indicate that this suppression (i) occurs only under prolonged, but not short exposure to stress/CORT, and mainly in males, (ii) is smaller than the impact of other neuroendocrine or paracrine responses – specifically, those of catecholamines or prostaglandins, (iii) is mostly ascribed to synergism of CORT with other stress responses, and (iv) can be completely abolished through blocking prostaglandins and/or catecholamines.
Specifically, the evidence supporting in vivo NK-suppressive effects of elevated CORT levels, when such suppression does occur, is derived from the combination of the findings that (i) prolonged stress or repeated CORT administrations increased LTR by 2-5 folds, and that (ii) these effects were attenuated by blocking GRs in NK-intact animals, and completely absent in NK-depleted animals. Thus, under these specific conditions, elevated CORT levels were involved in in vivo suppression of NKCC.
However, these effects occur only following prolonged exposure to high CORT levels, and are markedly less significant than the effects of catecholamines under physiological stress conditions. Specifically, dividing a single high dose of CORT to two administrations, given 3 hr apart, markedly increased its impact. Additionally, when a short 1 hr stress paradigm was used (either 3 hrs before tumor injection or immediately after tumor injection), which markedly increases CORT levels for ~2-3 hr [51], the blockade of GRs by RU-486 had no impact whatsoever, while an adrenergic blockade reduced 80-95% of the impact of these stress paradigms in both sexes. When a 5 hr stress paradigm was used (starting 3 hrs before tumor injection), the same GR blockade reduced 50% of the effect of stress – but still significantly less than the blockade of catecholamines, which reduced 75% of the effect of stress. It is noteworthy that (i) the blockade of catecholamines exerted its effect without reducing CORT levels, and that (ii) verifying the high effectiveness of the GR blockade used herein (through RU486), is its ability to reduce 80% of the impact of two CORT injections, which induced a much higher plasma CORT level than the stress paradigms.
It also seems that females are more resistant than males to the in vivo effects of high physiological levels of CORT on NKCC. In males, two injections of 1.5 mg/kg (3 hrs apart) were the minimal dose needed to impact LTR, while in females 4-fold higher doses (2 × 6 mg/kg) were needed. Similarly, following two injections of 3mg/kg of CORT, only males exhibited increase in the number of metastases developed three weeks later. Congruent with these differences, following the 5 hr stress paradigms where CORT was involved, females showed half the increase in LTR evident in males, although mounting a markedly higher endogenous CORT response. The mechanisms for the aforementioned lower impact of CORT in females may be related to (i) known higher levels of corticosteroid binding globulins in females [52], which reduce the effective levels of free CORT, (ii) shorter duration of hypothalamic-pituitary-adrenal axis responses to stress in females [52], (iii) sex-specific effects of stressors, which exacerbate the impact of stress in grouped-house males [53], and (iv) faster rate of CORT clearance in females, as is first reported herein following CORT administration (Fig. 1). Overall, despite their higher stress-induced CORT levels, female F344 rats seem to be significantly more resilient to the effects of elevated CORT levels on in vivo levels of NKCC. Following the one hr stress paradigms, where catecholamines, but not CORT, mediated the effects of stress on NKCC, females again showed half the increase in LTR compared to males. These findings suggest that female F344 rats are more resilient than males to stress-induced NK suppression in general, and specifically to effects mediated through activation of the SNS. These sexual dimorphisms are consistent with our previous studies employing other stress paradigms [48], and specifically with respect to the impact of β-adrenergic receptor activation on NKCC [54, 55].
The molecular mechanisms through which catecholamines and prostaglandins suppress NKCC have long been established. Both compounds have been repeatedly shown in vitro to suppress NKCC and most other aspects of cell mediated immunity (including CTL, macrophage, and dendritic cells) [23, 56-58]. These effects are mediated through activation of leukocyte membrane receptors for these ligands, and intracellular initiation of the cAMP-PKA cascade common to both receptor systems [59, 60]. Potentially underlying the aforementioned sex differences in the impact of catecholamines on NKCC are the (i) menstrual cycle and female gonadal hormones that were shown to affect human and rat NK cell susceptibility to adrenergic stimulation [24, 61], presumably through modulating expression levels of adrenergic receptors on leukocytes [62], and (ii) testosterone that was shown to increase rat susceptibility to adrenergic suppression of in vivo levels of NKCC [55].
