Abstract
Various pharmaceutical particles have been used in developing different drug delivery systems ranging from traditional tablets to state-of-the-art nanoparticle formulations. Nanoparticle formulations are unique in that the small size with huge surface area sometimes provides unique properties that larger particles and bulk materials do not have. Nanoparticle formulations have been used in improving the bioavailability of various drugs, in particular, poorly soluble drugs. Nanoparticle drug delivery systems have found their unique applications in targeted drug delivery to tumors. While nanoparticle formulations have been successful in small animal xenograft models, their translation to clinical applications has been very rare. Developing nanoparticle systems designed for targeted drug delivery, e.g., treating tumors in humans, requires clear understanding of the uniqueness of nanoparticles, as well as limitations and causes of failures in clinical applications. It also requires designing novel smart nanoparticle delivery systems that can increase the drug bioavailability and at the same time reduce the drug's side effects.
Keywords: Nanoparticle, Targeted drug delivery, Poorly soluble drug, Polymer micelle, Liposome, nanocrystal
1. Introduction
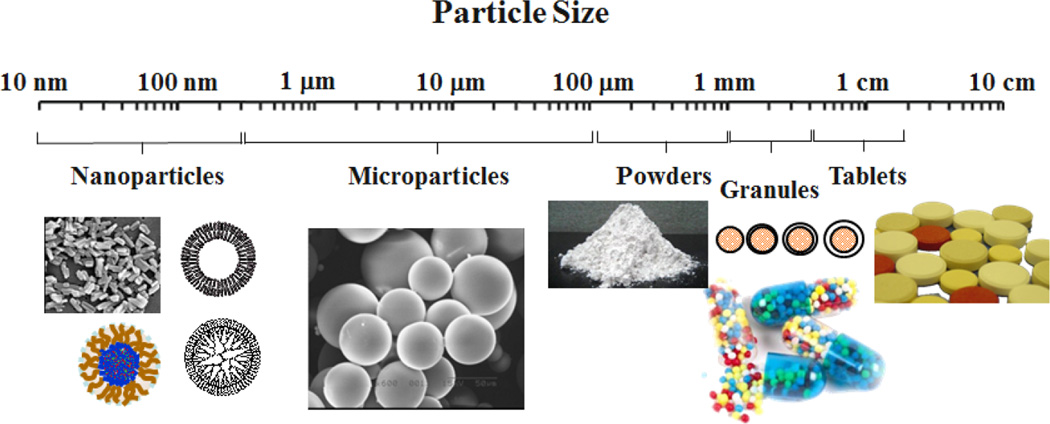
Pharmaceutical particles include a variety of sizes and shapes, ranging from traditional tablets and granules to microparticles and nanoparticles. The relative sizes of commonly used pharmaceutical particles are shown in Fig. 1. Tablets are most well-known and accepted formulations with a long history. Powders are processed and granules are made to make tablet formulations. Quite frequently, however, granules are used to make formulations different from traditional immediate release tablets. Drug-containing granules can be mixed or coated with pharmaceutical polymers to render them with delayed release or sustained release properties. In fact, the first sustained release drug delivery systems were made in 1952 by coating drug-containing cores with a polymer of varying thicknesses [1]. Microparticle and nanoparticle formulations are a more recent development in drug delivery. Microparticles are used to make long-term (i.e., weeks to months) depot formulations that can be injected by subcutaneous or intramuscular routes. The polymers used for long-term microparticle formulations are biodegradable so that the microparticles do not have to be removed after its lifetime is over, i.e., once all loaded drug is released. The most widely used biodegradable polymer is poly(lactic-co-glycolic acid) (PLGA). For more than a decade, nanoparticles have been used for developing formulations with special features, and the research on the nanoparticle-based drug delivery systems has dominated the literature. While significant advances have been made, the current nanoparticle-based formulations require drastic improvements to achieve their intended goals of developing unique delivery systems that others could not have achieved.
Fig. 1.
Relative sizes of various pharmaceutical particles ranging from nanoparticles to tablets.
Recent review articles describe many aspects of nanoparticles, such as history, advances, advantages, and potentials [2–10]. All nanoparticle-based drug delivery systems were developed largely by trial-and-error approach in a long chain of case-by-case studies without a rational formulation design [11]. While promises and potentials have been the main topics of most review articles, the real progress requires a clear understanding of the current status, mainly limitations, of nanoparticle technologies. Without defining the problem, its solution will not be found. The objective of this article is to examine the promises, in the context of limitations, of nanoparticles used in the drug delivery field. In particular, the current misconceptions blocking faster progress are discussed. The majority of the articles in the literature on nanoparticles deal with targeted drug delivery to tumors, only one aspect of numerous drug delivery technologies. To realize breakthroughs in the targeted drug delivery area as well as in other equally important areas, the strength and limitations of the current nanoparticle technology need to be carefully evaluated for opening up new opportunities.
