Abstract
Disruption of the dynamic properties of mitochondria (fission, fusion, transport, degradation, and biogenesis) has been implicated in the pathogenesis of neurodegenerative disorders, including Parkinson’s disease (PD). Parkin, the product of gene PARK2 whose mutation causes familial PD, has been linked to mitochondrial quality control via its role in regulating mitochondrial dynamics, including mitochondrial degradation via mitophagy. Models using mitochondrial stressors in numerous cell types have elucidated a PINK1-dependent pathway whereby Parkin accumulates on damaged mitochondria and targets them for mitophagy. However, the role Parkin plays in regulating mitochondrial homeostasis specifically in neurons has been less clear. We examined whether a stressor linked to neurodegeneration, glutamate excitotoxicity, elicits Parkin-mitochondrial translocation and mitophagy in neurons. We found that brief, acute exposure to glutamate causes Parkin translocation to mitochondria in neurons, in a calcium- and N-methyl-D-aspartate (NMDA) receptor-dependent manner. In addition, we found that Parkin accumulates on endoplasmic reticulum (ER) and mitochondrial/ER junctions following excitotoxicity, supporting a role for Parkin in mitochondrial-ER crosstalk in mitochondrial homeostasis. Despite significant Parkin-mitochondria translocation, however, we did not observe mitophagy under these conditions. To further investigate, we examined the role of glutamate-induced oxidative stress in Parkin-mitochondria accumulation. Unexpectedly, we found that glutamate-induced accumulation of Parkin on mitochondria was promoted by the antioxidant N-acetyl cysteine (NAC), and that co-treatment with NAC facilitated Parkin-associated mitophagy. These results suggest the possibility that mitochondrial depolarization and oxidative damage may have distinct pathways associated with Parkin function in neurons, which may be critical in understanding the role of Parkin in neurodegeneration.
Keywords: Parkinson’s Disease, Parkin, Mitochondria, Mitophagy, Endoplasmic Reticulum, Glutamate, NMDA receptor, Excitotoxicity, N-acetyl cysteine, antioxidant
INTRODUCTION
Dysregulation of the homeostatic mechanisms of mitochondrial maintenance (mitochondrial fission, fusion, transport, biogenesis and degradation - collectively termed mitochondrial dynamics), has been increasingly linked to neurodegeneration and Parkinson’s disease (PD) neuropathology (Chen and Chan, 2009; Subramaniam and Chesselet, 2013; Van Laar and Berman, 2009). The E3 ubiquitin ligase Parkin, mutations of which cause familial autosomal-recessive juvenile-onset PD, is associated with maintenance of mitochondrial dynamics (de Vries and Przedborski, 2013; Deng et al., 2008; Liu et al., 2012; Narendra et al., 2008; Park et al., 2008; Poole et al., 2008; Scarffe et al., 2014; Wang et al., 2011; Yu et al., 2011). Parkin has been specifically linked to mitochondrial quality control, whereby damaged, depolarized mitochondria are targeted for autophagic degradation (mitophagy) (Narendra et al., 2008). In this pathway, Parkin translocates to depolarized mitochondria via a PINK1-dependent mechanism, identifying them for mitophagic degradation (Narendra et al., 2008; Narendra et al., 2010; Vives-Bauza et al., 2010). This pathway is proposed to be important in PD as regulation of mitochondrial homeostasis is crucial to neuronal survival, and a build-up of damaged mitochondria could be detrimental (de Vries and Przedborski, 2013; Scarffe et al., 2014). However, it is not clear why loss of Parkin function would lead to a selective loss of PD-affected neurons.
Whereas the Parkin-mediated mitophagy pathway has been well described in cancer cell lines and other proliferating cells, its regulation in neurons remains less well defined (Grenier et al., 2013; Van Laar and Berman, 2013). Previously, we showed that the unique bioenergetics of post-mitotic neurons, which are dependent on mitochondrial respiration, appear to downregulate Parkin translocation following global mitochondrial depolarization by the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Van Laar et al., 2011). Under conditions that preserved ATP (by altering neuronal bioenergetics), we did observe CCCP-triggered Parkin-mitochondrial translocation occurring in neurons. Even with these conditions, however, increased mitophagy was not observed (Van Laar et al., 2011). Other studies have since confirmed that Parkin less readily undergoes mitochondrial translocation in neurons, observing increased Parkin-mitochondria association occurring under specific conditions such as long exposures to protonophores/ionophores (Cai et al., 2012; McCoy et al., 2014; Rakovic et al., 2013; Seibler et al., 2011), exposure to selective complex inhibitors (Wang et al., 2011), or exposure to stressors after replacing culture media with media lacking supplements and antioxidants (Joselin et al., 2012). Likewise, after Parkin translocation to mitochondria in neurons, subsequent mitophagic degradation in neurons has more rarely been clearly demonstrated. It has been expressly observed following prolonged CCCP exposure in the presence of anti-apoptotic agents (Cai et al., 2012) and, most recently, following localized mitochondrial damage in distal axons (Ashrafi et al., 2014). It was also suggested to occur after overexpression of an Alzheimer’s-linked NH2-tau fragment (Amadoro et al., 2014). On the other hand, mitophagy did not measurably occur despite induction of Parkin translocation to mitochondria following valinomycin exposure in iPS-derived neurons (Rakovic et al., 2013). Further, the relationship of some previously utilized conditions to in vivo settings is not clear.
We sought to determine whether neurochemically- and PD-relevant triggers of mitochondrial depolarization could affect Parkin translocation to mitochondria and subsequent mitophagy in neurons. One such trigger, glutamate excitotoxicity, has been proposed to play a role in neurodegenerative disease, including PD neuropathology (Blandini, 2010; Mehta et al., 2013). Glutamate, through activation of N-methyl-D-aspartate (NMDA) receptor calcium ion channels, causes an influx of calcium, which is taken up by mitochondria and subsequently causes mitochondrial depolarization (Schinder et al., 1996; Stout et al., 1998; White and Reynolds, 1996). This may be especially important in PD neuropathology, as calcium dysregulation has been linked to the selective vulnerability of neurons in PD (Chan et al., 2010; Chan et al., 2007; Hurley et al., 2013; Mosharov et al., 2009). Related to this, glutamate excitotoxicity has been proposed to play a role in the neurodegeneration observed in some PD models (Kress and Reynolds, 2005; Loschmann et al., 1994; Meredith et al., 2009; Plowey et al., 2014; Wu and Johnson, 2007). In addition, the PINK1/Parkin pathway has been reported to influence cellular response to excitotoxicity in neurons (Yu et al., 2011).
We therefore evaluated the effect of glutamate exposure on Parkin localization as well as mitophagy. To mimic acute excitotoxicity, we used a previously described model of short exposure to glutamate in primary rat neuron cultures (Reynolds and Hastings, 1995; Stout et al., 1998). We report that, in contrast to depolarizing protonophores, glutamate exposure induced Parkin accumulation at mitochondria. Accumulation occurred in a calcium- and NMDA receptor-dependent manner. In addition, glutamate-exposed neurons exhibited non-mitochondrial Parkin accumulations on endoplasmic reticulum (ER), and accumulations between mitochondria and ER. These findings have implications for the shared role of calcium handling between mitochondria and ER. We also investigated the role of reactive oxygen species (ROS) in Parkin-associated mitophagy in neurons. Whereas glutamate alone induced accumulation of Parkin on mitochondria in neurons, it did not induce mitophagy. However, unexpectedly, co-treatment with the antioxidant N-acetyl cysteine (NAC) promoted both glutamate-induced Parkin translocation to mitochondria and Parkin-associated mitophagy. Our results suggest that following physiologically relevant mitochondrial depolarization, both bioenergetic and oxidative stress pathways may regulate Parkin translocation and mitophagy. Elucidating the distinct roles these pathways play may be critical in understanding the role of Parkin in PD-related neurodegeneration.
MATERIALS AND METHODS
Cortical neuron culture, transfection, and treatment
Primary cortical neuron cultures were prepared from E17-18 Sprague-Dawley rats as previously described (Arnold et al., 2011; Van Laar et al., 2011; modified from Ghosh and Greenberg, 1995) and plated onto glass coverslips, glass-bottom MatTek dishes (MatTek Corp.), or plastic culture dishes coated with poly-D-lysine and mouse laminin. Cultures were maintained by feeding with ½ media changes every 3 days. Cells were transfected at day in vitro (DIV) 6, utilizing Lipofectamine 2000 by previously described methods (Arnold et al., 2011; Van Laar et al., 2011). Neurons were transfected where noted with plasmids expressing mitochondrially-targeted DsRed2 (mtDsRed2; Clontech), mitochondrially-targeted photo-activatable GFP (PA-mtGFP; (Karbowski et al., 2004)), full-length human Parkin (hu-Parkin; (Petrucelli et al., 2002)), GFP-tagged LC3 (Addgene plasmid 21073; deposited by T. Yoshimori (Kabeya et al., 2000)), and/or GFP-tagged Sec61β (Addgene plasmid 15108; deposited by T. Rapoport (Voeltz et al., 2006)). Of note, we routinely obtain 100% co-transfection of the multiple plasmids (Arnold et al, 2011).
Treatments were performed at DIV15 for all experiments. 1M stocks of glutamate and glycine (Sigma) were prepared in sterile 1xPBS, aliquoted, and stored at −20°C until day of use. For the glutamate treatments, media was collected from the cells and saved. Cells were rinsed with HBSS media (1x Hanks Balanced Salt Solution (Sigma, cat# H1641) supplemented with NaHCO3, (4.2mM), HEPES (10mM), and D-glucose (35mM)). Cells were then exposed to HBSS media alone (Control) or to HBSS media with glutamate (as indicated) plus glycine (1μM) for 10min in a 37°C, 5% CO2 tissue culture incubator. All glutamate exposures described included the co-agonist glycine. Calcium-free experiments substituted a Hank’s Solution lacking calcium (Sigma, cat# H4641) in the HBSS media. Immediately following treatment, cells were again rinsed with HBSS media. The saved culture media was replaced, and cells returned to the incubator for the indicated times.
