Abstract
Objective
The metabolic syndrome (MetS) may contribute to the pathogenesis of venous thromboembolism (VTE), but this association requires additional investigation.
Approach and Results
We performed a patient-level meta-analysis of case-control and cohort studies that evaluated the role of MetS and risk of unprovoked VTE. For case-control studies, odds ratios (ORs) and 95% confidence intervals (CI) were calculated using logistic regression analysis to estimate the influence of individual variables on the risk of VTE; Chi squared tests for trend were used to investigate the impact of increasing number of components of MetS on the risk of VTE, and to explore the influence of abdominal obesity on this relationship. For cohort studies, hazard ratios (HRs) and 95% CI were calculated by using multivariable Cox regression analysis.
Six case-control studies were included (908 cases with unprovoked VTE and 1794 controls): in multivariate analysis, MetS was independently associated with VTE (OR 1.91, 95% 1.57-2.33) and both MetS and abdominal obesity were better predictors of unprovoked VTE than obesity defined by the body mass index (BMI). Two prospective cohort studies were included (26.531 subjects, 289 unprovoked VTE events): age, obesity, and abdominal obesity, but not MetS were associated with VTE.
Conclusions
Case-control, but not prospective cohort studies support an association between MetS and VTE. Abdominal adiposity is a strong risk factor for VTE.
Keywords: Venous thromboembolism, metabolic syndrome, obesity, deep vein thrombosis, pulmonary embolism
Venous thromboembolism (VTE) is a multifactorial disease that can affect apparently healthy individuals as well as hospitalized patients. In most cases, the occurrence of VTE is associated with the presence of major provoking factors such as a recent surgical procedure, trauma, fracture, pregnancy or puerperium, use of oral contraceptives, prolonged immobilization or a severe medical disease (1). However, in up to 45% of cases none of these risk factors is identified and VTE remains classified as unprovoked (1-3).
Over recent years, a number of studies have reported an increased risk of cardiovascular events in patients with VTE (4-9). The results of a recent meta-analysis reported that the overall risk of cardiovascular events, as well as the risk of acute myocardial infarction or stroke after VTE is significantly higher in patients with unprovoked VTE than in patients with VTE secondary to a major provoking factor (9).
One of the hypotheses for this association between cardiovascular disease and VTE is that the two diseases may share common risk factors. Among traditional cardiovascular risk factors, obesity and age have been demonstrated to be consistently independent risk factors also for VTE (10,11). In a systematic review of the literature and meta-analysis, we found that also arterial hypertension, diabetes mellitus, and dyslipidemia are also associated with VTE (12). Although the estimated odds ratios for these variables were less robust than those reported for established major risk factors for VTE such as cancer or surgery, the findings are relevant since cardiovascular risk factors are more common, often coexist and their coexistence, at least for atherosclerotic disorders, is associated with an additive causative effect.
The metabolic syndrome is a cluster of cardiovascular risk factors including abdominal obesity, hypertension, insulin resistance, and dyslipidemia with high triglycerides levels and low HDL cholesterol (13). The interaction of these risk factors results in a significantly increased risk of developing coronary artery disease and ischemic stroke (14). Recent studies suggest that the metabolic syndrome may also be associated with VTE (15-21), but several issues remain unclear. In particular, whether this association is attributable to the metabolic syndrome or to abdominal obesity alone, with no additional contribution by the other components, and whether this association is gender specific remain to be established (19,20).
To further explore these issues, we carried out a systematic search of the literature and an individual patient level meta-analysis of published studies evaluating the association between unprovoked VTE and the metabolic syndrome.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
The results of study search and selection process are summarized in Figure 1. The inter-observer agreement for the study selection was excellent, with a k of 0.92. After the selection process, eight studies were eligible for this analysis (15,17,18,19,20, 21,22,23), six were case-control studies (15,17,18,21,22,23) and two were prospective cohort studies (19,20). All contacted investigators agreed to provide their full database of the study.
Figure 1.