2. Nanoparticle: Definition
The term "nanoparticle" has become fashionable and almost all scientific literature deals with nanoparticles in one way or another. In the drug delivery area, the first nanoparticles of 100 nm diameter were made of poly(methyl methacrylate) as a new adjuvant in 1976 [12]. Since then, literally hundreds of thousands of articles deal with nanoparticles, and yet, the clear definition of nanoparticles is lacking. According to the National Nanotechnology Initiative (www.nano.gov), nanotechnology is utilizing the unique physical, chemical, mechanical, and optical properties of materials that naturally occur at the nanoscale, i.e., the dimensions between approximately 1 and 100 nm. Both International Organization of Standardization (ISO) and American Society for Testing Materials (ASTM) have provided their definitions of nanoparticles which are practically the same [13, 14]. A nanoparticle is defined as a nano-object with all three dimensions in the size range from approximately 1 nm to 100 nm. Thus, nanoparticles are those within this size range. The IUPAC also defines nanoparticle as a particle of any shape with dimensions in the 1~100 nm range [15], however, there is no specific reason to use 100 nm as the size that separates nanoparticles from non-nanoparticles [16]. The only guiding principle of differentiating nanoparticles is that novel properties which bulk materials typically do not have can be developed, if the size is below 100 nm. Also, included in the IUPAC definition of nanoparticle is when the objects with only two dimensions are below 100 nm, e.g., tubes and fibers [15]. Thus, the definition of nanoparticle is not really based on the exact size of the particles, but rather depends on whether nanoparticles have novel properties that non-nanoparticles of the same material do not have.
2.1. Novel properties of nanoparticles
The fascination on the novel properties of nanoparticles mainly stems from the fact that nanoparticles have a huge surface area as compared with microparticles or other bulk materials. The assumption that goes together with this huge surface area is that the properties of nanoparticles are very different from larger particles. The relatively significant amount of atoms and molecules on the surface of nanoparticles is expected to bring interesting new properties. But the question is whether there have been any really interesting and unexpected properties that only nanoparticles have while their bigger counterparts do not. These novel properties should not include those which are already well known through traditional colloid chemistry. For example, colloidal gold particles have been made since the days of Michael Faraday in the middle of the 19th century [17], and it has been well known that the color of colloidal gold particles changes depending on the size of the gold particles. If such a well-known phenomenon is considered a representation of a novel property of nanoparticles, then current nanoparticles in general really do not provide any unique properties. Likewise, the nanoparticles that are supposed to have novel properties are not really new. Thus, the question is what novel properties do nanoparticles provide that have not been known. This question is important in applications of nanoparticles to the pharmaceutical industry, in particular, drug delivery systems where drug-loaded nanoparticles are usually larger than 100 nm. The current fever in nanoparticles is largely based on the assumed, yet unrecognized, novel properties.
2.2. Advantages of nanoparticles over small molecules
Although the nanoparticle itself may not possess any novel properties, nanoparticle formulations could provide new properties that may benefit drug delivery. Nanoparticles are distinguished from small molecules which represent free drugs that are not incorporated into nanoparticle systems. Nanoparticle delivery systems are designed and tested for the ultimate goal of developing clinically useful formulations to treat various diseases. Thus, the unique properties of nanoparticles need to be considered in the context of treating diseases, i.e., improving efficacy and safety. If nanoparticles indeed deliver more drugs to the target site as compared with the control, it should be able to lower the required doses of drugs, which in turn should result in reduced toxic side effects [18, 19]. Another beneficial property of nanoparticles is to improve the water solubility of poorly soluble drugs [2]. Many anticancer drugs, e.g., paclitaxel, are poorly water soluble. Making them into nanoparticle formulations can increase their water-solubility without using undesirable excipients, such as Cremophor EL or polysorbate which are used in Taxol® and Taxotere® formulations, respectively. Increase in water solubility without using harmful excipients is also expected to increase the safety, which in itself is a sufficient reason to use it, even if the efficacy is not improved. Other potentially beneficial properties of nanoparticles include fine tuning of the size less than 100 nm to evade macrophages in the reticuloendothelial system, surface modification to prolong the blood circulation time, enhancing the interaction with binding to the target cells, or delivery of multiple drugs in the same formulation [3]. These properties, however, have to be evaluated in terms of efficacy and safety of the formulation as a whole.