For experiments using pretreatments, where indicated, E-64d (Calbiochem; 15mM stock in DMSO), Pepstatin A (Fisher; 15mM stock in DMSO), MG132 (Sigma; 10mM stock in DMSO) and/or NAC (Sigma; 0.5M stock, prepared fresh on day of use in sterile culture-grade H2O) were added directly to the culture media, to the desired final concentration, 1hr before treatment. Following glutamate treatment, the saved media containing pretreament compounds was placed back on the respective cells. Thus, these compounds were also present during the post-exposure stage. For NAC conditions, the respective concentration of NAC (500 or 1000 μM) was also present during the 10min glutamate exposure.
Following post-treatment incubation, cells for confocal analyses were fixed in a 4% paraformaldehyde PBS solution for 20 min at RT, followed by three rinses with 1× PBS, and stored at 4°C. Cells for ATP analyses and Western blot analyses were immediately collected by scraping into ice cold 1xPBS, pelleted by centrifugation (2,000×g, 5 min), and resuspended in a urea/CHAPS lysis buffer (9M urea, 2% CHAPS, in 30mM Tris, with 1x protease inhibitor cocktail). Lysates were stored at −80°C until use.
Immunocytochemistry, Imaging, and Quantification
Immunocytochemical staining of fixed neurons was completed as previously described (Arnold et al., 2011; Van Laar et al., 2011), and coverslips mounted onto glass slides using ProLong Gold antifade mounting media (Invitrogen). Primary antibodies used included PRK8 mouse-anti-Parkin (1:20; Santa Cruz, cat# sc-32282) and rabbit-anti-GFP (1:200; Cell Signaling, cat# 2956S), and were detected using AlexaFluor 647-conjugated anti-mouse and 488-conjugated anti-rabbit secondaries, respectively (1:500; Invitrogen).
Images for analysis were captured using an Olympus Fluoview 1000 confocal microscope (60x oil immersion lens, NA: 1.42). Random individual cells (5–30 per coverslip) were imaged as z-stacks encompassing the entire depth of the cell (0.25–0.5μm slices, using sequential laser imaging and Kalman filter correction). Blinded images were analyzed on an individual slice-by-slice basis, using Olympus Fluoview Viewer software, as described previously (Van Laar et al., 2011), to assess cells positive for Parkin accumulation on mitochondria, on endoplasmic reticulum (ER), between mitochondria and ER, or on mitochondria in association with LC3 accumulation (indicative of mitophagosomes). Any cell exhibiting at least one instance of mitochondria, ER, and/or LC3 puncta with a clearly colocalized accumulation of Parkin fluorescence was considered positive for their respective assays. For publication, representative acquired images were selected, and a minimal number of consecutive z-stack slices necessary to demonstrate the described localization (1–4 slices) were compressed into a single image. Image files were assembled into figures using Photoshop CS4 software (Adobe).
Live Imaging with TMRM and H2-DCF-DA
Tetramethyl rhodamine methyl ester (TMRM) analysis was used to determine that brief glutamate exposure was sufficiently depolarizing cells using previously described methods (Van Laar et al., 2011; Ward, 2010), with minor modifications. Rat primary cortical neurons plated on glass-bottom MatTek dishes were transfected with PA-mtGFP as described above. On DIV15, cells were pre-incubated in 20nM TMRM in media for 30min in a tissue culture incubator. Media was then removed, and replaced with an incubation media (MEM pH 7.4, supplemented with 2% GlutaMax, 20mM HEPES, 33mM glucose, 1mM Na-pyruvate) containing 20nM TMRM. Living cells were imaged on an Olympus Fluoview FV1000 confocal scanning microscope with a 37°C heated stage chamber. PA-mtGFP was photoactivated by briefly exposing the cells to epifluorescence illumination using a DAPI filter. Photoactivated cells were then imaged for PA-mtGFP (488nM) and TMRM (568nm), 10μs/pixel. A baseline fluorescence image was obtained, and then 1-fold media added containing either 20nM TMRM alone, or TMRM with a 2x concentration of glutamate/glycine. Cells were then immediately imaged over time at one image every 20sec to capture fluorescence loss, indicative of mitochondrial depolarization. For TMRM analyses with NAC, cells were pretreated with 1mM NAC for 1hr. After 1hr, TMRM was then added directly to the media and cells incubated for 30min. Media was then replaced with incubation media containing TMRM and 1mM NAC. After a baseline fluorescence image was obtained, 1-fold media was added containing TMRM with 1mM NAC, or TMRM, 1mM NAC, and a 2x concentration of glutamate/glycine, and cells imaged over time.
Analyses for ROS production were performed using previously described methods (Choi et al., 2011; Reynolds and Hastings, 1995), with minor modification. Primary neurons were plated on glass-bottom MatTek dishes as described. On DIV15, cells were treated as in previous experiments with one of the following conditions: control HBSS media, 100μM glutamate, 1mM NAC (with 1hr NAC pretreatment), or 100μM glutamate plus 1mM NAC (with 1hr NAC pretreatment). Following treatment, cells were incubated in their respective culture media for 2hr. At 2hr following treatment, media was removed and cells were incubated in HBSS media containing 10μM of the 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCF-DA) ROS probe for 15min. The probe-containing media was removed and replaced with fresh HBSS media, and cells were immediately imaged live to observe the resulting 2,7 -dichlorofluorescien (DCF) formation and fluorescence, indicative of ROS formation, with an Olympus FluoView1000 confocal scanning microscope with a 37°C heated stage chamber, utilizing an excitation wavelength at 488nm and pinhole at 80μm. 10–20 random single-slice fields per individual plate were imaged at 40x magnification. Identical acquisition and laser settings were used throughout all fields and all conditions imaged. Fluorescent intensity was determined for each field individually using Olympus Fluoview Viewer software. Intensities were averaged for all fields for each individual plate. Averages of the plates were then determined per condition, with three plates per condition.
Cell Fractionation for Mitochondria-, ER-, and MAM-enriched Fractions
Neurons were plated onto 10cm culture dishes and maintained until treatment at DIV15. Cells were treated as described above (HBSS control or 100μM glutamate, 10mim). Following a 2hr post-exposure period, cells were collected by scraping into ice-cold PBS. For each independent experiment, twelve (12) dishes were pooled per condition, and cells were pelleted by centrifugation (170×g, 10min, 4°C). Cells were washed by gently resuspending in ice-cold 1×PBS, then re-pelleted (170×g, 5min, 4°C). Cells were then resuspended in a sucrose homogenization medium (SHM; 250mM sucrose, 10mM HEPES, pH 7.4), and an aliquot taken (whole cell aliquot). The remaining cell suspension was fractionated using methods modified from Bozidis et al., and Wieckowski et al. (Bozidis et al., 2007; Wieckowski et al., 2009). Cells were disrupted in SHM using Knotes 2mL glass tissue homogenizers with glass, followed by disruption in 1mL glass tissue homogenizers with Teflon pestles. Homogenate was centrifuged at 600×g, 5min, 4°C, and the supernatant was saved. The pellet was re-homogenized in the 1mL homogenizer, homogenate centrifuged, and supernatant combined with the previous supernatant. The pooled supernatant was centrifuged at 10,300×g for 10min at 4°C to generate a crude mitochondrial enrichment and crude endoplasmic reticulum/cytosol (ER/cytosol) supernatant. The mitochondria-enriched pellet was resuspended in mannitol buffer A (250mM mannitol, 0.5mM EGTA, 5mM HEPES, pH 7.4), an aliquot taken (mitochondria crude-enrichment aliquot), and the rest subjected to ultracentrifugation to isolate mitochondrial-associated membrane (MAM).
The remaining mitochondrial resuspension was layered over a solution of 30% Percoll in mannitol buffer B (235mM mannitol, 1mM EGTA, 25mM HEPES, pH 7.4), then centrifuged at 95,000×g for 65min at 4°C. Using glass pipettes, supernatant layers corresponding to the enriched MAM fraction were removed and saved. The crude ER/cytosol supernatant was centrifuged 3x, each at 10,300×g, 10min, 4°C, to remove extra mitochondrial contaminate, and the final supernatant was saved (resulting pellets were discarded). Finally, the MAM-containing supernatant and ER-containing supernatant were centrifuged separately at 100,000×g for 60min at 4°C.
The resulting ER enriched pellet, MAM-enriched pellet, mitochondrial crude-enrichment aliquot, and whole cell aliquot were each immediately resuspended and lysed in a small volume of urea/CHAPS lysis buffer. Lysates were stored at −80°C until protein quantification and Western blot analysis.
Western Blot Analyses
Whole-cell lysates and cell fraction lysates were prepared as described above, and protein concentrations were determined by the Bradford method (Bradford, 1976). Samples were diluted in a reducing sample buffer, and subjected to SDS-PAGE using Peirce pre-cast Tris-HEPES gels. A BioRad SemiDry Transfer apparatus was used for gel transfer to PVDF. The resulting Western blots were probed using the following primary antibodies: PRK8 mouse-anti-Parkin (1:200; Santa Cruz), rabbit-anti-Mitofusin-2 (1:2000; Sigma, cat# 110M4842), mouse-anti-HSP60 (1:1200; Stressgen, cat# SPA-806), rabbit-anti-calreticulin (1:1000; Abcam, cat# ab92516), rabbit-anti-GAPDH (1:1200; Santa Cruz, cat# sc25778), rabbit-anti-actin (1:12,000; Abcam, cat# ab8227), rabbit-anti-FACL4 (1:5000; Abcam, cat# ab137525). For secondaries, corresponding Li-Cor Odyssey compatible IR680- and IR800-conjugated antibodies were used for detection, and blots were imaged and analyzed using a Li-Cor Odyssey system (Li-Cor).