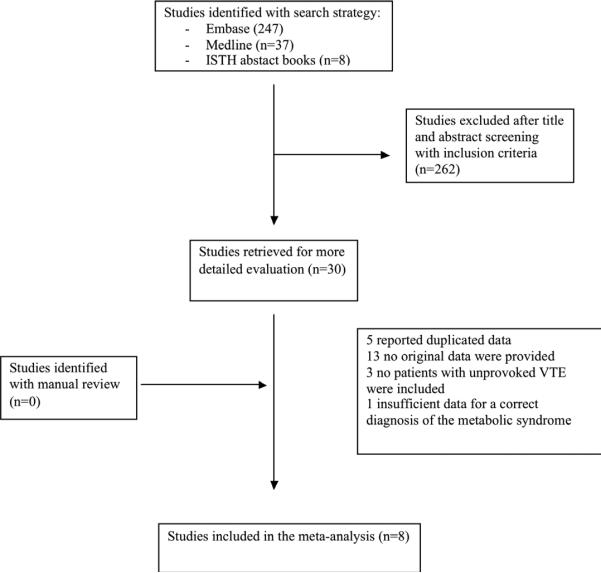
Process of study selection
Table 1 summarizes the characteristics of the six case-control studies included in the analysis and Table 2 summarizes the characteristics of the two prospective cohort studies. Briefly, among case-control studies, one study included patients with deep vein thrombosis only (15), while the remaining studies included patients with both deep vein thrombosis and pulmonary embolism (17,18,21,22,23). Five studies enrolled patients with a single episode of VTE (15,18,21,22,23), and one study enrolled patients with recurrent VTE (17). One study was conducted in Asian patients only (18), and the other five studies enrolled Caucasian patients (15,17,21,22,23). Mean age of study patients in case-control studies was under 50 years in four studies (17,18,21,22), between 50 and 60 years in one study (23) and over 60 years in one study (15). According to our predefined quality score for case-control studies, two studies scored 6, thus were high-quality (18,22), four studies scored 5, thus were medium-quality (15,17,21,23). According to our predefined quality score for cohort studies, both studies scored 6 and were thus high-quality (19,20)
TABLE 1.
Description of case-control studies included in the meta-analysis
| Study (reference) | Inclusion criteria | Definition of unprovoked VTE | Timing between VTE and assessment of MS | Quality of studies |
|---|---|---|---|---|
| Ageno 2006 (15) | Cases: consecutive patients with DVT Controls: suspected and objectively excluded DVT. Negative history of VTE or cancer |
Absence of surgery, trauma, fracture, acute medical disease, pregnancy, immobilization, oral contraceptives, cancer | Less than 6 months | 5 |
| Ay 2007 (17) | Cases: patients with recurrent VTE, at least one unprovoked Controls: healthy, unrelated individual with negative history of VTE |
Absence of surgery, trauma with immobilization, pregnancy, cancer | Median 2.55 years | 5 |
| Jang 2009 (18) | Cases: consecutive patients with VTE Controls: Individuals attending an health center for periodic health examination. Negative history of VTE or cancer |
Absence of recent surgery, trauma, fracture, immobilization, severe medical disease, pregnancy, oral contraceptives, known hypercoagulable disease, cancer | 6 months | 6 |
| Vayà 2011 (21) | Cases: patients with VTE (VTE related to pregnancy or cancer excluded) Controls: healthy, unrelated individuals with negative history of VTE |
Absence of surgery, immobilization, oral contraceptives and other non-defined risk factors. | 3 to 6 months | 5 |
| Di Minno 2011 (22) | Cases: consecutive patients aged <50 years with unprovoked VTE Controls: healthy subjects with negative history of VTE or cancer |
Absence of pregnancy, cancer, surgery, trauma, fracture, immobilization, acute medical illness, oral contraceptives, long distance travel | Less than 6 months | 6 |
| Rattazzi 2013 (23) | Cases: patients with at least one episode of VTE Controls: healthy, unrelated individuals with negative history of VTE |
Absence of surgery, trauma, fracture, immobilization, acute medical disease, oral contraceptives, hormonal replacement therapy, or pregnancy | Median 60 months | 5 |
DVT: Deep vein thrombosis; VTE: Venous thromboembolism; MS: Metabolic syndrome
TABLE 2.
Description of prospective cohort studies included in the meta-analysis
| Study (reference) | Inclusion criteria | Definition of unprovoked VTE | Mean time between assessment of VTE and MS | Quality of studies |
|---|---|---|---|---|
| Borch 2009 (19) | Population study of individuals assessed for the MS and with no prior VTE | Absence of major surgery, trauma, or acute medical condition within 8 weeks prior to event; active cancer at time of event, immobilization within 14 days prior to event | 6.7 years | 6 |
| Steffen 2009 (20) | Population study of individuals assessed for the MS and with no prior VTE | Absence of surgery, trauma, recent hospitalization, or severe immobility. Cancer related VTE excluded patients from analyses | 7.6 years | 6 |
DVT: Deep vein thrombosis; VTE: Venous thromboembolism; MS: Metabolic syndrome
In prospective cohort studies the mean time elapsed between the measurement of the features of the metabolic syndrome and VTE events was 7.6 years (range 0.6-15.1) (19) in one study and 6.7 years (range 0.2-12.7 years) in the second study (20). Information was collected on both deep vein thrombosis and pulmonary embolism (19,20). At baseline, mean age of study subjects was 58 years in one study (19) and between 60 and 63 years (metabolic syndrome negative and positive patients, respectively) in the second study (20). The definition of unprovoked VTE slightly differed among all selected studies (Tables 1 and 2).