3. Nanoparticles in Pharmaceutical Applications
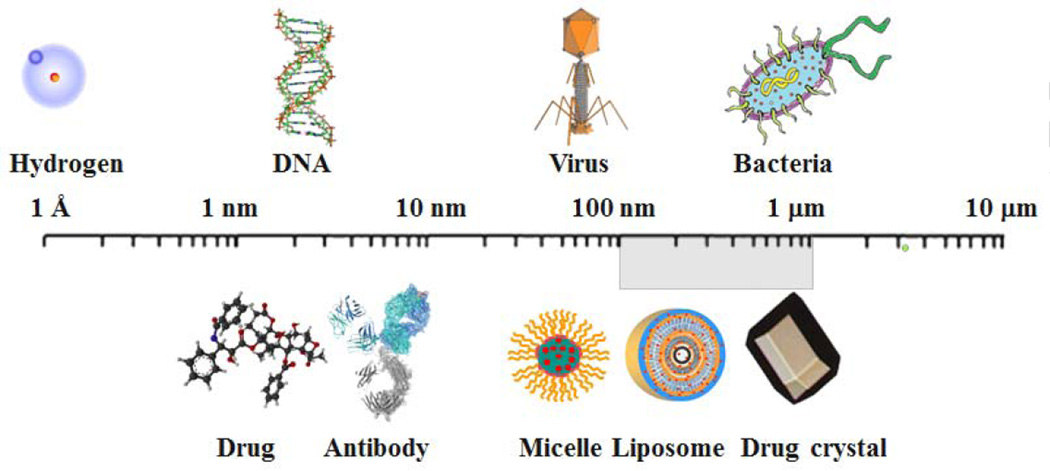
Fig. 2 shows relative sizes of examples of nanoparticles in relation to the sizes of familiar examples. Various proteins, including albumin and antibodies, have been used to deliver drugs. Polymer micelles, liposomes, and drug nanocrystals have been used to improve drug delivery, i.e., deliver more drugs to the targets and/or increase the overall bioavailability. Incidentally, all these formulations accompany the increase in solubility of poorly soluble drugs, and this is one property that contributes to the increased bioavailability. Since there is no clear boundary that separates nanoparticles from non-nanoparticles based on the size, the exact upper limit of nanoparticle size cannot be determined. If the size is above 1 µm, however, a particle clearly becomes a microparticle, and thus, for now, any particles less than 1 µm possessing unique properties that larger size particles of the same material do not have can be called nanoparticles. As shown in Fig. 2, there is a grey area defining the upper limit of nanoparticles in the 100 nm ~1 µm range. Such flexibility, in fact, is required in utilizing the nanoparticle concept in drug delivery, because it is the unique properties of nanoparticles that are useful, instead of the size itself. This leads to the question as to what unique properties of nanoparticles can be exploited for improving drug delivery.
Fig. 2.
Examples of nanoparticles and their relative sizes.
In the drug delivery field, the nanosized drug delivery systems have been used for more than six decades. The liposome was first developed in 1964 [20] and the term "nanoparticle" was first used in 1976 to describe 100 nm polymer particles [12]. Thus, the idea of using nanoparticles in drug delivery began almost four decades ago, and the unique abilities of the nanoparticles were already appreciated by drug delivery scientists. Almost three decades ago, drug delivery scientists started exploiting the unique properties of drug nanocrystals in improving drug bioavailability. The majority of new chemical entities and currently used drugs are poorly water-soluble. The bioavailability of the poorly soluble drugs is known to improve by making the drug in nanosizes to increase the surface area for improved drug dissolution [21]. The improved drug dissolution, in turn, results in a high drug concentration gradient for improved absorption, leading to improved bioavailability [22, 23]. The drug nanocrystal technology has been used to develop several clinically successful drugs, such as sirolimus, aprepitant, fenofibrate, megestrol, and paliperdione [24]. Only a limited number of nanocrystal formulations in clinical applications indicates the difficulties involved in the development of such formulations, and clearly more advances need to be made for wider applications of the nanocrystal formulation. Preparing stable nanocrystal formulations requires an engineering solution.
4. Nanoparticles for Targeted Drug Delivery to Tumors
Targeted drug delivery is a holy grail of drug delivery. It is especially important in treating tumors, and naturally, the majority of the articles published in the drug delivery field have been focused on this topic. In essence, the current approach of targeted drug delivery to tumors has been relying on two assumptions. First, the nanoparticle drug delivery system will accumulate more than its control formulation at the tumor due to the widely accepted enhanced permeation and retention (EPR) effect. Second, increasing the blood circulation time of nanoparticles by coating the surface with poly(ethylene glycol) (PEG), known as PEGylation, will improve the nanoparticle accumulation at the target site. These assumptions were thought to be correct as evidenced by numerous successes observed in the studies using nanoparticle-based treatment of tumors in mouse xenograft models. The EPR effect was first described by Matsumura and Maeda in 1986 [25]. Numerous publications have shown that the EPR effect in mouse models increases the drug accumulation by nanoparticle formulations by 200~500%. This increase may be impressive, but in the big picture, such an increase is still not sufficient to effectively treat tumors. The amount of the drug that accumulates at the tumor is still only 2~5% of the total administered dose [26]. The 2~5 fold increase in drug concentration may be able to shrink the tumor more than the control formulations, but it has not been able to completely eradicate tumors.