ATP Measurements
Intracellular ATP was measured from lysates of whole cells. Neurons were collected and resuspended in an urea/CHAPS lysis buffer as described above. ATP levels were determined using a luciferase-based ATP Determination Kit (Molecular Probes) and an L Max II Luminometer (Molecular Devices) as previously described (Arnold et al., 2011; Van Laar et al., 2011). ATP data were normalized to individual sample protein concentrations as determined by Bradford assay (Bradford, 1976).
Statistics
Significance in confocal analyses for Parkin localization was determined using the z-test of proportions followed by post-hoc analysis (Sidak correction) using alpha = 0.05. Significance in DCF analyses for ROS and Western blot analyses for mitofusin-2 was determined using one-way ANOVA or Student’s T-tests, where appropriate, followed by suitable post-hoc analysis (Tukey’s or Bonferroni correction, respectively) using alpha = 0.05. Significance in Western blot analyses of Parkin localization in the cell fractionation studies was determined by first completing natural log transformation of the Parkin/loading control densitometry ratios. Mean differences between glutamate treatment and control groups were then calculated and analyzed using 2-sided paired t-test with alpha = 0.05. Both R and Microsoft Excel software programs were utilized for data analyses.
RESULTS
Brief exposure to glutamate causes Parkin translocation to mitochondria in neurons
We first examined the effects of a brief exposure to glutamate on Parkin localization in neurons. Rat cortical neurons were transfected with plasmids expressing human full-length Parkin (hu-Parkin) and mitochondrially-targeted DsRed2 fluorescent protein (mtDsRed2) at day in vitro (DIV) 6, and overexpression was sustained for several weeks after transfection, as we have previously demonstrated (Van Laar et al., 2011). Previous work has shown that, due to the developmental expression of glutamate NMDA receptors in vitro, primary cortical neurons do not exhibit glutamate sensitivity until after DIV11 (Mizuta et al., 1998). Thus, cultures were maintained until DIV15 for treatment. Neurons were exposed to a 10min pulse of glutamate (30–500μM) plus co-agonist glycine (1μM), or to control HBSS media, as described in Methods. We utilized glutamate concentrations that are thought to be similar to estimated synaptic and extrasynaptic concentrations of glutamate (Bergles et al., 1997; Clements et al., 1992; Dzubay and Jahr, 1999). Treatment was followed by HBSS washout, and normal media was then replaced. The cells were fixed at 2 or 6hr post-exposure, as noted.
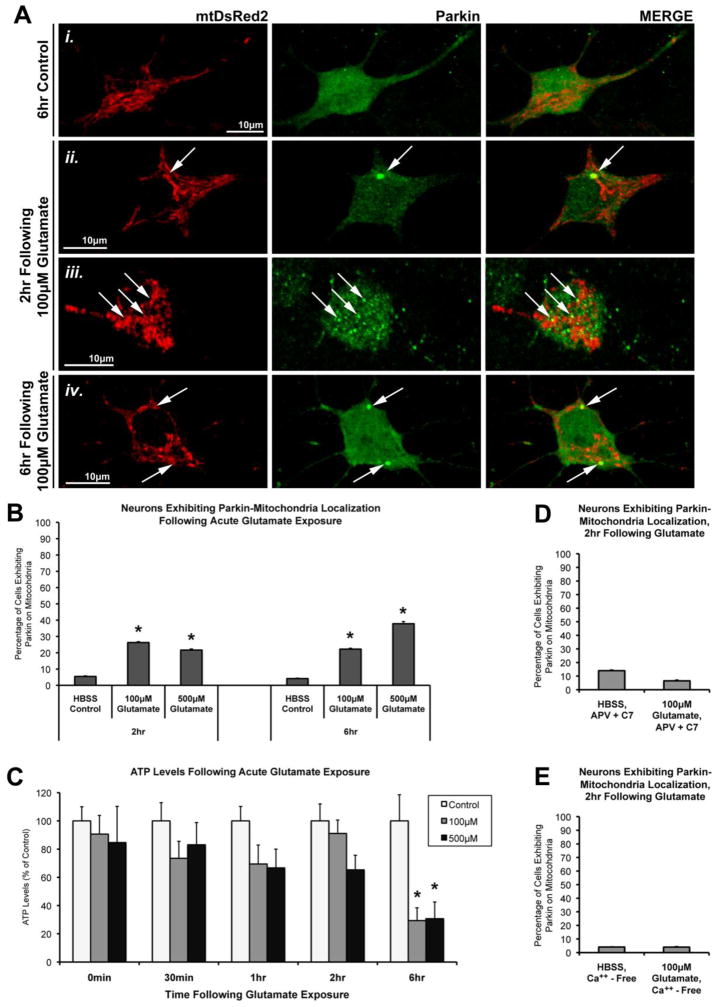
Blinded confocal images of cells were assessed for colocalization of Parkin protein and mitochondria. As expected, we observed a characteristic mitochondrial morphological remodeling in response to glutamate exposure in most neurons (Figure 1; Supplemental Figure 1), which is in accordance with previously observed spherical contraction, or “rounding,” of mitochondria noted in neurons following glutamate exposure (Rintoul et al., 2003; Brustovetsky et al., 2009). We found that a short pulse of glutamate (100μM or 500μM, 10min) leads to Parkin accumulation on mitochondria within 2hr in a significant percentage of the neurons as compared to their timed HBSS-exposed control neurons (Figure 1A, B). Lower concentrations of glutamate (30μM) were also sufficient to induce Parkin translocation to mitochondria in neurons (Supplemental Figure 1C).
Figure 1. The effect of acute glutamate exposure on Parkin localization and ATP levels in cortical neurons.
(A) Rat cortical neurons were co-transfected with full-length human Parkin and mitochondrially-targeted DsRed2 (mtDsRed2) at DIV6. At DIV15, neurons were exposed to a brief (10min) treatment with either HBSS media (Control) or Glutamate (100μM or 500μM), followed by a 2hr or 6hr incubation period in culture media. Cells were imaged for mtDsRed2 fluorescence (red) and Parkin immunofluorescence (green) using the PRK8 antibody. (Ai) Control cells exhibited normal mitochondrial morphology with ubiquitous Parkin distribution. (Aii, iii, iv) Glutamate-exposed cells exhibited a variable degree of mitochondrial remodeling, and a proportion of cells exhibit Parkin accumulations on mitochondria (arrows). (B) Graph of the observed percentage of neurons exhibiting Parkin-mitochondrial localization following glutamate exposure (37–72 individual cells per condition, across 5–6 independent neuronal preparations; * = p<0.05 from respective time-point control, +/− SEM). (C) Non-transfected neurons were treated on DIV15, and collected at the indicated time points following acute glutamate exposure. ATP levels were determined for whole-cell lysates at these time points (* = p< 0.05 from respective time-point control, +/−SEM). (D) Graph of the observed percentage of neurons exhibiting Parkin-mitochondrial localization following glutamate exposure in the presence of NMDA receptor inhibitors (2R)-amino-5-phosphonovaleric acid (APV) and 7-chlorokynurenic acid (C7) (n=47–50 cells per condition; +/− SEM). (E) Graph of the observed percentage of neurons exhibiting Parkin-mitochondrial localization following glutamate exposure in the absence of calcium (Ca++-free; n=49–50 cells per condition; +/− SEM).
The degree of Parkin-mitochondrial translocation that we observed at both 2hr and 6hr following glutamate exposure is similar to that which we previously observed after CCCP-induced mitochondrial depolarization in the presence of oligomycin (Van Laar et al., 2011), and similar to numbers reported by others in neurons with prolonged exposure to CCCP (Cai et al., 2012; McCoy et al., 2014). We also found that in a majority of the neurons positive for the phenomenon, only a small subset of the mitochondria exhibited Parkin accumulations (in many cases only 1–2 mitochondria per neuron). This observation is also similar to our previous findings with CCCP plus oligomycin (Van Laar et al., 2011).
Glutamate exposure causes mitochondrial depolarization and delayed loss of ATP
Using tetramethyl rhodamine methyl ester (TMRM) analysis, we verified that, as expected, glutamate exposure resulted in the rapid induction of cell-wide mitochondrial depolarization in neurons (Supplemental Figure 1). We also examined ATP levels after glutamate exposure. We had previously demonstrated that ATP levels inversely correlated with the ability of Parkin to accumulate on mitochondria after depolarization with CCCP in neurons (Van Laar et al., 2011). Here, we observed that ATP levels are preserved in neurons for up to 2 hrs following acute glutamate exposure (Figure 1C). A significant loss of ATP, however, was measured by 6hr following glutamate exposure (Figure 1C). This is in contrast to our previous findings with CCCP exposure, in which neuronal ATP levels dropped significantly within 15min of exposure (Van Laar et al., 2011).
Parkin translocation to mitochondria after glutamate exposure is NMDA receptor-dependent and calcium-dependent
Glutamate excitotoxicity is elicited via excessive cellular influx of calcium ions through NMDA receptor activation. To rule out non-calcium effects, we evaluated the role of NMDA calcium channel function in our experiments. Neurons were treated with either 100μM glutamate or HBSS control in the presence of both (2R)-amino-5-phosphonovaleric acid (APV; 200μM), a competitive antagonist of the NMDA receptor; and 7-Chlorokynurenic acid (C7; 200μM), an antagonist of the NMDA receptor glycine modulatory site. The NDMA receptor inhibitors completely blocked glutamate-induced Parkin translocation (Figure 1D). We also exposed neurons to 100μM glutamate in the presence of calcium-free HBSS, which completely abolished glutamate’s effects on Parkin localization as well (Figure 1E). Therefore, the observed effects of glutamate exposure on Parkin translocation are dependent on NMDA receptor activation and calcium influx into the cell.