Results of the analysis of case-control studies
We initially analyzed aggregate data, and the presence of the metabolic syndrome was significantly associated with VTE with an OR 2.64 (95% CI 2.19-3.18). There was no heterogeneity among the studies (I2=0%) (Figure 2).
Figure 2.
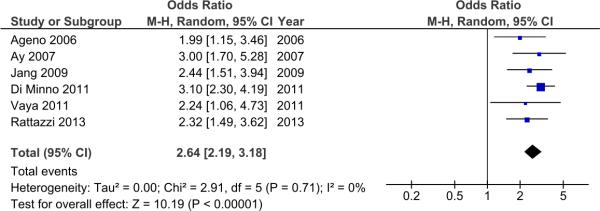
Forest plot of case control studies
We subsequently carried out the patient level analysis. Overall, we received data for 908 patients with unprovoked VTE and 1794 controls. No cases or controls were excluded from the analysis for insufficient data. Baseline characteristics of the 2702 cases and controls enrolled in this study are summarized in Table 3. The two groups were significantly different according to gender, mean age and the prevalence of obesity. Dyslipidemia was significantly more prevalent in cases of unprovoked VTE than in controls. The metabolic syndrome and each of its components was also significantly more prevalent in the group of cases than controls.
TABLE 3.
Baseline characteristics of the study population and prevalence of the metabolic syndrome and of its components in case-control studies
| Total population | VTE patients | Controls | P | |
|---|---|---|---|---|
| Number | 2702 | 908 | 1794 | - |
| Males, n (%) | 1162 (43.0) | 475 (52.3) | 687 (38.3) | <0.001 |
| Mean age, y (SD) | 51.4 (14.3) | 52.7 (15.3) | 50.7 (13.7) | 0.001 |
| Obesity*, n (%) | 686 (25.4) | 313 (34.5) | 373 (20.8) | <0.001 |
| Cholesterol >200 mg/dL°, n (%) | 1253 (46.4) | 475 (52.3) | 778 (43.4) | <0.001 |
| LDL cholesterol >160 mg/dL°, n (%) | 548 (20.3) | 257 (28.3) | 291 (16.2) | <0.001 |
| Metabolic syndrome, n (%) | 703 (26.0) | 341 (37.6) | 362 (20.2) | <0.001 |
| Abdominal obesity†, n (%) | 1066 (39.5) | 466 (51.3) | 600 (33.4) | <0.001 |
| Fasting glucose ≥100 mg/dL°, n (%) | 720 (26.6) | 314 (34.6) | 406 (22.6) | <0.001 |
| Blood pressure ≥130/85 mmHg°, n (%) | 1052 (38.9) | 440 (48.5) | 612 (34.1) | <0.001 |
| HDL cholesterol <40/50 mg/dL°, n (%) | 773 (28.6) | 365 (40.2) | 504 (28.1) | <0.001 |
| Triglycerides≥150 mg/dL°, n (%) | 641 (23.7) | 283 (31.2) | 358 (20.0) | <0.001 |
VTE: venous thromboembolism, SD: standard deviation
Caucasian patients: BMI > 30 Kg/m2 Asian patients: BMI > 25 Kg/m2
Caucasian patients: waist circumference > 102 cm for men and > 88 cm for women; Asian patients: waist circumference > 90 cm for men and > 80 cm for women
Patients currently receiving drug therapy for hypertension, diabetes, or dyslipidemia (only statins) were defined as having those disorders.
In our subgroup analyses, the OR for VTE for metabolic syndrome in men was 1.88 (95% CI 1.46-2.42), and in women 2.77 (95% CI 2.16-3.56). The OR for VTE with metabolic syndrome in patients younger than 50 years was 1.99 (95% CI 1.57-2.53), and in patients aged 50 years or older 2.07 (95% CI 1.61-2.66). Heterogeneity between male and female patients was significant (chi-square 4.63, p for interaction =0.03), whereas there was no statistically significant difference according to age (p for interaction >0.10).