The EPR effect simply describes accumulation of extravasated nanoparticles at the tumor slightly more than the control, due to the less efficient back diffusion to the blood from the interstitial space than the dissolved drug molecules. Since, it is the free drug molecules that kill tumor cells, release of the drug from the nanoparticles at the right time is critical. Thus, mere presence of nanoparticles around the tumor does not necessarily impact killing tumor cells. There may be instances where blank nanoparticles cause necrosis or apoptosis of cells. If the blank nanoparticles are bioactive, however, they will also have the same effects on normal cells, leading to undesirable results. The biodistribution of paclitaxel nanocrystals in tumor-bearing mice was examined using paclitaxel nanocrystals that contain both tritium-labeled paclitaxel and fluorescent probe molecules [27]. It was discovered that only about 1% of the total injected paclitaxel accumulated at the tumor site after intravenous injection through the tail vein. Both nanocrystals and Taxol®, a solution formulation, showed similar amounts of paclitaxel at the tumor with similar activity. This makes sense, because paclitaxel in Taxol® is likely to be in the micellar form of Cremophor EL [2].
The main point of all the studies done in small animal models is that most of them have shown efficacy in inhibiting tumor growth in small animal xenograft models, and the efficacy has been almost always higher than the control non-nanoparticle formulations. But even in the small animal xenograft models, no tumors were fully eradicated. Most small animal model studies show the data for the first few weeks or a month. The decrease in tumor size may occur in the beginning, but the small animals are dying anyway despite repeated injections. If the nanoparticle formulations were indeed highly effective in treating tumors, such formulations could have been administered repeatedly to completely destroy the tumor. Unfortunately, no small animal studies have shown such real success. The above two assumptions on the EPR effect and PEGylation of nanoparticles may not represent the in vivo processes, especially in the human body.
4.1. Clarification of "passive" and "active" targeting
There has been a misunderstanding of the concepts of so-called "passive" and "active" targeting. These terminologies were used to describe the differences between nanoparticle formulations that have surface-bound targeting moieties, such as ligands or antibodies, against specific binding sites on the target tumor cell surface. In vitro studies using cell culture systems clearly showed superior uptake of nanoparticles with ligands or antibodies over the control nanoparticles. But when nanoparticles are introduced to the circulating blood, delivery of nanoparticles to the target tumor is simply based on the blood circulation and subsequent extravasation near the tumor [28]. This is where the EPR concept has been widely applied despite the fact that the increase in drug accumulation is only marginal [29]. Whether the nanoparticle has a targeting moiety or not, the number of nanoparticles reaching the target tumor remains the same [9, 30]. The presence of ligands or antibodies may help interaction with the target cells and consequent endocytosis. Thus, strictly speaking, there is no "passive" or "active" targeting. These decades-old terminologies should not be used anymore, as they imply the wrong impression of the ability of nanoparticles.
A recent study using liposomes with a targeting ligand has clearly demonstrated that the accumulation of the targeted particle was significantly reduced as compared with the non-targeted particles over the 4 weeks of treatment [31]. The expression of the target receptor changes over time, and thus, the expectation of targeting tumor cells based on overexpression of a target receptor is naïve at best. Furthermore, almost all studies on "active" targeting have never produced the quantitative data on the density of expressed receptors. The future studies of targeted drug delivery based on "active" targeting should provide information on the receptor density on the target cells which changes over time. Furthermore, the concept of active targeting is largely based on the observation that cancer cells "overexpress" certain receptors to be hyper-responsive to the low levels of growth factors and other ligands present in their surroundings. Levels of cell surface receptors can increase by as much as 50-fold [32]. The hyper-responsiveness toward ligands of cancer cells is limited by the amount of nanoparticles reaching the target cancer cells, which is only 2–4 folds larger than the control. On the other hand, the presence of an "active" targeting moiety on nanoparticles may inadvertently allow preferential interaction with normal cells which also express the same receptors, albeit 50-fold less. Since there are a significantly larger number of normal cells than cancer cells, a 50-fold increase in receptors on the cancer cell surface will make little difference in delivery of nanoparticles to the target cancer cells. If anything, the presence of an "active" targeting moiety is likely to reduce the number of nanoparticles reaching the target because they may be picked up by normal cells which express the same receptors. There have been situations where antibody-based drugs result in substantial benefits to certain cancer patients, but these are exceptions rather than the norm.