Another important finding of this study is a marked synergism between the effects of CORT and epinephrine, where CORT potentiated the effects of epinephrine, but not vise versa. Specifically, in ADX rats (that cannot mount a systemic CORT or epinephrine response), the combined effect of epinephrine and CORT administration was an 83-fold increase in LTR, which is much greater than the multiplication of the separate effects of each drug: 3-fold for CORT, and 12-fold for epinephrine. This synergism was completely absent in sham-operated animals, in which administration of each hormone separately may have naturally induced the release of the other hormone or interacted with its baseline levels [49, 50], and where the combined administration reached the same final LTR levels as in ADX animals. Indicating a role for CORT in potentiating the effects of epinephrine, but not vise versa, are the findings that CORT administration increased LTR to similar levels in sham (to 1.4% LTR) and in ADX animals (to 1.2% LTR), while the impact of epinephrine was more than 3 fold greater in sham-operated animals (to 17% LTR) compared to ADX animals (to 5% LTR) (Fig. 6). Last, in stressed animals the blockade of beta-adrenergic receptors rendered the additional blockade of GR ineffective, but not vise versa (Fig. 8 & 9). Overall, these findings strongly suggest that under endogenous stress responses catecholamines act both independently of CORT and synergistically with it, while CORT has a minor impact alone and acts mainly through potentiating the effects of catecholamines.
Several factors may explain the profound differences between the reported robust in vitro effects of CORT on NKCC at presumably physiological concentrations, leading to its nearly complete abolishment [15], and the much smaller in vivo effects under stressful conditions evident herein. First, corticosteroid binding globulin is present only in the in vivo milieu and binds up to 90% of CORT molecules [63], reducing the effective concentrations of CORT. Second, in the in vivo milieu, but not in vitro, leukocytes continuously redistribute, enabling re-population of critical immune compartments by CORT-insensitive or unaffected cells. Third, a host of in vivo compounds modulate NKCC and NK response to stress hormones, including IL-12, IFN-gamma, and IL-2 [1, 51], the latter two were also shown to induce a state of NK cell resistance to suppression by glucocorticoids, catecholamines, or surgery [23, 64, 65]. Thus, these characteristics of the in vivo milieu may dramatically mitigate the effects of stress-induced elevated CORT levels, rendering it relatively insignificant as to impacting in vivo levels of NKCC.
An important clinical setting, where stress and surgery may have significant impact through modulating immunity, is a removal of a primary tumor. Animal and clinical studies have implicated anti-metastatic NKCC in controlling minimal residual disease, and pointed at postoperative endocrine responses and immune suppression as independent risk factors for cancer recurrence [1, 10, 20, 66]. Importantly, we were able to significantly attenuate or abolish postoperative NK suppression and double recurrence-free long-term survival rate by perioperatively employing a prostaglandin synthesis inhibitor and a beta-adrenergic blocker [10, 11, 35], as was also shown herein with respect to the MADB106 LTR index, which involves both NK-dependent and NK-independent mechanisms [40]. Given that in the clinical context of surgery, direct blockade of CORT would have several contraindications, it is important to note that at least with respect to the in vivo levels of NKCC, the combined blockade of prostaglandins and catecholamines, which is clinically feasible [67], was at least as effective as the blockade of CORT.
Overall, although surgery-induced or stress-induced elevated CORT levels may contribute to in vivo suppression of NKCC levels, this effect of CORT becomes significant only at prolonged stress responses, mostly in males, is mostly mediated through potentiating the NK-suppressive effects catecholamines or other factors, and can be blocked as effectively or more effectively by antagonizing catecholamines and/or prostaglandins rather than by antagonizing GRs. Thus, in the clinical context this suppression may either be insignificant relative to the effects of catecholamines and prostaglandins, or may be antagonized through their receptor blockade, which was suggested to have several additional advantages in the context of oncological surgeries [67, 68].