Glutamate excitotoxicity triggers translocation of Parkin to ER
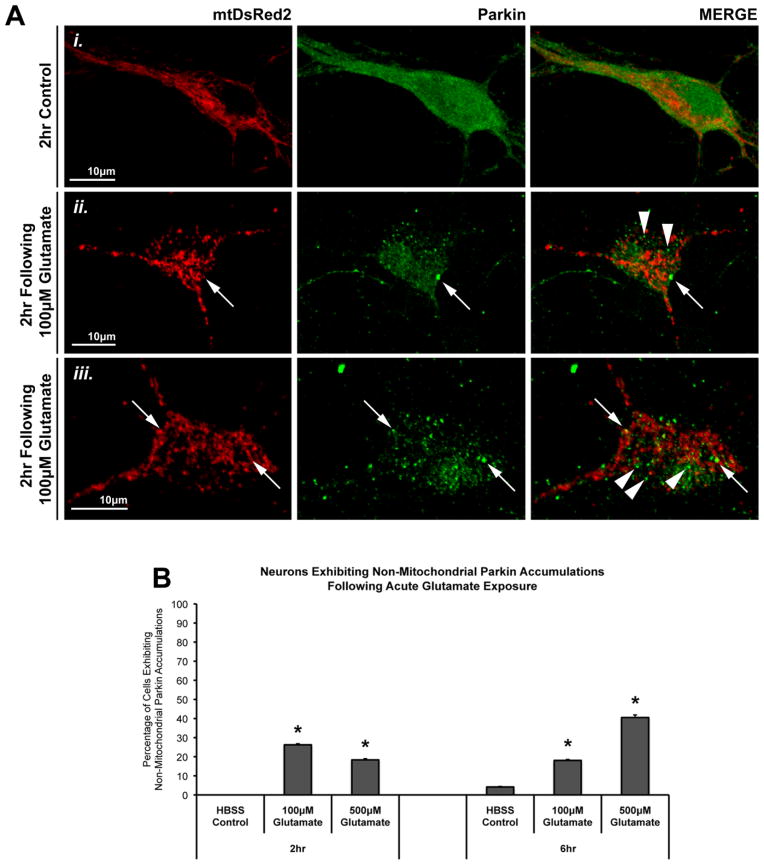
Interestingly, we noted that glutamate exposure also caused many non-mitochondrial accumulations of Parkin (Figure 2A). These punctate accumulations of Parkin often occurred in areas near or between mitochondria. Quantification revealed that a significant number of neurons exhibited these non-mitochondrial Parkin accumulations following glutamate exposure, at levels similar to the number of neurons exhibiting Parkin-mitochondrial accumulation (Figure 2B).
Figure 2. Mitochondrial and non-mitochondrial Parkin accumulations following glutamate exposure.
In the same studies described in Figure 1, we observed that, in addition to Parkin (green) localizing to mitochondria (mtDsRed2; red) (Aii, iii, arrows), some glutamate-exposed cells exhibited punctate Parkin accumulations that were not associated with mitochondria. These Parkin puncta were located either unassociated with any mitochondria or near, but not on, mitochondria (Aii, iii, arrowheads). (B) Graph of the observed percentage of neurons exhibiting non-mitochondrial Parkin localization following glutamate exposure (37–72 individual cells across 5–6 independent neuronal preparations; * = p<0.05 from respective time-point control, +/− SEM). For this assessment, a cell exhibiting non-mitochondrial Parkin accumulation was considered positive independent of whether or not that cell also exhibited Parkin-mitochondria localization.
We had not observed such non-mitochondrial accumulations in our previous studies of CCCP-oligomycin treatments. We hypothesized that endoplasmic reticulum (ER) might be involved, as the ER and mitochondria share an interactive role in regulating calcium (Csordas and Hajnoczky, 2009). Further, a recent study by Cali et al. identified a possible role for Parkin in facilitating mitochondrial-ER interactions and calcium crosstalk (Cali et al., 2013). We therefore examined whether Parkin was accumulating at ER and/or ER-mitochondrial junctions after glutamate.
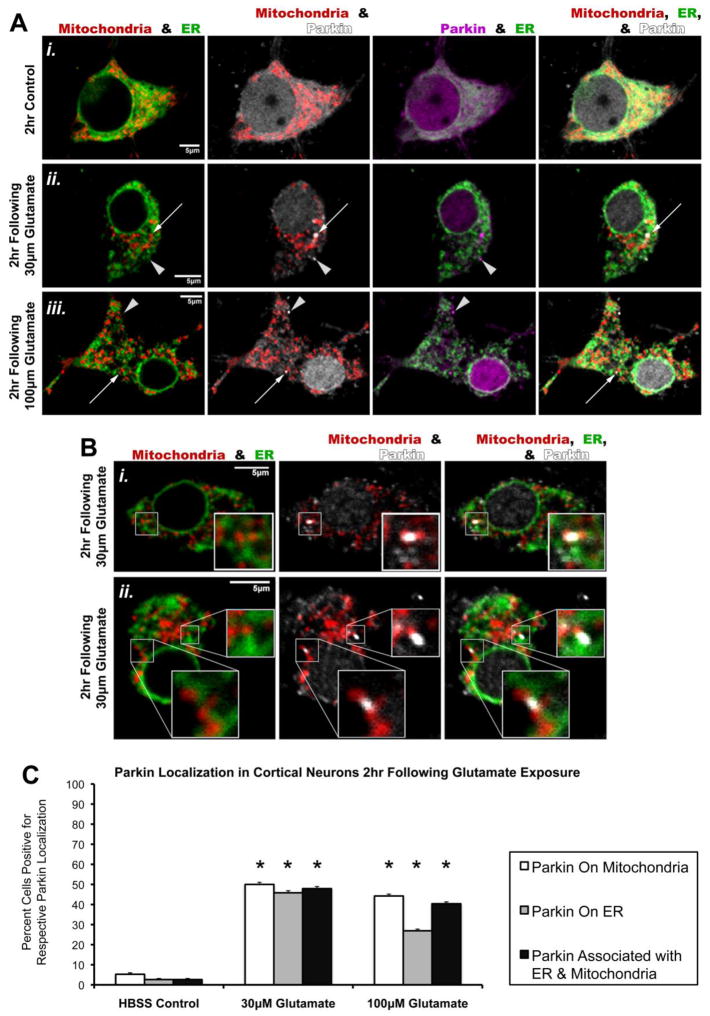
Cortical neurons were transfected with hu-Parkin, mtDsRed2, and GFP-tagged Sec61β (GFP-Sec61β), an ER membrane protein, and then treated with pulses of 30μM or 100μM glutamate as detailed above. Confocal imaging and blinded analysis revealed that glutamate exposure at both concentrations indeed led to significant numbers of cells exhibiting colocalization of Parkin accumulations with ER within 2hr (Figure 3). This included Parkin on ER and Parkin in spaces between mitochondria and ER. In the latter case, these Parkin accumulations appeared to overlap parts of both ER and tightly-neighboring mitochondria at sites suggestive of ER-mitochondrial junctions (Figure 3B). We also confirmed the previously demonstrated Parkin accumulation onto mitochondria (Figure 3). These data suggest that glutamate exposure induces translocation of Parkin to ER as well as mitochondria.
Figure 3. Glutamate exposure also triggers Parkin localization to ER and to regions between mitochondria and ER.
Rat cortical neurons were co-transfected with full-length human Parkin, mtDsRed2 (Mitochondria), and GFP-tagged ER-membrane protein Sec61β (ER) at DIV6. At DIV15, neurons were exposed to a brief (10min) treatment with either HBSS media (Control, Ai) or Glutamate (30μM, Ai; 100μM, Aii), followed by a 2hr incubation period in culture media. Note that the same Parkin immunofluorescence as shown in white in the Mitochondria & Parkin column was pseudocolored to magenta for the Parkin & ER column of images to better observe distribution. Images were assessed for Parkin accumulations on mitochondria (arrows) and ER (arrowheads). (B) Representative images displaying Parkin accumulations associating with both ER and nearby mitochondria, thus appearing to localize to mitochondria-ER junctions (inserts). (C) Graph of the observed percentage of neurons exhibiting individual Parkin accumulations on mitochondria alone (white bars), individual Parkin accumulations on ER alone (grey bars), and/or individual Parkin accumulations associated with both mitochondria and nearby ER following glutamate exposure (black bars) (31–52 individual cells per condition across 3 independent neuronal preparations; * = p<0.05 from respective HBSS control, +/− SEM). For this assessment, a cell exhibiting any one of the assessed Parkin accumulation types was considered positive independent of whether or not that cell also exhibited either of the other two Parkin accumulation types.