4.2. Smart nanoparticle systems
The key to the successful treatment of tumors is to deliver as much drug as possible to the target tumor. Of the many nanoparticulate systems used for tumor-targeted drug delivery, liposomes and polymer micelles constitute major portions. Liposomes have been widely used to develop tumor-targeted drug delivery systems. Many anticancer drugs are hydrophobic and they reside inside the liposomal lipid bilayer, and thus are prone to transfer to other hydrophobic sites in the blood, such as blood proteins. A study using a rat model showed that liposomes with more rigid bilayers transferred the loaded drug to lipoproteins at higher transfer rates than liposomes with more flexible bilayers [33]. It was also shown that PEGylated liposomes release a hydrophobic drug at a higher rate than the control. The fast drug release during blood circulation is not limited to liposomes. Polymer micelles may release their contents even faster, presumably through interacting with blood proteins [34]. Liposomal drug carriers or polymer micelles lose the loaded drug by the diffusional process as well as interacting with blood components. Maintaining the loaded drug inside the delivery vehicle is important, not just for liposome formulations, but also for all other intravenously administered formulations. Thus, it becomes critically important to develop drug delivery vehicles with a very slow drug release in the blood but with fast drug release when activated by environmental factors, which are often referred to as smart drug delivery systems. Smart nanoparticles are those that are capable of releasing more drug molecules to the surrounding environment upon stimulation. The stimuli include physical (temperature, light, magnetic field, and electricity), chemical (pH, and ions), and biological (enzymes, antibodies, and small molecules) components.
Smart drug delivery systems have been engineered to respond to external factors, such as ultrasound, radiofrequency, light, and temperature. Ultrasound, a pressure wave, can be delivered with high spatial and temporal resolution. Ultrasound can lead to heating as well as non-thermal mechanical effects by the energy dissipated during interaction with the tissues. Ultrasound, in combination with microbubbles, has been used in drug delivery. Microbubbles can generate diverse mechanical forces when exposed to ultrasound. Sonoporation is induction of a transient permeabilization of cellular membranes by ultrasound. The sonoporation can be used to enhance extravasation of drugs from blood to a surrounding interstitial space by treating a specific area with focused ultrasound [35, 36]. A series of self-assembling micelles were prepared to incorporate photosensitive Pt(IV)–azide prodrugs derived from cisplatin [37]. The micelles released biologically active Pt(II) quickly upon UVA irradiation. In the H22 murine hepatocarcinoma model the UVA irradiated animal showed significantly improved drug efficacy over the control. Currently, most chemotherapy focuses on primary tumors, even though metastatic disease is responsible for the majority of cancer deaths. Recently, a new design of a multicomponent nanochain formulation was designed to take the microenvironment of micrometastasis into consideration for cancer treatment [38]. Three iron oxide nanospheres are chemically linked to one doxorubicin-loaded liposome to make a linear, chain-like assembly. The unique advantage of the nanochain particles is that they allow multivalent attachment on the vascular target, which in turn results in about 6% of the administered dose accumulated in micrometastases in the lungs in a mouse model. In comparison, control liposomes exhibited less than 1% accumulation in lung micrometastases. To release the drug from the congregated nanochains to the metastatic cancer cells, a “mild” radiofrequency field was applied outside near the body to cause the iron oxide nanospheres of the nanochain to vibrate, break open the liposome spheres and spread the drug to the entire volume of micrometastatic sites.
One of the most promising smart drug delivery systems is thermosensitive liposomes. Increasing the temperature at the tumor site to 41 °C for 1 hour [39] or applying ultrasound for 20 min [35] is known to induce hyperpermeable tumor vessels for maximal extravasation. Subsequent increases to higher temperatures, e.g., 42 °C, are made to induce fast release of the drug from the nanoparticles [35, 39]. This two-step approach, of course, requires special nanoparticle formulations, such as low-temperature sensitive liposomes that change their release properties by a small increase in temperature. Since it is critical to keep the drug inside the delivery vehicles until they are near the tumor and to release the drug upon receiving external stimulus, developing more refined nanoparticles that respond reliably to the external factors is necessary.