In multivariate logistic regression analysis, the metabolic syndrome remained associated with odds of VTE after adjusting for age, sex and obesity, with an OR of 1.91 (95% CI 1.57-2.33) (Table 4). The analysis was repeated after the exclusion of the study by Ay et al (17), because it was the only study including patients with recurrent VTE only. The results were unchanged (data not shown, available upon request). The association between the metabolic syndrome and VTE was significant both in male and in female patients (OR 1.61 95% CI 1.22-2.20 and 2.32, 95% CI 1.74-3.09, respectively) after adjusting for age and obesity. The observed association remained statistically significant when the multivariate analysis was repeated after the exclusion of patients with increased waist circumference with an OR of 2.19 (95% CI 1.51-3.16) after adjusting for age and obesity.
TABLE 4.
Results of multi-adjusted regression models of case-control studies on the association between the metabolic syndrome, its components and VTE
| Metabolic syndrome and VTE | ||
|---|---|---|
| Risk factor | OR | 95% CI |
| Metabolic syndrome | 1.91 | 1.57-2.33 |
| Age | 1.00 | 0.99-1.01 |
| Obesity* | 1.56 | 1.28-1.89 |
| Male sex | 1.67 | 1.42-1.97 |
| Components of the metabolic syndrome and VTE | ||
| Abdominal obesity† | 1.66 | 1.36-2.02 |
| HDL cholesterol <40/50 mg/dL° | 1.48 | 1.24-1.77 |
| Blood pressure ≥130/85 mmHg° | 1.41 | 1.17-1.70 |
| Fasting glucose ≥100 mg/dL° | 1.26 | 1.04-1.53 |
| Triglycerides≥150 mg/dL° | 1.24 | 1.02-1.51 |
| Age | 1.00 | 0.99-1.01 |
| Obesity* | 1.22 | 0.99-1.52 |
| Male sex | 1.64 | 1.38-1.94 |
Caucasian patients: BMI > 30 Kg/m2 Asian patients: BMI > 25 Kg/m2
Caucasian patients: waist circumference > 102 cm for men and > 88 cm for women; Asian patients: waist circumference > 90 cm for men and > 80 cm for women
Patients currently receiving drug therapy for hypertension, diabetes, or dyslipidemia (only statins) were defined as having those disorders.
Results of multi-adjusted regression models on the association of the individual components of the metabolic syndrome and VTE are reported in Table 4.
Results of the analysis of prospective cohort studies
We initially analyzed aggregate data, and the presence of the metabolic syndrome was significantly associated with VTE, with an OR 1.15 (95% CI 0.90-1.47). There was no heterogeneity among the studies (I2=0%)(Figure 3). We subsequently carried out the patient level analysis. Overall, we received data on 26,544 subjects who had 552 VTE events during follow up. Excluding 13 subjects with incomplete data and considering only unprovoked events, 26,531 participants had 289 events during follow-up.
Figure 3.

Forest plot of cohort studies
Baseline characteristics of patients who developed and who did not develop unprovoked VTE during follow-up are summarized in Table 5. The two groups were significantly different according to mean age, prevalence of obesity, abdominal obesity and hypertension, whereas other baseline characteristics and prevalence of metabolic syndrome were similar in the two groups.
TABLE 5.
Baseline characteristics of the study population and prevalence of the metabolic syndrome and of its components in cohort studies
| Total population | Patients with VTE | No VTE | P | |
|---|---|---|---|---|
| Number | 26531 | 289 | 26242 | - |
| Males, n (%) | 11986 (45.2) | 128 (44.3) | 11858 (45.2) | 0.77 |
| Mean age, y (SD) | 59.4 (10.0) | 63.3 (8.9) | 59.3 (10.1) | <0.001 |
| Obesity*, n (%) | 5987 (22.6) | 105 (36.3) | 5882 (22.4) | <0.001 |
| Cholesterol >200 mg/dL°, n (%) | 12142 (45.8) | 126 (43.6) | 12016 (45.8) | 0.46 |
| LDL cholesterol >160 mg/dL°, n (%) | 4742 (17.9) | 44 (15.2) | 4698 (18.9) | 0.24 |
| Metabolic syndrome, n (%) | 9720 (36.6) | 113 (39.1) | 9607 (36.6) | 0.38 |
| Abdominal obesity†, n (%) | 11724 (44.2) | 172 (59.5) | 11552 (44.0) | <0.001 |
| Fasting glucose ≥100 mg/dL°, n (%) | 10507 (39.6) | 115 (39.8) | 10392 (39.6) | 0.92 |
| Blood pressure ≥130/85 mmHg°, n (%) | 14740 (55.6) | 187 (64.7) | 14553 (55.5) | 0.002 |
| HDL cholesterol <40/50 mg/dL°, n (%) | 9282 (35.0) | 88 (30.4) | 9194 (35.0) | 0.11 |
| Triglycerides≥150 mg/dL°, n (%) | 8206 (30.9) | 81 (28.0) | 8125 (31.0) | 0.26 |
VTE: venous thromboembolism, SD: standard deviation
Caucasian patients: BMI > 30 Kg/m2 Asian patients: BMI > 25 Kg/m2
Caucasian patients: waist circumference > 102 cm for men and > 88 cm for women; Asian patients: waist circumference > 90 cm for men and > 80 cm for women
Patients currently receiving drug therapy for hypertension, diabetes, or dyslipidemia (only statins) were defined as having those disorders
In the random effects Cox model age ≥ 65 years (HR 1.80, 95% CI 1.39, 2.34), obesity (HR 1.56, 95% CI 1.15, 2.11), and abdominal obesity (HR 1.55, 95% CI 1.15, 2.08) were significantly associated with the development of unprovoked VTE during follow-up, whereas hypertension was not associated (HR 1.12, 95% CI 0.86-1.46). The metabolic syndrome was not significantly associated with an increased risk of developing VTE when added to a multivariate model with obesity and age ≥ 65 years. The metabolic syndrome was also not significantly associated with an increased risk of VTE in a multivariate model that included all other covariates that were significantly associated with VTE in univariate analysis or considering the pre-specified subgroups according to age and sex (data not shown). Given the absence of association between the metabolic syndrome and VTE, no further analysis on the additive role of the individual components was performed.