Endogenous Parkin also accumulates on mitochondria and ER after glutamate exposure
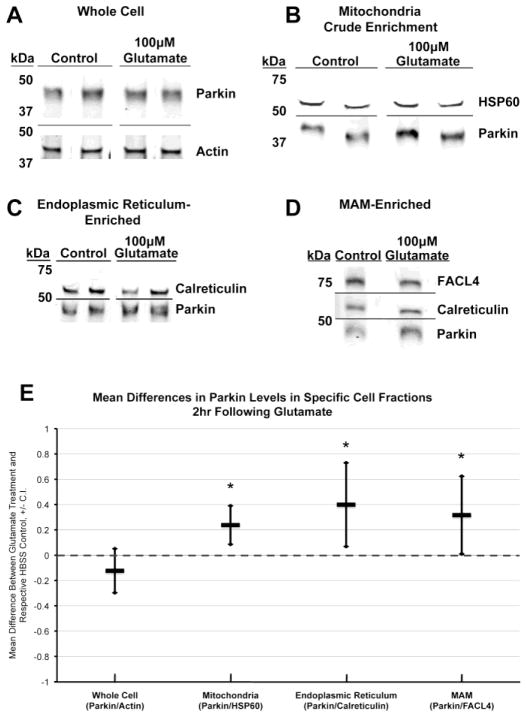
To ensure that the Parkin translocation was not an artifact of hu-Parkin overexpression, we also examined whether glutamate excitotoxicity triggers endogenous Parkin translocation to mitochondria, ER, and mitochondria-ER junctions. 2hr following pulses of 100μM glutamate or HBSS control, non-transfected neurons were subjected to cell fractionation to obtain mitochondria-enriched, ER-enriched, and mitochondrial associated membrane (MAM)-enriched subfractions (Supplemental Figure 2). Lysates from whole cells and cellular subfractions were assessed for Parkin content via Western blot analyses (Figure 4A–D). We found that total whole-cell Parkin levels did not significantly change. However, Parkin levels were significantly increased in the mitochondrial crude-enrichment fraction, ER-enriched fraction, and MAM-enriched fraction following glutamate exposure, as compared to their respective HBSS controls (Figure 4E). These results provide supportive evidence that excitotoxicity induces translocation of endogenous Parkin to mitochondria, ER, and mitochondria-ER junctions.
Figure 4. Western blot analysis of endogenous Parkin localization in cortical neurons after glutamate exposure.
Non-transfected cortical neurons were treated and harvested on DIV15 for cell fractionation. Western blot analyses were completed for Parkin levels and respective loading controls on the following cellular fractions: (A) whole cell, (B) mitochondria crude enrichment, (C) endoplasmic reticulum-enriched, and (D) mitochondria-associated membrane-enriched (MAM-enriched). Note that in the MAM-enriched fraction (D), presence of both FACL4 and calreticulin was assessed to verify presence of MAM. FACL4 was used as the loading control. (E) Quantitative results from Western blot analyses presented as the difference of the mean, calculated as treatment minus respective control (n= 4–5; * = p<0.05, +/− 95% C.I.).
Parkin translocation to mitochondria after glutamate exposure is promoted by N-acetyl cysteine
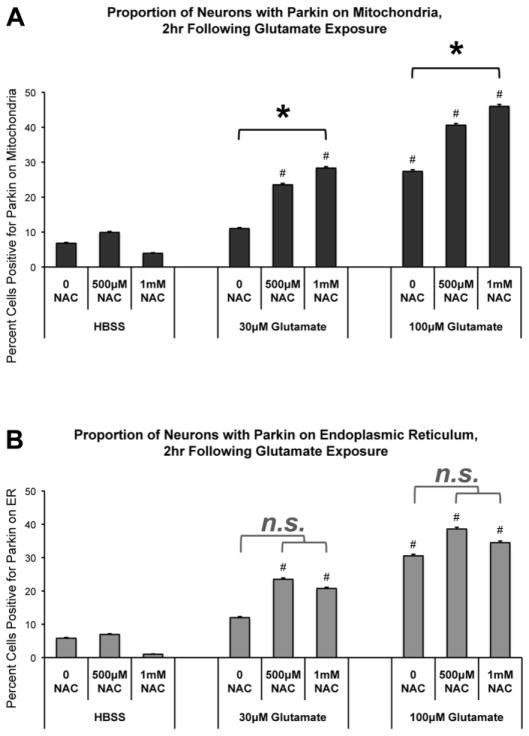
A role for ROS in Parkin-mitochondria translocation, and subsequent mitophagy, has been suggested (Joselin et al., 2012). It has also been shown that excitotoxicity causes increased ROS production and cellular oxidative stress (Mehta et al., 2013; Rego and Oliveira, 2003; Reynolds and Hastings, 1995). Additionally, studies show that co-treatment with the antioxidant N-acetyl cysteine (NAC) prevents glutamate-induced neurotoxicity (Choi et al., 2011; Fukui et al., 2009). Therefore, we assessed the effects of NAC on Parkin translocation after glutamate exposure. We evaluated Parkin-mitochondrial and Parkin-ER associations in neurons co-treated with glutamate and NAC as compared to glutamate alone, NAC alone, or HBSS control exposures, as described in Methods. We hypothesized that by preventing ROS damage, NAC would prevent glutamate-induced Parkin translocation to mitochondria. Contrary to our initial predictions, we found that addition of NAC consistently promoted translocation of Parkin to mitochondria after brief glutamate exposure (Figure 5A). NAC alone had no effect on Parkin translocation compared to control. However, co-treatment of glutamate with NAC resulted in significant increases in cells positive for Parkin-mitochondria accumulations as compared to glutamate alone (Figure 5A). In contrast, NAC co-treatment did not significantly alter Parkin accumulation on ER following glutamate (Figure 5B).
Figure 5. Co-treatment with the antioxidant N-acetyl cysteine (NAC) promotes Parkin localization to mitochondria following glutamate exposure.
Rat cortical neurons were co-transfected with Parkin, mtDsRed2, and GFP-Sec61β at DIV6. At DIV15, neurons were pretreated with NAC (500μM or 1mM) or control media for 1hr. Neurons were then pulsed with either (1) glutamate (30μM or 100μM) plus NAC (500μM or 1mM), (2) glutamate alone, (3) NAC alone, or (4) HBSS control. The 10min treatment exposure was followed by a 2hr incubation period in the corresponding culture media used for pretreatment. Cells were then assessed by confocal analysis for Parkin accumulation on mitochondria or endoplasmic reticulum (ER). (A) *: Co-treatment of NAC with glutamate increased the proportion of neurons with Parkin-mitochondrial accumulation as compared to glutamate alone (* = p<0.05 between respective NAC/glutamate condition and glutamate alone). #: Exposure to glutamate, with or without NAC co-treatment, increased the numbers of neurons positive for Parkin-mitochondria accumulation at 2hr following treatment as compared to respective non-glutamate HBSS controls (# = p<0.05). (B) n.s. bars: NAC co-treatment did not significantly alter the number of cells exhibiting Parkin accumulation on ER as compared to glutamate alone. #: As in earlier experiments, glutamate exposure increased the numbers of neurons with Parkin-ER accumulation as compared to respective non-glutamate HBSS controls (# = p<0.05). 87–106 individual cells per condition, across 4 independent neuronal preparations; +/− SEM.
We verified that NAC did prevent formation of ROS after glutamate exposure under our conditions. As expected, NAC did reduce the formation of ROS as detected 2hr following glutamate exposure (Supplemental Figure 3). We also observed that NAC did not prevent mitochondrial depolarization associated with glutamate treatment (Supplemental Figure 4).
After glutamate exposure, increased Parkin-associated mitophagy was only detected in the presence of NAC
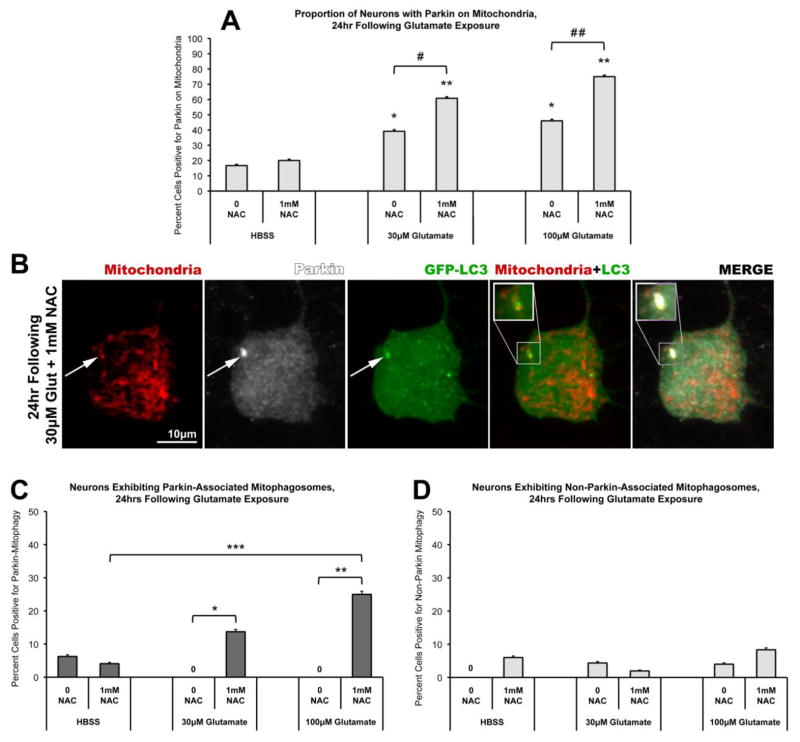
We next evaluated mitophagy in neurons treated with glutamate and NAC, compared to glutamate alone or control exposures. Neurons were co-transfected with Parkin, mtDsRed2, and green fluorescence protein-tagged LC3 (GFP-LC3). To inhibit autophagic flux and enhance detection of mitophagic vesicles in primary neurons, neurons were pretreated with lysosomal inhibitors (10μM Pepstatin A and 10μM E-64d; Cai et al., 2012; Tanida et al, 2005). Neurons were pretreated with the lysosomal inhibitors, with or without NAC, for 1hr, followed by glutamate or control exposure for 10min. NAC and/or lysosomal inhibitors were also present in the post-exposure period. The neurons were then evaluated at 2hr or 24hr post-glutamate exposure. Parkin-mitophagosome formation was assessed as co-localization of mtDsRed2, Parkin puncta, and GFP-LC3 accumulation.