4.3. Capabilities of nanoparticles: presumed vs. real
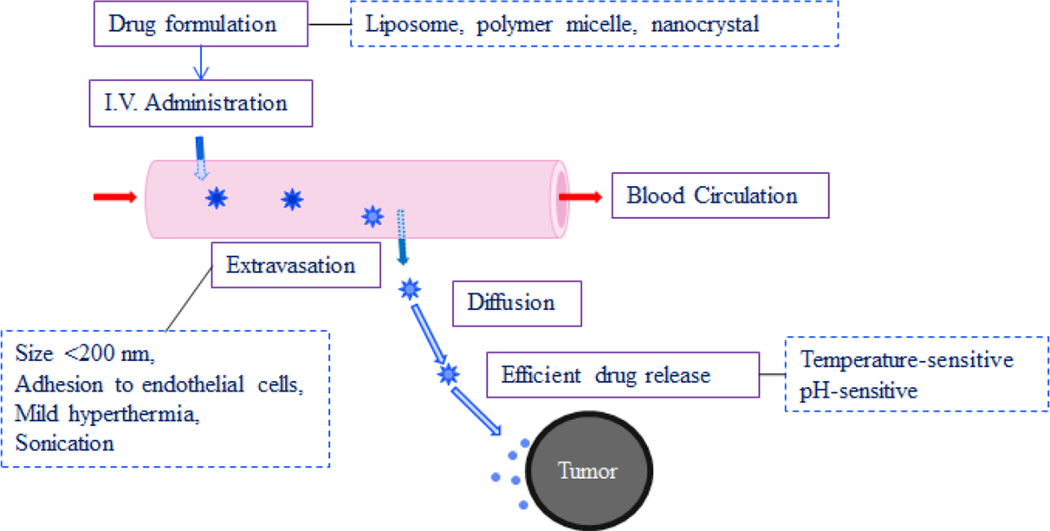
Improved efficacy of many nanoparticle formulations in small animal models has induced significant hope of targeted drug delivery in human patients. In theory, the drug delivery systems that work so well in small animal models should work, although not as well, in humans. Yet, a seemingly most promising low temperature-sensitive liposome approach has not produced the target response in clinical trials to date. This is despite the fact that treatment of tumors with mild hyperthermia was shown to be beneficial when used in combination with radiotherapy or chemotherapy [40]. There are a couple of reasons why this approach may not produce desirable effects in clinical applications. A precise sequence of events have to occur at the right times. First, the nanoparticle delivery systems have to extravasate from the blood into the surrounding tissue when the local area is activated, e.g., temperature of a local area is increased, and the local region is exposed to UV light, radiofrequency or magnetic field. Second, the nanoparticles accumulated near the tumor have to release their contents fast to establish a necessary drug concentration gradient when another signal is received. This sequence of processes is described in Fig. 3. Nanoparticle formulations, such as liposomes, polymer micelles, and drug nanocrystals, need to extravasate from the blood to the interstitial tissue near a tumor. In this process, a nanoparticle size smaller than 200 nm is preferred, because they are known to have low uptake by the reticuloendothelial system, leading to long circulation time [41], and the pore sizes of the endothelial cell lining near the tumor are known to be less than 380 nm [42]. In this process, application of mild hyperthermia (e.g., temperature of 41 °C), or ultrasound, is known to cause hyperpermeability to nanoparticles. The extravasated nanoparticles have to diffuse through the extracellular matrix near the tumor and undergo an efficient drug release to establish a high drug concentration gradient. Here, the second application of hyperthermia (e.g., temperature of 42 °C and higher) can accelerate the drug release from thermosensitive liposomes. In addition, nanoparticles with pH-sensitive polymers can increase the drug release near tumors where the pH is around 6.5.
Fig. 3.
Ideal sequence of targeted drug delivery to a tumor. (Adapted from Reference [43]).
It is rather intuitive that an accumulation of drug-containing nanoparticles per se is not enough to exert any anti-tumor effect. It is the concentration gradient of free drug molecules that is important in achieving antitumor activity. Nanoparticles cannot penetrate into the core of a solid tumor as effectively as free drugs. Thus, designing nanoparticle drug carriers possessing the fast drug release property upon activation by external or internal stimulators has become even more important than previously thought. Equally important is to keep the drug inside the nanoparticle carriers during blood circulation. There needs to be an assurance that the external or internal triggers can be activated in a reliable manner in clinical applications. The inability to accurately control the exact temperature necessary for fast releasing doxorubicin in clinical studies is thought to be a major reason for the failure of thermo-sensitive liposomes in humans [44].
5. The Future of Nanoparticles in Drug Delivery
Treating tumors requires a lot more than simply delivering a certain amount of a drug to the tumor site using nanoparticles. It seems that the drug delivery scientists have become complacent in their design of nanoparticle formulations. The current tumor-targeted drug delivery is mostly based on the EPR effect of nanoparticles. Many recent publications, however, indicate that the impact of the EPR effect is marginal at best [27]. This explains the absence of any development of nanoparticle formulation in clinical applications. The results obtained from the mouse studies are disconnected from clinical practice. It has been two decades since the EPR effect was used as the reason for the success of nanoparticles in small animal xenograft models. The time has come to reexamine the validity of the EPR effect and its real contribution, if it exists, in treating tumors in human patients. The poor translation of nanoparticle formulations to clinical efficacy has caused questions on the inflamed claims based on small animal xenograft models [45]. When several nanoparticle formulations used in clinical applications were compared with their solution counterpart formulations, there were no substantial differences except Abraxane® which is a paclitaxel-albumin conjugate formulation [46].