DISCUSSION
The results of this patient-level meta-analysis do not convincingly support the role of the metabolic syndrome as an independent risk factor for VTE, but support the role of abdominal adiposity, as assessed by waist circumference, as a strong risk factor for VTE. The association between the metabolic syndrome and unprovoked VTE was confirmed in the analysis of case-control studies in different patient subgroups, but not in the analysis of prospective cohort studies. Likewise, individual components of the metabolic syndrome were also independently associated with VTE, and their coexistence resulted in an additive effect in case-control studies, but not in longitudinal studies, with the exception of abdominal obesity.
The fact that the importance of the co-clustering of the various components of the metabolic syndrome or their complete expression did not play a role in prospective studies on VTE occurrence may suggest a minor contribution of the “atherogenic” pattern found in the metabolic syndrome to the pathogenesis of VTE. Conversely, the state of chronic, low-grade inflammation that is associated with the metabolic syndrome, but that most of all depends on the presence of abdominal adiposity, may result in a prothrombotic state that predisposes to VTE. Both chronic inflammation and insulin resistance, which plays a key role in the pathogenesis of the syndrome, are associated with increased levels of fibrinogen and low grade chronic inflammation has also been associated with increased release of soluble tissue factor and factor VII (24). The simultaneous increase in both soluble tissue factor and factor VII clearly enhances the risk of activation of the coagulation cascade. Furthermore, increased levels of plasminogen activator inhibitor-1 and decreased plasma tissue plasminogen activator activity are common in these patients, thus leading to a hypofibrinolytic state (24). Recent studies have shown that plasminogen, thrombin-activatable fibrinolysis inhibitor, plasminogen activator inhibitor-1, and tissue plasminogen activator are all associated with the risk of venous thrombosis, thus supporting the potential relevance of hypofibrinolysis as a link between VTE and both abdominal obesity and the metabolic syndrome (26,26). Furthermore, hypofibrinolysis was also reported to be associated with an increased risk of recurrent VTE (27). Taken together, these coagulation abnormalities have the potential to predispose patients to an increased risk of VTE. This hypothesis was initially suggested by the results of case-case control studies (15-18,21,22,23), and to a much lesser extent, by the results of prospective cohort studies (19,20). In fact, the main finding of one of the two longitudinal studies, the Tromso study, was that abdominal obesity is a necessary component for the association between the metabolic syndrome and VTE, because after the removal of abdominal obesity this association is lost. This observation, substantially confirmed by the results of the present study, is relevant because abdominal adipose tissue, which is best viewed as an endocrine organ (28), plays a central role in the etiology of the metabolic syndrome and in predisposing to the chronic inflammatory state. For this reason, the International Diabetes Federation proposed the presence of abdominal obesity as a necessary condition for the diagnosis of the metabolic syndrome (29). In the second longitudinal study, the LITE study, this finding was observed in men only (20). In this study, abdominal obesity, but not the metabolic syndrome was related to VTE in women. In men, the metabolic syndrome was associated with VTE, but when abdominal obesity was removed, this association between the metabolic syndrome and VTE was no longer statistically significant. Gender related differences have been reported by some authors also for the association between the metabolic syndrome and cardiovascular disease (30,31), but have not been confirmed by other studies, and the mechanisms underlying this finding have not been elucidated. The results of our individual patient data meta-analysis of case-control studies confirmed a statistically significant interaction by gender, but, differently from the study by Steffen et al (20), the association between the metabolic syndrome and VTE, with or without abdominal obesity, remained significant in both males and females. This association could not be confirmed when the individual patients data of the two prospective cohort studies were analysed.