No evidence of mitophagosome formation was observed at 2hr following glutamate, with or without NAC co-treatment (data not shown). At 24hr after exposure, co-treatment with NAC (1mM) and glutamate (100μM) still showed significantly increased Parkin-mitochondrial accumulation as compared to glutamate alone (Figure 6A). Analysis revealed no detectable evidence of Parkin-associated mitophagy in neurons treated with glutamate alone (Figure 6C). However, the presence of NAC co-treatment with glutamate was associated with a significant increase in Parkin-associated mitophagosomes (Figure 6B–C). Notably, we also observed rare instances of mitophagosome formation not associated with Parkin. However, the presence of glutamate and/or NAC had no effect on levels of non-Parkin mitophagosomes (Figure 6D). These data suggest, unexpectedly, that NAC co-treatment specifically fosters Parkin-associated mitophagy following glutamate exposure.
Figure 6. NAC co-treatment facilitates Parkin-associated mitophagy following glutamate exposure.
Rat cortical neurons were co-transfected with full-length human Parkin, mtDsRed2, and GFP-tagged LC3 (GFP-LC3) at DIV6. At DIV15, neurons were exposed to a brief (10min) treatment with either HBSS media or Glutamate (30μM or 100μM), with or without 1mM NAC, followed by a 24hr incubation period in culture media. Cells were then assessed by confocal analysis for localization of Parkin immunofluorescence relative to mtDsRed2 fluorescence (Mitochondria) and GFP immunofluorescence (GFP-LC3). (A) Glutamate-induced Parkin localization to mitochondria was observed 24hr following both 30μM and 100μM glutamate exposure (46–51 individual cells per condition, across 4 independent neuronal preparations; * = p<0.05 from HBSS, 0 NAC; ** = p<0.05 from HBSS, 1mM NAC; +/− SEM). Parkin localization was significantly increased by 1mM NAC co-treatment with glutamate, as compared to glutamate alone (# = p<0.05 between 30μM glutamate, 0 NAC and 30μM glutamate, 1mM NAC; # # = p<0.05 between 100μM glutamate, 0 NAC and 100μM glutamate, 1mM NAC; +/− SEM). (B) Instances of Parkin-associated mitophagosome formation were observed as co-localization of both Parkin (white) and GFP-LC3 (green) accumulations on a mitochondrion (red) (inset). (C) Graph of the observed percentage of neurons exhibiting Parkin-associated mitophagosomes 24hr following glutamate exposure with or without NAC co-treatment (46–51 individual cells per condition, across 4 independent neuronal preparations; * = p<0.05 between 30μM glutamate, 0 NAC and 30μM glutamate, 1mM NAC; ** = p<0.05 between 100μM glutamate with, 0 NAC and 100μM glutamate, 1mM NAC; *** = p<0.05 between HBSS, 1mM NAC and 100μM glutamate, 1mM NAC; +/− SEM). (D) Instances of mitophagosome formation not associated with Parkin were also observed in these experiments (measured as colocalization of GFP-LC3-labeled autophagosomes with mitochondria, but not containing Parkin). The proportions of cells exhibiting this mitophagy were not significantly different from controls nor significantly influenced by NAC co-treatment (+/− SEM).
Proteasome-dependent loss of Mitofusin-2 does not explain the differential effect of glutamate on neuronal mitophagy in the absence or presence of NAC
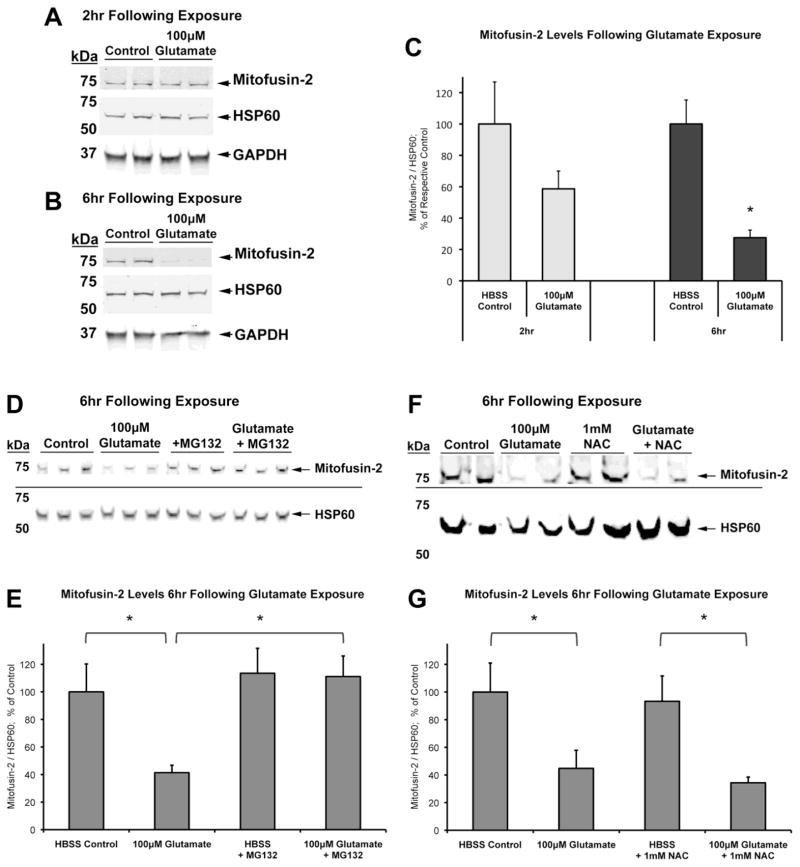
Previous studies have shown that upon mitochondrial depolarization, Parkin ubiquitinates the mitochondrial fusion proteins mitofusins 1 and 2 (Mfn1 and Mfn2), among others, targeting them for proteasomal degradation ahead of mitophagy (Chen and Dorn, 2013; Gegg et al., 2010; Poole et al., 2010; Ziviani et al., 2010). Evidence suggests that the Parkin-mediated loss of mitofusins prevents the fusion of damaged mitochondria, thus promoting fission events necessary for mitophagy (Gegg et al., 2010; Poole et al., 2010; Rakovic et al., 2011; Ziviani et al., 2010). We wondered whether differences in mitofusin degradation might explain the NAC-dependent Parkin-associated mitophagy. We therefore examined whether the presence of NAC promoted degradation of Mfn2 after excitotoxic exposure. We evaluated Mfn2 levels at time points prior to the detection of mitophagy under these conditions.
Non-transfected neurons were exposed to either HBSS or 100μM glutamate, as described in previous experiments, and whole-cell lysates were collected at 2hr and 6hr (prior to detectable mitophagy). We found that at both time points after glutamate exposure, levels of HSP60, a mitochondrial matrix protein, did not significantly alter relative to either actin or GAPDH levels (data not shown). Thus we used HSP60 as a mitochondrial loading control to analyze Mfn2 levels (Figure 7).
Figure 7. Effect of acute glutamate exposure on cellular levels of mitofusin-2.
On DIV15, non-transfected cortical neurons were treated (HBSS Control or 100μM Glutamate) and harvested. Western blot analyses were conducted for mitofusin-2 (Mfn2) and HSP60 at (A) 2hr and (B) 6hr following treatment. (C) Graph of Mfn2 levels with respect to HSP60 mitochondrial loading control at 2hr and 6hr (n=8–9; * = p<0.05 from respective time-point control, +/− SEM). (D) Western blot analysis of neurons co-treated with or without proteasome inhibitor MG132 and 100μM glutamate, 6hr following exposure. (E) Graph of Mfn2 levels with respect to HSP60 mitochondrial loading control at 2hr and 6hr (n=3; * = p<0.05, +/− SEM). (F) Western blot analysis of neurons co-treated with or without 1mM NAC and 100μM glutamate, 6hr following exposure. (G) Graph of Mfn2 levels with respect to HSP60 mitochondrial loading control at 6hr (n=6; * = p<0.05, +/− SEM).
We found that Mfn2 levels dropped over time following glutamate exposure. Detectable full-length Mfn2 decreased slightly but not significantly at 2hr after glutamate exposure, and dropped significantly at 6hr (Figure 7A–C). In replicate experiments, co-treatment with the proteasome inhibitor MG132 (10μM) completely prevented the loss of Mfn2 (Figure 7D–E). These results suggest that glutamate-induced depolarization of mitochondria does result in a proteasome-dependent loss of Mfn2 in neurons. We then evaluated the effect of co-treatment with NAC on Mfn2 levels. NAC alone did not affect Mfn2 levels, and NAC co-treatment with glutamate did not significantly alter the observed loss of Mfn2 as compared to glutamate alone at 6hr following exposure (Figure 7F–G). Thus, while Mfn2 is a key player in Parkin recruitment and mitophagy (Chen and Dorn, 2013), Mfn2 loss alone may not be sufficient to trigger Parkin-associated mitophagy in neurons after glutamate exposure. In addition, since proteasome-dependent loss of Mfn2 occurred equally in both glutamate conditions, this cannot be the explanation for the difference observed in Parkin-associated mitophagosome formation between the absence or presence of NAC after glutamate.
DISCUSSION
These studies report two significant, novel findings: First, we provide the first evidence that glutamate excitotoxicity in neurons, proposed to have a contributing role in neurodegeneration and PD pathogenesis, can trigger Parkin translocation to mitochondria, as well as to ER and mitochondria-ER junctions. This suggests an interaction between excitotoxicity, Parkin function, and dynamic functions of mitochondria and ER. Secondly, we have demonstrated an unexpected regulation of Parkin-associated mitochondrial quality control in neurons, discovering that mitophagy is promoted after glutamate exposure only when the antioxidant N-acetyl cysteine (NAC) is present. This suggests that in neurons, Parkin mobilization and mitochondrial quality control could be regulated by both the bioenergetic status and redox status of the cell. Given the many links of oxidative stress and bioenergetics in neurodegenerative disease and, in particular, PD, this has implications for understanding the etiology of PD neurodegeneration.