Studies on nanoparticle drug carriers have shown that the drug efficacy may not increase over the control formulation, but the side effects associated with the drug can be reduced [27, 31]. This may occur through adjusting the biodistribution of the same drug using nanoparticle formulations. The presence of nanoparticles may alter the drug release rate in normal cells, leading to reduced side effects. Rosiglitazone (RSG), a member of the thiazolidinedione class of drugs, modulates macrophage inflammation. Unfortunately, however, RSG has also been known to increase fatality from heart dysfunction, and this side effect dramatically limits its clinical use. RSG was reformulated into 200 nm nanoparticles to eliminate the side effect and improve drug biodistribution and bioavailability. RSG was incorporated into a hydrophobic PLGA core which was covered by a poly(vinyl alcohol) hydrophilic layer [47]. This nanoparticle formulation was shown to accumulate in circulating monocytes and resident macrophages, and subsequently dissolved in the acidic endosomes to release RSG.
Nanoparticle formulations, with proper engineering, can be used to overcome various difficulties in navigating the body in search of cancer cells. Typically, when a patient is diagnosed with cancer, the first-line treatment includes surgery to remove the primary tumor, followed by chemotherapy to eradicate any residual disease, including micrometastases at distant organs. Nanoparticle-based drug delivery may be useful in well-vascularized tumors that are several millimeters in diameter, but it is ineffective against micrometastases, which presents small clusters of malignant cells dispersed within variable tissue types [38].
Nanoparticle formulations are made and tested for the ultimate goal of treating, or preventing, diseases. There are many diseases to be treated, e.g., diabetes, heart diseases, Alzheimer's disease, macular degeneration, lung diseases, and cancer, to name a few. Of the many diseases, targeted drug delivery to tumors has been the dominant, if not the only, topic for nanotechnology-based drug delivery systems. If nanoparticles possess truly novel properties and innovational, then one wonders why nanoparticles have not been tested for other equally important diseases.
Regardless of the future engineering of nanoparticle drug delivery systems, one thing the drug delivery scientists need to be aware of is that nanoparticles will have to rely on blood circulation to reach target sites for efficacy. This inherently limits the percentage of the drug reaching the tumors. Since it is known that only a low percentage of the total administered dose actually reaches the target tumor by nanoparticle formulations, it is critically important to maximize the efficacy of the drug near the target. This requires new thinking of designing nanoparticle formulations. At the same time, efforts need to be made in reducing the side effects of the drug by altering the biodistribution and/or preventing drug release at the non-target sites. Problems in the difficulty of treating tumors and other diseases can be overcome by first identifying and understanding the problems.
Highlights.
Provide correct information on nanoparticle-based targeted drug delivery to tumors.
Nanoparticles are not magic bullets and have various limitations in drug delivery.
New smart nanoparticles require overcoming physiological barriers.
Need to exploit reduced side effects by nanoparticles via altered biodistribution
Acknowledgments
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dokoumetzidis A, Macheras P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006;321:1–11. doi: 10.1016/j.ijpharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clinical Pharmacology & Therapeutics. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 3.Cho K, Wang Xu, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 4.Irvine DJ. Drug delivery: One nanoparticle, one kill. Nat Mater. 2011;10:342–343. doi: 10.1038/nmat3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florence AT. “Targeting” nanoparticles: The constraints of physical laws and physical barriers. J. Control. Release. 2012;164:115–124. doi: 10.1016/j.jconrel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Advanced Drug Delivery Reviews. 2012;64(Supplement):206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Crommelin DJA, Florence AT. Towards more effective advanced drug delivery systems. International Journal of Pharmaceutics. 2013;454:496–511. doi: 10.1016/j.ijpharm.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: The past and the future. Advanced Drug Delivery Reviews. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Advanced Drug Delivery Reviews. 2014;69–70:29–41. doi: 10.1016/j.addr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wacker M. Nanocarriers for intravenous injection—The long hard road to the market. International Journal of Pharmaceutics. 2013;457:50–62. doi: 10.1016/j.ijpharm.2013.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Kreuter J, Speiser P. In vitro studies of poly(methylmethacrylate) adjuvants. J. Pharm. Sci. 1976;65:1624–1627. doi: 10.1002/jps.2600651115. [DOI] [PubMed] [Google Scholar]
- 13.ISO/TS, 27687:2008 Nanotechnologies - Terminology and definitions for nano objects - nanoparticle, nanofibre and nanoplate. http://www.iso.org/iso/home/standards_development/list_of_iso_technical_committees/iso_technical_committee.htm?commid=381983.
- 14.ASTM, 2456-06 Standard terminology relating to nanotechnology. http://www.astm.org/Standards/E2456.htm.