The reasons for the observed discrepancies are, at least in part, attributable to the study designs. In the case-control studies included in our meta-analysis, the components of the metabolic syndrome were measured within 6 months after VTE in four studies (15,18,21,22), and after a median of 2.5 years and 60 months in two studies (17,23). In the longitudinal studies, the features of the metabolic syndrome were measured at baseline and VTE events occurred after years (19,20). Because all components of the metabolic syndrome are modifiable, and individuals with a higher cardiovascular risk profile at baseline may have been managed more aggressively, there is a chance that the effect of some of the metabolic risk factors may have been diluted at the time of the VTE event. Regular physical activity and a healthy diet are key elements for the primary intervention in patients with the metabolic syndrome and have been shown to reduce the incidence of VTE in some (32,33), but not all studies (34,35). Likewise, statins are among the main therapeutic strategies in patients with the metabolic syndrome and have also been recently shown to reduce the risk of VTE (36). Another potential explanation for the observed discrepancies is that the prevalence of the metabolic syndrome increases with increasing age (37,38), and the distribution of risk factors may have changed in longitudinal studies over time between patients who subsequently developed VTE and patients without VTE. Of note, in four of the five case-control studies (17,18,21,22), the mean age of study patients ranged between 41 and 47 years, and only in one study the mean age of patients with VTE was 63 (15). Conversely, the mean age at baseline in the two prospective cohort studies was around 60 years (19,20), and, thus, VTE occurred in these studies at an older age compared to the case-control studies. However, in the case-control studies we separately assessed the association between the metabolic syndrome and VTE in patients aged 50 years or younger and in patients older than 50 years, this association remained statistically significant in both groups and the magnitude of the association was actually greater in older persons. Finally, a third explanation of the discrepancy between study designs is that the retrospective nature of case-control studies may have contributed to overestimation of the magnitude of the observed associations. In addition, also the time elapsed between VTE and the measurement of the components of the metabolic syndrome in the case-control studies included in our analysis might have contributed to bias, since obesity is a known risk factor for VTE and weight loss counselling after VTE in overweight individuals might have been proposed to at least some of the patients. On the other hand, reduced mobility following acute VTE may have favoured weight gain with consequent increased waist circumference and changes in lipid profile, thus resulting in a revers association.
There are several limitations to our study. First, inclusion criteria and the definition of unprovoked VTE differed among studies. When the analysis was repeated for case control-studies with the exclusion of the study that used different inclusion criteria (patients with recurrent VTE as opposed to patients with a first episode) (17), the results were unchanged. With regard to the different definitions of unprovoked VTE, these mainly related to the inclusion of hormone-associated VTE in two studies (17,21) and of long distance travel in another study (22). However, both hormone-therapy and long distance travel are considered as weak risk factors for VTE, and it is unlikely that the low number of patients included with either one of these risk factors may have significantly affected our results. Second, one of the studies was only carried out in Asian patients. To reduce the potential bias of ethnical differences, we used ethnic-specific definitions of obesity and components of the metabolic syndrome (39,40).
In conclusion, only the individual patient data meta-analysis of case-control studies confirmed an association between unprovoked VTE and the metabolic syndrome. This association was not present in longitudinal studies. Conversely, the meta-analysis of both case-control and longitudinal studies supports the role of abdominal adiposity as a strong risk factor for VTE. The modifiable nature of the individual components of the metabolic syndrome, but also bias that are intrinsic in the design of case-control studies may explain this discrepancy. Future studies that are specifically designed to address this issue taking into account all potential sources of bias are warranted. Furthermore, the role of the metabolic syndrome in the pathogenesis of VTE secondary to traditional major risk factors should also be explored.
Supplementary Material
SIGNIFICANCE.
This very large individual patient data meta-analyses on more than 30,000 subjects explored the association between the metabolic syndrome and its individual components and venous thromboembolism. The study provides some important confirmation on the role of obesity and visceral obesity as risk factors for venous thrombosis, with important clinical implications for both the primary and the secondary prevention. The results of this meta-analysis do not fully support the role of the metabolic syndrome, given the discrepancy between the results of case-control studies and prospective cohort studies. The modifiable nature of the individual components of the metabolic syndrome, but also bias that are intrinsic in the design of case-control studies may explain this discrepancy and suggest the need for additional studies that are specifically designed to address this clinical question.