Linking glutamate excitotoxicity, Parkin, ER, and mitochondrial quality control: potential relevance to PD and neurodegeneration
Glutamate excitotoxicity has been proposed to contribute to neuronal vulnerability in PD and other neurodegenerative disorders (Blandini, 2010; Mehta et al., 2013). Here, we discovered that brief exposure to physiologically relevant concentrations of glutamate leads to significant Parkin accumulation on mitochondria in neurons, via activation of NMDA receptors and calcium influx. These results demonstrate that a known physiologic stressor linked to neurodegeneration, which is specific to neurons and leads to calcium-induced mitochondrial depolarization, regulates translocation of Parkin to mitochondria.
We also observed previously-unreported non-mitochondrial accumulations of Parkin following excitotoxicity-induced mitochondrial depolarization, which were not seen in our previous studies using CCCP. Our imaging and biochemical analyses demonstrated that glutamate excitotoxicity triggered Parkin accumulation both on ER and between ER and mitochondria. To our knowledge, this is the first evidence of glutamate excitotoxicity triggering Parkin translocation to ER in neurons. Previous studies do suggest a link between Parkin function and ER stress. Parkin expression is increased in response to ER stress (Wang et al., 2007), and accumulation of the Parkin substrate Pael-R leads to ER stress (Imai et al., 2001; Kitao et al., 2007). Further, findings by Yamamoto et al. suggest that ER stress results in a decrease in the phosphorylation rate of Parkin concordant with an increase in ubiquitin ligase activity (Yamamoto et al., 2005). Given these results, it is not surprising that Parkin would be targeted to ER following excitotoxic, calcium-induced stress.
The function of Parkin, once localized to ER and ER-mitochondria junctions, however, is as yet unknown. Interestingly, it has been reported that ER is linked to selective escape of proteins from mitochondria during mitophagy (Saita et al., 2013), providing a possible link between Parkin, mitochondria, and ER. In addition, Drp1-dependent fission appears to be facilitated at ER-mitochondrial junction sites (Friedman et al., 2011), demonstrating a connection between ER and mitochondrial fission/fusion dynamics. This is intriguing, considering that fission is proposed to be a precursor to mitophagy, whereby severely damaged portions of mitochondria are selectively divided off for degradation (Twig et al., 2008). Facilitation of this function may be particularly important for maintaining healthy mitochondrial-ER calcium ion crosstalk following excitotoxic stress. Indeed, one recent study found that overexpression of Parkin facilitated calcium crosstalk and sustained cellular bioenergetics in part by increasing interaction between ER and mitochondria, though the specific mechanism for this enhancement remains unknown (Cali et al., 2013). Of note, we observed glutamate-induced accumulations of Parkin on ER whether or not we observed mitophagy. This suggests that Parkin translocation to ER is not likely to be solely for purposes of facilitating mitophagy. Further elucidating the interrelationships of Parkin with mitochondrial and ER homeostases may shed light on neuronal vulnerability in Parkin-associated PD.
NAC facilitates Parkin translocation and mitophagy in neurons following excitotoxic insult – a possible unexpected role for oxidative stress
Despite translocation of Parkin to mitochondria after glutamate exposure, we only observed Parkin-associated mitophagy when the antioxidant NAC was present. While we have not ruled out an alternative action of NAC, these findings suggest the possibility that inhibition of ROS can promote Parkin-associated mitophagy in neurons. We had hypothesized the opposite, that ROS would promote Parkin translocation to mitochondria and subsequent mitophagy, and that inhibiting ROS would prevent Parkin translocation. A previous study had suggested a possible role for ROS in promoting the Parkin translocation process (Joselin et al., 2012). Glutamate excitotoxicity is known to increase production of ROS (Mehta et al., 2013; Rego and Oliveira, 2003; Reynolds and Hastings, 1995), and therefore, we had expected that NAC would inhibit Parkin-mitochondrial translocation.
However, to our initial surprise, we observed that glutamate-induced Parkin translocation to mitochondria was instead enhanced by the addition of NAC. Furthermore, the presence of NAC in glutamate-exposed neuron cultures promoted Parkin-mediated mitophagy, which we had not observed with glutamate alone. Parkin translocation to mitochondria has been observed in neurons under certain conditions of mitochondrial stress or depolarization (Amadoro et al., 2014; Ashrafi et al., 2014; Cai et al., 2012; Joselin et al., 2012; McCoy et al., 2014; Narendra et al., 2008; Rakovic et al., 2013; Seibler et al., 2011; Van Laar et al., 2011; Vives-Bauza et al., 2010; Wang et al., 2011). However, unlike many other cell types, specific Parkin-associated mitophagy itself has more rarely been clearly demonstrated in neurons (Ashrafi et al., 2014; Cai et al., 2012), although evidence of non-Parkin-associated neuronal mitophagy has also been observed (Chu et al., 2013; Sterky et al., 2011). Thus, this study represents one of the few clearly observed triggers of Parkin-associated neuronal mitophagy. In addition, we found mitophagic events occurred rarely within neurons, involving only one or two mitochondria per neuron, even though global mitochondrial depolarization was observed. These data are consistent with other observations that only subpopulations of neuronal mitochondria recruit Parkin following depolarization (Ashrafi et al., 2014; Cai et al., 2012; Van Laar et al., 2011), and further support additional regulatory mechanisms for mitophagy in neurons, as might be expected for cells dependent on mitochondria.
The mechanism for the unexpected effect of NAC on excitotoxicity-mediated neuronal mitophagy is not yet known. However, there are interesting potential explanations that may be particularly relevant in the neuronal environment related to PD degeneration. For instance, studies have suggested that conserved cysteines of Parkin, including a cysteine critical to ubiquitin ligase function, must be preserved in order for Parkin to accumulate on mitochondria and initiate mitophagy (Narendra et al., 2010; Zheng and Hunter, 2013). However, cysteine residues are highly susceptible to oxidative modification, and cysteine modification by oxidation products such as dopamine quinone inactivates Parkin (LaVoie et al., 2005). It is possible that ROS produced by glutamate excitotoxicity could oxidatively modify some of the cytosolic Parkin pool, reducing the translocation to mitochondria. Thus the addition of NAC could potentially result in a larger pool of functional Parkin, facilitating Parkin-mitochondria interactions.
Studies have shown NAC can prevent the effects of mitochondrially-produced ROS (Kulisz et al., 2002; Moreira et al., 2007; Seaton et al., 1997), and we show that NAC clearly reduced cellular ROS levels following glutamate. However, we cannot fully exclude a role for localized ROS damage at the mitochondria. Further study to determine subcellular localization of ROS production and inhibition following glutamate-antioxidant co-treatments will be important in understanding the mechanism of the NAC-promoted mitophagy. Related to this, a recent study found that localized induction of ROS production at the mitochondria induced Parkin accumulation and subsequent mitophagy in distal axons (Ashrafi et al., 2014). The mitophagy-promoting effects of NAC in the present studies, then, suggest that glutamate-induced calcium influx into mitochondria induces complex mechanisms of mitophagic signaling. In addition, NAC could be working through unknown mechanisms independent of its antioxidant capacity. However, we suspect the effects are related to its antioxidant functions, as we have preliminary studies in which we observe that the membrane-permeable radical scavenger 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) similarly promotes Parkin translocation to mitochondria after glutamate (unpublished data). In addition, we show here that while NAC does inhibit ROS formation, it does not prevent glutamate-induced mitochondrial depolarization. This suggests that the effects of NAC function downstream of the initial excitotoxic insult. Nevertheless, other unknown mechanisms, perhaps involving cysteine or glutathione, are possible as well.
These findings also raise the possibility that bioenergetics and oxidative stress interact with different pathways related to Parkin function. Recent studies have identified a Parkin-dependent mitochondrial quality control pathway that is distinct from mitophagy, consisting of mitochondria-derived vesicles (MDVs) that are shuttled to the lysosome in a mitophagy-independent manner (McLelland et al., 2014; Soubannier et al., 2012a; Soubannier et al., 2012b). This pathway, interestingly, appears to be particularly important in degradation of ROS-damaged mitochondrial components (Soubannier et al., 2012b). Thus, one could hypothesize distinct pathways of mitochondrial maintenance: In the absence of antioxidants, glutamate excitotoxicity may trigger the selective degradation of oxidatively damaged mitochondrial components through Parkin-dependent shuttling of MDVs. In contrast, when oxidative damage is prevented by NAC, the resulting mitochondrial depolarization and calcium influx may result in more wholesale degradation of mitochondrial organelles. Although under the limitations of our studies, we did not detect any obvious MDV formation in the absence of NAC, further exploration will help to elucidate whether this pathway plays an important role in neurons. In addition, there are other non-mitophagic functions of Parkin, such as selective degradation of electron transport chain components (Vincow et al., 2013) or inhibition of Bax translocation to mitochondria (Charan et al., 2014).
It is possible that in the more highly oxidative environment of PD-vulnerable DA neurons (Sofic et al., 2006), one or more of these non-mitophagic functions of Parkin might be critical. The present studies were performed in cortical neurons, as the low percentage of DA neurons in midbrain cultures limits the ability to assess DA neurons directly. It is important to note, however, that while DA neurons are among the most vulnerable in PD, it is clear that multiple non-DA neuron populations, including cortical, are also affected in PD progression (Braak et al., 2004). Nevertheless, evaluating whether the more oxidative environment of DA neurons alters the regulation of mitophagy will be important in order to dissect mitochondrial quality control pathways in neurodegenerative environments.