Acknowledgments
Grant support: This work was supported by NIH/NCI grant # CA125456 (SBE), and by the Israel-USA bi-national Science Foundation # 2005331 (SBE & GGP)
Abbreviations used in this article
- NKCC
NK cell cytotoxicity
- CORT
corticosterone
- LTR
lung tumor retention
- ADX
adrenalectomy/adrenalectomized
- PLSD
protected least significant differences
- GRs
glucocorticoid receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarr AJ, et al. beta-Adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain Behav Immun. 2012;26(8):1226–38. doi: 10.1016/j.bbi.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyburn H, et al. Human NK cells: their ligands, receptors and functions. Immunol Rev. 1997;155:119–25. doi: 10.1111/j.1600-065x.1997.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen BL, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90(1):30–6. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Eliyahu S, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80(6):880–8. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Levi B, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain, Behavior, and Immunity. 2011;25(4):727–35. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga C, et al. Anxiety and pain suppress the natural killer cell activity in oral surgery outpatients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):654–8. doi: 10.1067/moe.2001.115465. [DOI] [PubMed] [Google Scholar]
- 8.Witek-Janusek L, et al. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22(6):969–81. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraki S, et al. Interleukin-18 restores immune suppression in patients with nonseptic surgery, but not with sepsis. Am J Surg. 2007;193(6):676–80. doi: 10.1016/j.amjsurg.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Benish M, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–52. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasner A, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 12.Vallejo R, et al. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol. 2003;22(2):139–46. doi: 10.1615/jenvpathtoxoncol.v22.i2.70. [DOI] [PubMed] [Google Scholar]
- 13.Cox WI, Holbrook NJ, Friedman H. Mechanism of glucocorticoid action on murine natural killer cell activity. Journal of the National Cancer Institute. 1983;71(5):973–81. [PubMed] [Google Scholar]
- 14.Van Ierssel AJ, et al. Suppression of intestinal mucosal natural killer cells by corticosteroids. Aliment Pharmacol Ther. 1997;11(2):347–53. doi: 10.1046/j.1365-2036.1997.138314000.x. [DOI] [PubMed] [Google Scholar]
- 15.Shakhar G, et al. Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: a potential approach to reduce postoperative metastasis. Ann Surg Oncol. 2007;14(2):841–52. doi: 10.1245/s10434-006-9078-9. [DOI] [PubMed] [Google Scholar]
- 16.Tseng RJ, et al. Stress-induced modulation of NK activity during influenza viral infection: role of glucocorticoids and opioids. Brain Behav Immun. 2005;19(2):153–64. doi: 10.1016/j.bbi.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Shakhar G, Blumenfeld B. Glucocorticoid involvement in suppression of NK activity following surgery in rats. J Neuroimmunol. 2003;138(1-2):83–91. doi: 10.1016/s0165-5728(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 18.Pruett SB, et al. Quantitative relationships between the suppression of selected immunological parameters and the area under the corticosterone concentration vs. time curve in B6C3F1 mice subjected to exogenous corticosterone or to restraint stress. Toxicol Sci. 1999;49(2):272–80. doi: 10.1093/toxsci/49.2.272. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Eliyahu S, et al. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–64. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 20.Inbar S, et al. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6(4):e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irwin M, et al. Sympathetic nervous system mediates central corticotropin-releasing factor induced suppression of natural killer cytotoxicity. J Pharmacol Exp Ther. 1990;255(1):101–7. [PubMed] [Google Scholar]
- 22.Bodner G, Ho A, Kreek MJ. Effect of endogenous cortisol levels on natural killer cell activity in healthy humans. Brain Behav Immun. 1998;12(4):285–96. doi: 10.1006/brbi.1998.0533. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrand K, Hermodsson S. An immunopharmacological analysis of adrenaline-induced suppression of human natural killer cell cytotoxicity. International Archives of Allergy and Applied Immunology. 1989;89(4):334–41. doi: 10.1159/000234972. [DOI] [PubMed] [Google Scholar]
- 24.Shakhar K, et al. Timing within the menstrual cycle, sex, and the use of oral contraceptives determine adrenergic suppression of NK cell activity. Br J Cancer. 2000;83(12):1630–1636. doi: 10.1054/bjoc.2000.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bankhurst AD. The modulation of human natural killer cell activity by prostaglandins. J Clin Lab Immunol. 1982;7(2):85–91. [PubMed] [Google Scholar]
- 26.Yakar I, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. Journal of Immunology. 1998;160(7):3251–8. [PubMed] [Google Scholar]
- 28.Dhabhar FS, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24(1):127–37. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonnesen E, Tonnesen J, Christensen NJ. Augmentation of cytotoxicity by natural killer (NK) cells after adrenaline administration in man. Acta Pathologica, Microbiologica, et Immunologica Scandandinavica Section C. 1984;92(1):81–3. [PubMed] [Google Scholar]
- 30.Ben-Eliyahu S. Can we really know if a stressor increases or decreases natural killer cell activity? Brain Behav Immun. 2012;26(8):1224–5. doi: 10.1016/j.bbi.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Meron G, et al. PGE(2) suppresses NK activity in vivo directly and through adrenal hormones: Effects that cannot be reflected by ex vivo assessment of NK cytotoxicity. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(Suppl):S32–40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingu K, et al. Kinetics of the early recruitment of leukocyte subsets at the sites of tumor cells in the lungs: natural killer (NK) cells rapidly attract monocytes but not lymphocytes in the surveillance of micrometastasis. Int J Cancer. 2002;99(1):74–81. doi: 10.1002/ijc.10279. [DOI] [PubMed] [Google Scholar]