Acknowledgements
Study concept and design: Ageno, Dentali, Squizzato; Acquisition of data: Ageno, Dentali, MND Di Minno, Ay, Jang, Steffen, Rattazzi, Braekkan, Bonet; Analysis and interpretation of data: Ageno, Dentali, Hansen, Steffen, Vayà, Pabinger, Oh, G Di Minno, Cushman, Pauletto, Squizzato; Drafting of the manuscript: Ageno, Dentali, Squizzato; Critical revision of the manuscript for important intellectual content: MND Di Minno, Ay, Hansen, Steffen, Vayà, Rattazzi, Pabinger, Oh, G Di Minno, Cushman, Pauletto, Squizzato, Dentali; Final approval of the article: Ageno, MND Di Minno, Ay, Jang, Hansen, Steffen, Vayà, Rattazzi, Pabinger, Oh, G Di Minno, Braekkan, Cushman, Bonet, Pauletto, Squizzato, Dentali. Ageno and Dentali had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of all the data analysis.
Sources of funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) and the Longitudinal Investigation of Thromboembolism Etiology was funded by grant HL59367 from the NHLBI. CHS research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank the staff and participants of the ARIC and CHS studies for their important contributions.
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- VTE
Venous thromboembolism
- OR
Odds Ratio
- HR
Hazard Ratio
Footnotes
Disclosure
The authors have no conflicts of interest to disclose related this manuscript
REFERENCES
- 1.Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, Melton LJ Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 2.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 3.Agnelli G, Verso M, Ageno W, Imberti D, Moia M, Palareti G, Rossi R, Pistelli R. MASTER investigators. The MASTER registry on venous thromboembolism: description of the study cohort. Thromb Res. 2008;121:605–610. doi: 10.1016/j.thromres.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Becattini C, Agnelli G, Prandoni P, Silingardi M, Salvi R, Taliani MR, Poggio R, Imberti D, Ageno W, Pogliani E, Porro F, Casazza F. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J. 2005;26:77–83. doi: 10.1093/eurheartj/ehi018. [DOI] [PubMed] [Google Scholar]
- 5.Prandoni P, Ghirarduzzi A, Prins MH, Pengo V, Davidson BL, Sorensen H, Pesavento R, Iotti M, Caviglia E, Iliceto S, Pagnan A, Lensing AWA. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost. 2006;4:1891–1896. doi: 10.1111/j.1538-7836.2006.02058.x. [DOI] [PubMed] [Google Scholar]
- 6.Schulman S, Lindmarker P, Holmstrom M, Larfars G, Carlsson A, Nicol P, Svensson E, Ljungberg B, Viering S, Nordlander S, Leijd B, Jahed K, Hjorth M, Linder O, Beckman M. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4:734–742. doi: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 7.Bova C, Marchiori A, Noto A, Rossi V, Daniele F, Santoro C, Ricchio R, De Lorenzo R, Umbaca R, Prandoni P. Incidence of arterial cardiovascular events in patients with idiopathic venous thromboembolism. A retrospective cohort study. Thromb Haemost. 2006;96:132–136. [PubMed] [Google Scholar]
- 8.Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370:1773–1779. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 9.Becattini C, Vedovati MC, Ageno W, Dentali F, Agnelli G. Incidence of arterial cardiovascular events after venous thromboembolism: a systematic review and a meta-analysis. J Thromb Haemost. 2010;8:891–897. doi: 10.1111/j.1538-7836.2010.03777.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansson PO, Eriksson H, Welin L, Svardsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the Study of Men Born in 1913”. Arch Intern Med. 1999;159:1886–1890. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 11.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 12.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2496. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Ageno W, Prandoni P, Romualdi E, Ghirarduzzi A, Dentali F, Pesavento R, Crowther M, Venco A. The metabolic syndrome and the risk of venous thrombosis. A case control study. J Thromb Haemost. 2006;4:1914–1918. doi: 10.1111/j.1538-7836.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosetti M, Ageno W, Salerno M, Pedretti RF, Salerno-Uriarte JA. Metabolic syndrome as a risk factor for deep vein thrombosis after acute cardiac conditions. Thromb Res. 2007;120:815–818. doi: 10.1016/j.thromres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Ay C, Tengler T, Vormittag R, Simanek R, Dorda W, Vukovich T, Pabinger I. Venous thromboembolism – a manifestation of the metabolic syndrome. Haematologica. 2007;92:373–379. doi: 10.3324/haematol.10828. [DOI] [PubMed] [Google Scholar]