Taken together, our work and the work of others may suggest a larger role for Parkin in maintaining mitochondrial homeostasis and cellular bioenergetics in neurons. These new findings suggest that neurons utilize both bioenergetic status and redox status to regulate mitochondrial degradation, and that there is a role for Parkin in mitochondrial/ER interactions. This carries critical implications for mitochondrial health in the specific neurons that are vulnerable in PD. These neurons are poorly myelinated, necessitating a high energy requirement, and may have lower antioxidant capacity (Braak et al., 2004; Perry et al., 1982; Sofic et al., 2006). Thus the need for mitochondrial quality control is readily evident. As these neurons may also be susceptible to excitotoxic damage, the combination of ROS and calcium influx damage could overwhelm the system, and may contribute to the selective vulnerability of these neurons in PD.
Supplementary Material
HIGHLIGHTS.
Glutamate excitotoxicity in neurons triggers Parkin translocation to mitochondria.
Excitotoxicity also triggers Parkin translocation to endoplasmic reticulum.
Antioxidant N-acetyl cysteine promoted the Parkin accumulation on mitochondria.
Glutamate-induced Parkin-mitophagy occurred only in the presence of N-acetyl cysteine.
Acknowledgments
This work was supported by funding from the National Institutes of Health (R01NS077954 and K08NS059576 to S.B.B, and NS07391 to V.S.V.), an Ellison Medical Foundation/American Federation for Aging Research Fellowship (to V.S.V.), and a Parkinson Disease Foundation Fellowship (PDF-FBS-1107 to V.S.V.).
ABBREVIATIONS
- APV
(2R)-amino-5-phosphonovaleric acid
- C7
7-Chlorokynurenic acid
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- DCF
2,7 -dichlorofluorescien
- DIV
day in vitro
- ER
endoplasmic reticulum
- GFP-Sec61β
green fluorescent protein-tagged Sec61beta protein
- GFP-LC3
green fluorescent protein-tagged LC3 protein
- H2-DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- hu-Parkin
full-length human Parkin
- mtDsRed2
mitochondrially-targeted DsRed2 protein
- MAM
mitochondrial-associated membrane
- MDVs
mitochondria-derived vesicles
- NAC
N-acetyl cysteine
- NMDA
N-methyl-D-aspartate
- PA-mtGFP
mitochondrially-targeted photoactivatable green fluorescent protein
- PINK1
PTEN induced putative kinase 1
- PD
Parkinson’s disease
- SHM
sucrose homogenization medium
- TMRM
tetramethyl rhodamine methyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadoro G, Corsetti V, Florenzano F, Atlante A, Ciotti MT, Mongiardi MP, Bussani R, Nicolin V, Nori SL, Campanella M, Calissano P. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol Dis. 2014;62:489–507. doi: 10.1016/j.nbd.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Arnold B, Cassady SJ, VanLaar VS, Berman SB. Integrating multiple aspects of mitochondrial dynamics in neurons: age-related differences and dynamic changes in a chronic rotenone model. Neurobiol Dis. 2011;41:189–200. doi: 10.1016/j.nbd.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014 doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci U S A. 1997;94:14821–5. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F. An update on the potential role of excitotoxicity in the pathogenesis of Parkinson’s disease. Funct Neurol. 2010;25:65–71. [PubMed] [Google Scholar]
- Bozidis P, Williamson CD, Colberg-Poley AM. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts. Curr Protoc Cell Biol. 2007;Chapter 3(Unit 3–27) doi: 10.1002/0471143030.cb0327s37. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Li V, Brustovetsky N. Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium. 2009;46:18–29. doi: 10.1016/j.ceca.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial Parkin Translocation and Degradation of Damaged Mitochondria via Mitophagy in Live Cortical Neurons. Curr Biol. 2012 doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta. 2013;1832:495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S63–70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Charan RA, Johnson BN, Zaganelli S, Nardozzi JD, LaVoie MJ. Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin. Cell Death Dis. 2014;5:e1313. doi: 10.1038/cddis.2014.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–76. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Kang KS, Fukui M, Zhu BT. Critical role of the JNK-p53-GADD45alpha apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br J Pharmacol. 2011;162:175–92. doi: 10.1111/j.1476-5381.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–62. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RL, Przedborski S. Mitophagy and Parkinson’s disease: be eaten to stay healthy. Mol Cell Neurosci. 2013;55:37–43. doi: 10.1016/j.mcn.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubay JA, Jahr CE. The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci. 1999;19:5265–74. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–62. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–70. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–47. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Grenier K, McLelland GL, Fon EA. Parkin- and PINK1-Dependent Mitophagy in Neurons: Will the Real Pathway Please Stand Up? Front Neurol. 2013;4:100. doi: 10.3389/fneur.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MJ, Brandon B, Gentleman SM, Dexter DT. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain. 2013;136:2077–97. doi: 10.1093/brain/awt134. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW, Shen J, Slack RS, Park DS. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum Mol Genet. 2012;21:4888–903. doi: 10.1093/hmg/dds325. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–9. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao Y, Imai Y, Ozawa K, Kataoka A, Ikeda T, Soda M, Nakimawa K, Kiyama H, Stern DM, Hori O, Wakamatsu K, Ito S, Itohara S, Takahashi R, Ogawa S. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum Mol Genet. 2007;16:50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Reynolds IJ. Dopaminergic neurotoxins require excitotoxic stimulation in organotypic cultures. Neurobiol Dis. 2005;20:639–45. doi: 10.1016/j.nbd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1324–9. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–21. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson’s Disease-Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschmann PA, Lange KW, Wachtel H, Turski L. MPTP-induced degeneration: interference with glutamatergic toxicity. J Neural Transm Suppl. 1994;43:133–43. [PubMed] [Google Scholar]
- McCoy MK, Kaganovich A, Rudenko IN, Ding J, Cookson MR. Hexokinase activity is required for recruitment of parkin to depolarized mitochondria. Hum Mol Genet. 2014;23:145–56. doi: 10.1093/hmg/ddt407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. Embo J. 2014;33:282–95. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp Neurol. 2009;219:334–40. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I, Katayama M, Watanabe M, Mishina M, Ishii K. Developmental expression of NMDA receptor subunits and the emergence of glutamate neurotoxicity in primary cultures of murine cerebral cortical neurons. Cell Mol Life Sci. 1998;54:721–5. doi: 10.1007/s000180050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee G, Chung J. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Perry TL, Godin DV, Hansen S. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–10. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–19. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Johnson JW, Steer E, Zhu W, Eisenberg DA, Valentino NM, Liu YJ, Chu CT. Mutant LRRK2 enhances glutamatergic synapse activity and evokes excitotoxic dendrite degeneration. Biochim Biophys Acta. 2014;1842:1596–603. doi: 10.1016/j.bbadis.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic A, Grunewald A, Kottwitz J, Bruggemann N, Pramstaller PP, Lohmann K, Klein C. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS One. 2011;6:e16746. doi: 10.1371/journal.pone.0016746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic A, Shurkewitsch K, Seibler P, Grunewald A, Zanon A, Hagenah J, Krainc D, Klein C. Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons. J Biol Chem. 2013;288:2223–37. doi: 10.1074/jbc.M112.391680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–74. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–27. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–8. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun. 2013;4:1410. doi: 10.1038/ncomms2400. [DOI] [PubMed] [Google Scholar]
- Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37:315–24. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–33. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton TA, Cooper JM, Schapira AH. Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res. 1997;777:110–8. doi: 10.1016/s0006-8993(97)01034-2. [DOI] [PubMed] [Google Scholar]
- Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–6. doi: 10.1523/JNEUROSCI.4441-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Sapcanin A, Tahirovic I, Gavrankapetanovic I, Jellinger K, Reynolds GP, Tatschner T, Riederer P. Antioxidant capacity in postmortem brain tissues of Parkinson’s and Alzheimer’s diseases. J Neural Transm Suppl. 2006:39–43. doi: 10.1007/978-3-211-33328-0_5. [DOI] [PubMed] [Google Scholar]
- Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012a;22:135–41. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One. 2012b;7:e52830. doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108:12937–42. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1:366–73. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet. 2011;20:927–40. doi: 10.1093/hmg/ddq531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson’s disease. Exp Neurol. 2009;218:247–56. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol Dis. 2013;51:43–55. doi: 10.1016/j.nbd.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci U S A. 2013;110:6400–5. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–83. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–86. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Imai Y, Kataoka A, Takahashi R. Cell type-specific upregulation of Parkin in response to ER stress. Antioxid Redox Signal. 2007;9:533–42. doi: 10.1089/ars.2006.1522. [DOI] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MW. Quantitative Analysis of Membrane Potentials. In: Papkovsky DB, editor. Live Cell Imaging, Methods in Molecular Biology. Vol. 591. Humana Press; 2010. pp. 335–351. [DOI] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–97. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4:1582–90. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- Wu YN, Johnson SW. Rotenone potentiates NMDA currents in substantia nigra dopamine neurons. Neurosci Lett. 2007;421:96–100. doi: 10.1016/j.neulet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Friedlein A, Imai Y, Takahashi R, Kahle PJ, Haass C. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J Biol Chem. 2005;280:3390–9. doi: 10.1074/jbc.M407724200. [DOI] [PubMed] [Google Scholar]
- Yu W, Sun Y, Guo S, Lu B. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet. 2011;20:3227–40. doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Hunter T. Parkin mitochondrial translocation is achieved through a novel catalytic activity coupled mechanism. Cell Res. 2013;23:886–97. doi: 10.1038/cr.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–23. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.