- 15.Vert M, Doi Y, Hellwich K-H, Hess M, Hodge P, Kubisa P, Rinaudo M, Schué F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012) Pure Appl. Chem. 2012;84:377–410. [Google Scholar]
- 16.Ruzer LS. Unattached fraction of radon progeny as an experimental tool in the assessment of the risk of nanoparticles. In: Ruzer LS, Harley NH, editors. Aerosol Handbook: Measurement Dosimetry, and Health Effects. 2nd Edn. Baca Raton: CRS Press, Taylor & Francis Group; 2013. pp. 415–438. [Google Scholar]
- 17.Faraday M. Experimental relations of gold (and other metals) to light. Phil. Trans. R. Soc. Lond. 1857;147:145–181. [Google Scholar]
- 18.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012;64:1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 19.Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. Journal of Controlled Release. 2013;166:182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface active agents as observed in the electron microscope. J. Mol. Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 21.Cooper ER. Nanoparticles: A personal experience for formulating poorly water soluble drugs. J. Control. Release. 2010;141:300–302. doi: 10.1016/j.jconrel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: In vivo performances. J. Control. Release. 2012;160:418–430. doi: 10.1016/j.jconrel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release. 2013;170:15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010;62:1569–1579. doi: 10.1111/j.2042-7158.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 26.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release. 2013;172:12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae YH, Park K. Targeted Drug Delivery to Tumors: Myths, Reality, and Possibility. J. Control. Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 31.Paoli EE, Ingham ES, Zhang H, Mahakian LM, Fite BZ, Gagnon MK, Tam S, Kheirolomoom A, Cardiff RD, Ferrara KW. Accumulation, internalization and therapeutic efficacy of neuropilin-1-targeted liposomes. Journal of Controlled Release. 2014;178:108–117. doi: 10.1016/j.jconrel.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg RA. The Biology of Cancer. Second Edition. New York, NY: Garland Science; 2013. [Google Scholar]
- 33.Decker C, Schubert H, May S, Fahr A. Pharmacokinetics of temoporfin-loaded liposome formulations: Correlation of liposome and temoporfin blood concentration. J. Control. Release. 2013;166:277–285. doi: 10.1016/j.jconrel.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Kim S, Li L, Wang S, Park K, Cheng J-X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yudina A, Lepetit-Coiffé M, De Smet M, Langereis S, Grüll H, Moonen C. In vivo temperature controlled ultrasound-mediated intracellular delivery of cell-impermeable compounds. J. Control. Release. 2012;161:90–97. doi: 10.1016/j.jconrel.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Sanches PG, Rossin R, Böhmer M, Tiemann K, Grüll H. Real-time imaging and kinetics measurements of focused ultrasound-induced extravasation in skeletal muscle using SPECT/CT. J. Control. Release. 2013;168:262–270. doi: 10.1016/j.jconrel.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Xiao H, Noble GT, Stefanick JF, Qi R, Kiziltepe T, Jing X, Bilgicer B. Photosensitive Pt(IV)–azide prodrug-loaded nanoparticles exhibit controlled drug release and enhanced efficacy in vivo. J. Control. Release. 2014;173:11–17. [PubMed] [Google Scholar]
- 38.Peiris PM, Toy R, Abramowski A, Vicente P, Tucci S, Bauer L, Mayer A, Tam M, Doolittle E, Pansky J, Tran E, Lin D, Schiemann WP, Ghaghada KB, Griswold MA, Karathanasis E. Treatment of cancer micrometastasis using a multicomponent chain-like nanoparticle. J. Control. Release. 2014;173:51–58. doi: 10.1016/j.jconrel.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, ten Hagen TLM, Haeri A, Soullié T, Scholten C, Seynhaeve ALB, Eggermont AMM, Koning GA. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J. Control. Release. 2014;174:202–208. doi: 10.1016/j.jconrel.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, Vujaskovic Z, Wessalowski R, Jauch KW, Dürr HR, Ploner F, Baur-Melnyk A, Mansmann U, Hiddemann W, Blay JY, Hohenberger P. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;1:561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lia Y-P, Yuan-Ying, Zhang X-Y, Gub Z-H, Zhoub Z-H, Yuanb W-F, Zhoub J-J, Zhua J-H, Gaoa X-J. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J. Control. Release. 2001;71:203–211. doi: 10.1016/s0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 42.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessles: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee BK, Yun YH, Park K, Sturek M. Introduction to biomaterials for cancer therapeutics. In: Park K, editor. Biomaterials for Cancer Therapeutics. Oxford, UK: Woodhead Publishing Ltd.; 2013. [Google Scholar]
- 44.Needham D. Reverse engineering the low temperature sensitive liposome (LTSL) for treating cancer. In: Park K, editor. Biomaterials for Cancer Therapeutics. Oxford, UK: Woodhead Publishing Ltd.; 2013. [Google Scholar]
- 45.Grainger DW. Connecting drug delivery reality to smart materials design. International Journal of Pharmaceutics. 2013;454:521–524. doi: 10.1016/j.ijpharm.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 46.Stirland DL, Nichols J, Miura S, Bae YH. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release. 2013;172:1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mascolo DD, Lyon C, Aryal S, Ramirez MR, Wang J, Candeloro P, Guindani M, Hsueh WA, Decuzzi P. Rosiglitazone-loaded nanospheres for modulating macrophage-specific inflammation in obesity. J. Control. Release. 2013;170:460–468. doi: 10.1016/j.jconrel.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]