- 18.Jang MJ, Choi W, Bang SM, Lee T, Kim YK, Ageno W, Oh D. Metabolic Syndrome Is Associated With Venous Thromboembolism in the Korean Population. Atheroscl Thromb Vasc Biol. 2009;29:311–315. doi: 10.1161/ATVBAHA.109.184085. [DOI] [PubMed] [Google Scholar]
- 19.Borch K, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome – The Tromso Study. J Thromb Haemost. 2009;7:739–745. doi: 10.1111/j.1538-7836.2008.03234.x. [DOI] [PubMed] [Google Scholar]
- 20.Steffen LM, Cushman M, Peacock JM, Heckbert SR, Jacobs DR, Rosamond WD, Folsom AR. Metabolic syndrome and risk of venous thromboembolism: Longitudinal Investigation of Thromboembolism Etiology (LITE). J Thromb Haemost. 2009;7:746–751. doi: 10.1111/j.1538-7836.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vayà A, Martinez-Triguero ML, Espana F, Todolì JA, Bonet E, Corella D. The metabolic syndrome and its individual components: its association with venous thromboembolism in a Mediterranean population. Metab Syndr Relat Disord. 2011;9:197–201. doi: 10.1089/met.2010.0117. [DOI] [PubMed] [Google Scholar]
- 22.Di Minno MND, Tufano A, Guida A, Di Capua M, De Gregorio AM, Cerbone AM, Tarantino G, Di Minno G. Abnormally high prevalence of major components of the metabolic syndrome in subjects with early-onset idiopathic venous thromboembolism. Thromb Res. 2011;127:193–197. doi: 10.1016/j.thromres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Rattazzi M, Villalta S, Galliazzo S, et al. Low CD34(+) cells, high neutrophils and the metabolic syndrome are associated with an increased risk of venous thromboembolism. Clin Sci (Lond) 2013;125:211–218. doi: 10.1042/CS20120698. [DOI] [PubMed] [Google Scholar]
- 24.Dentali F, Squizzato A, Ageno W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin Thromb Hemost. 2009;35:451–457. doi: 10.1055/s-0029-1234140. [DOI] [PubMed] [Google Scholar]
- 25.Lisman T, de Groot PhG, Meijers JCM, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer ME, Lisman T, de Groot PG, Meijers JC, le Cessie S, Doggen CJ, Rosendaal FR. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–121. doi: 10.1182/blood-2010-02-267740. [DOI] [PubMed] [Google Scholar]
- 27.Meltzer ME, Bol L, Rosendaal FR, Lisman T, Cannegieter SC. Hypofibrinolysis as a risk factor for recurrent venous thrombosis; results of the LETS follow-up study. J Thromb Haemost. 2010;8:605–607. doi: 10.1111/j.1538-7836.2009.03715.x. [DOI] [PubMed] [Google Scholar]
- 28.Opie LH. Metabolic syndrome. Circulation. 2007;115:32–35. doi: 10.1161/CIRCULATIONAHA.106.671057. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 30.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ. Metabolic syndrome and 10-year cardiovascular disease in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 32.van Stralen KJ, Le Cessie S, Rosendaal FR, Doggen CJ. Regular sports activities decrease the risk of venous thrombosis. J Thromb Haemost. 2007;5:2186–2192. doi: 10.1111/j.1538-7836.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- 33.Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the longitudinal investigation of thromboembolism etiology. Circulation. 2007;115:188–195. doi: 10.1161/CIRCULATIONAHA.106.641688. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost. 2012;10:189–198. doi: 10.1111/j.1538-7836.2011.04588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen-Krone IJ, Enga KF, Njølstad I, Hansen JB, Braekkan SK. Heart healthy diet and risk of myocardial infarction and venous thromboembolism. The Tromsø Study. Thromb Haemost. 2012;108:554–560. doi: 10.1160/TH11-11-0818. [DOI] [PubMed] [Google Scholar]
- 36.Squizzato A, Galli M, Romualdi E, Dentali F, Kamphuisen PW, Guasti L, Venco A, Ageno W. Statins, fibrates and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248–1256. doi: 10.1093/eurheartj/ehp556. [DOI] [PubMed] [Google Scholar]
- 37.Alexander CM, Landsman PB, Grundy SM. The influence of age and body mass index on the metabolic syndrome and its components. Diabetes Obes Metab. 2008;10:246–250. doi: 10.1111/j.1463-1326.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 38.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factors findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Asia–Pacific perspective: redefining obesity and its treatment. Australia: 2000. Steering Committee of the WHO Western Pacific Region, IASO & IOTF. [Google Scholar]
- 40.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC., Jr Harmonizing the Metabolic Syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