- 34.Melamed R, et al. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33(1):16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Barlozzari T, et al. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. Journal of Immunology. 1985;134(4):2783–9. [PubMed] [Google Scholar]
- 37.Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. Journal of Immunology. 1983;131(2):1024–7. [PubMed] [Google Scholar]
- 38.Ben-Eliyahu S, Page GG. In vivo assessment of natural killer cell activity in rats. Progress in Neuroendocrineimmunology. 1992;5:199–214. [Google Scholar]
- 39.Ben-Eliyahu S, et al. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996;2(4):457–60. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb Y, et al. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253(4):798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30(2):135–52. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 42.Page G, Ben-Eliyahu S, Taylor A. The development of sexual dimorphism in natural killer cell activity and resistance to tumor metastasis in the Fischer 344 rat. Journal of Neuroimmunology. 1995;63(1):69–77. doi: 10.1016/0165-5728(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 43.Seifarth JE, McGowan CL, Milne KJ. Sex and life expectancy. Gend Med. 2012;9(6):390–401. doi: 10.1016/j.genm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175(2):323–8. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Page GG, et al. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54(1):21–8. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 46.Chambers WH, et al. Functional heterogeneity between NKR-P1bright/Lycopersicon esculentum lectin (L.E.)bright and NKR-P1bright/L.E.dim subpopulations of rat natural killer cells. Journal of Immunology. 1992;148(11):3658–65. [PubMed] [Google Scholar]
- 47.Chambers WH, et al. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. Journal of Experimental Medicine. 1989;169(4):1373–89. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naor R, et al. Metastatic-promoting effects of LPS: Sexual dimorphism and mediation by catecholamines and prostaglandins. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gwosdow AR, et al. Interleukin-1-induced corticosterone release occurs by an adrenergic mechanism from rat adrenal gland. Am J Physiol. 1992;263(3 Pt 1):E461–6. doi: 10.1152/ajpendo.1992.263.3.E461. [DOI] [PubMed] [Google Scholar]
- 50.O’Connell NA, et al. Interleukin-1 regulates corticosterone secretion from the rat adrenal gland through a catecholaminedependent and prostaglandin E2-independent mechanism. Endocrinology. 1994;135(1):460–7. doi: 10.1210/endo.135.1.8013385. [DOI] [PubMed] [Google Scholar]
- 51.Shaashua L, et al. In vivo suppression of plasma IL-12 levels by acute and chronic stress paradigms: potential mediating mechanisms and sex differences. Brain Behav Immun. 2012;26(6):996–1005. doi: 10.1016/j.bbi.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58(6):1085–9. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 54.Goldfarb Y, et al. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother. 2009;32(3):280–91. doi: 10.1097/CJI.0b013e31819a2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page GG, et al. Male--female differences in the impact of betaadrenoceptor stimulation on resistance to experimental metastasis: exploring the effects of age and gonadal hormone involvement. J Neuroimmunol. 2008;193(1-2):113–9. doi: 10.1016/j.jneuroim.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koren HS, Leung KH. Modulation of human NK cells by interferon and prostaglandin E2. Mol Immunol. 1982;19(10):1341–6. doi: 10.1016/0161-5890(82)90302-9. [DOI] [PubMed] [Google Scholar]
- 57.Broug-Holub E, et al. Effects of stress on alveolar macrophages: a role for the sympathetic nervous system. Am J Respir Cell Mol Biol. 1998;19(5):842–8. doi: 10.1165/ajrcmb.19.5.3103. [DOI] [PubMed] [Google Scholar]
- 58.Seiffert K, Granstein RD. Neuroendocrine regulation of skin dendritic cells. Ann N Y Acad Sci. 2006;1088:195–206. doi: 10.1196/annals.1366.011. [DOI] [PubMed] [Google Scholar]
- 59.Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochemical Journal. 1990;272(2):327–31. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torgersen KM, et al. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272(9):5495–500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Eliyahu S, et al. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. British Journal of Cancer. 1996;74(12):1900–7. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landmann R. Beta-adrenergic receptors in human leukocyte subpopulations. European Journal of Clinical Investigation. 1992;1:30–6. [PubMed] [Google Scholar]
- 63.Lewis JG, Elder PA. Intact or “active” corticosteroid-binding globulin (CBG) and total CBG in plasma: Determination by parallel ELISAs using monoclonal antibodies. Clin Chim Acta. 2012 doi: 10.1016/j.cca.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 64.Holbrook NJ, Cox WI, Horner HC. Direct suppression of natural killer activity in human peripheral blood leukocyte cultures by glucocorticoids and its modulation by interferon. Cancer Reseach. 1983;43(9):4019–25. [PubMed] [Google Scholar]
- 65.Oosterling SJ, et al. Perioperative IFN-alpha to avoid surgically induced immune suppression in colorectal cancer patients. Histol Histopathol. 2006;21(7):753–60. doi: 10.14670/HH-21.753. [DOI] [PubMed] [Google Scholar]
- 66.Lee JW, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18(18):4895–902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201–6. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]


