Abstract
We report a structure-activity relationship of lithocholic acid amphiphiles for their anticancer activities against colon cancer. We synthesized ten cationic amphiphiles differing in nature of cationic charged head groups using lithocholic acid. We observed that anticancer activities of these amphiphiles against colon cancer cell lines are contingent on nature of charged head group. Lithocholic acid based amphiphile possessing piperidine head group (LCA-PIP1) is ~10 times more cytotoxic as compared to its precursor. Biochemical studies revealed that enhanced activity of LCA-PIP1 as compared to lithocholic acid is due to greater activation of apoptosis.LCA-PIP1 induces sub G0 arrest and causes cleavage of caspases. A single dose of lithocholic acid-piperidine derivative is enough to reduce the tumor burden by 75% in tumor xenograft model.
Keywords: Bile acids, Cancer, Amphiphiles, Anticancer activities, Colon Cancer
Introduction
Colon cancer is third most malignant tumor type involving uncontrolled growth of cells in colon.1 Many small molecule and antibody based therapeutics have been engineered and developed for cancer therapy to target this disease effectively.2 Apart from genetic reasons, prolonged high consumption of western-type diet augments risk of colon cancer in majority of patients. Increase uptake of fatty diets need extensive recirculation of bile acids for its digestion. Therefore, colon epithelial cells gets enhanced exposure to these high concentration of bile acids. Clinical studies have revealed elevated levels of fecal secondary bile acids from patients diagnosed with colon cancer.3 Therefore, bile acids are critical for pathogenesis of colon cancer progression and renewal of colon epithelium.
Bile acids are cholesterol-derived amphiphile molecules that help in absorption of fats and fat-soluble vitamins. 4 Primary bile acids like chenodeoxycholic acid and cholic acid are synthesized and secreted from liver and get re-circulated via portal system. 5 Gut microflora convert these primary bile acids to secondary bile acids, lithocholic acid and deoxycholic acid by de-hydroxylation.6 High concentration of bile acids and its accumulation in the gut can induce apoptosis of colon epithelium.7 Bile acid induced cytotoxicity causes colon cancer progression by disrupting the balance between cell growth and apoptosis, and helps in selection of bile acid resistant colon cancer cells.8
Bile acids exercise their cytotoxic effect via different cellular mechanisms that is contingent on structure, hydrophobicity, and stereochemistry of bile acids.9 Glycine and taurine conjugated primary bile acids are least toxic due to their low hydrophobicity, whereas unconjugated hydrophobic secondary bile acids are more cytotoxic.10 Diverse cellular mechanisms including membrane disruptions, role of PKC, MAPK pathways, and nuclear factor-kappa β activation have been proposed for toxicity of bile acids.11 Stenson and coworkers suggested the enantiospecific bile-acid mediated apoptosis in colon cancer cells.12 Natural bile acids induce apoptosis, escalated cellular detachment and enhanced caspases-3 and -9 cleavage as compared to synthetic enantiomers of bile acids in HT-29 and HCT-116 cells. 13
Cationic lipid amphiphiles and their drug conjugates have been explored for potential biological activities as anticancer and antibacterial candidates as they have ability to interact with cellular membranes effectively due to their charged hydrophobic character.14 Chikkara et al. synthesized lipophilic derivatives of anticancer drug doxorubicin for anticancer therapy15 and Emtricitabine based prodrugs for enhanced anti-HIV activity.16 Banerjee and co-workers synthesized haloperidol conjugated cationic lipids17 and lipids targeting estrogen receptors for target delivery to cancer cells.18 Sreekanth et al synthesized charged bile acid tamoxifen conjugates for breast cancer therapy and revealed that conjugation of multiple tamoxifen molecules to cationic cholic acid induces membrane perturbations and enhanced activity.19 We have recently shown that conjugation of single-charged head groups on bile acids induces favorable interactions with model membranes and cancer cells for their cytotoxicity, whereas multi-headed amphiphiles does not interact with mammalian cells due to enhanced hydration barrier between amphiphiles and cellular membranes.20
In this paper, we hypothesize that anticancer activity of amphiphiles is contingent on nature of the single-charged head group on lithocholic acid amphiphile. Therefore, we synthesized ten lithocholic acid based amphiphiles differing in nature of the charged head group attached to hydroxyl group of lithocholic acid. Anticancer activities of these amphiphiles were investigated against three human colon cancer cell lines. Annexin-FITC and cell cycle analysis were performed to study the mechanism of cellular death by these amphiphiles. We then evaluated in vivo anticancer potential of amphiphiles in tumor xenograft models, and analyzed the mechanism of activity in tumors.
Results and discussion
Synthesis of amphiphiles
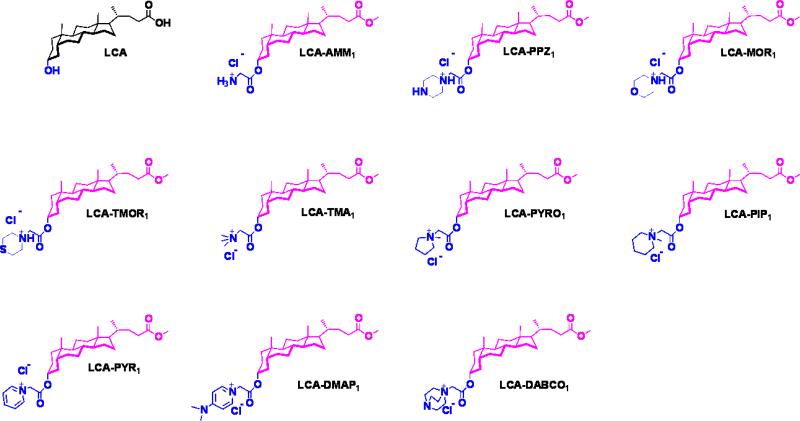
We synthesized and characterized ten amphiphiles (Fig. 1) differing of charged head group using lithcholic acid. All the lithocholic acid amphiphiles were synthesized and characterized (except LCA-PPZ1, LCA-MOR1, LCA-TMOR1) as described previously.20 Amphiphiles LCA-PPZ1, LCA-MOR1, LCA-TMOR1 were synthesized by reaction of chloroacetyl derivative of lithocholic acid methyl ester with corresponding amine, and purified by column chromatography as described in experimental section. All the synthesized amphiphiles were purified and characterized by 1H-NMR, 13C-NMR, mass spectrometry, and HPLC (Waters, RI-410).
Fig. 1.
Molecular structures of lithocholic acid amphiphiles synthesized and studied.
Anticancer activities in colon cancer cell lines (MTT Assay)
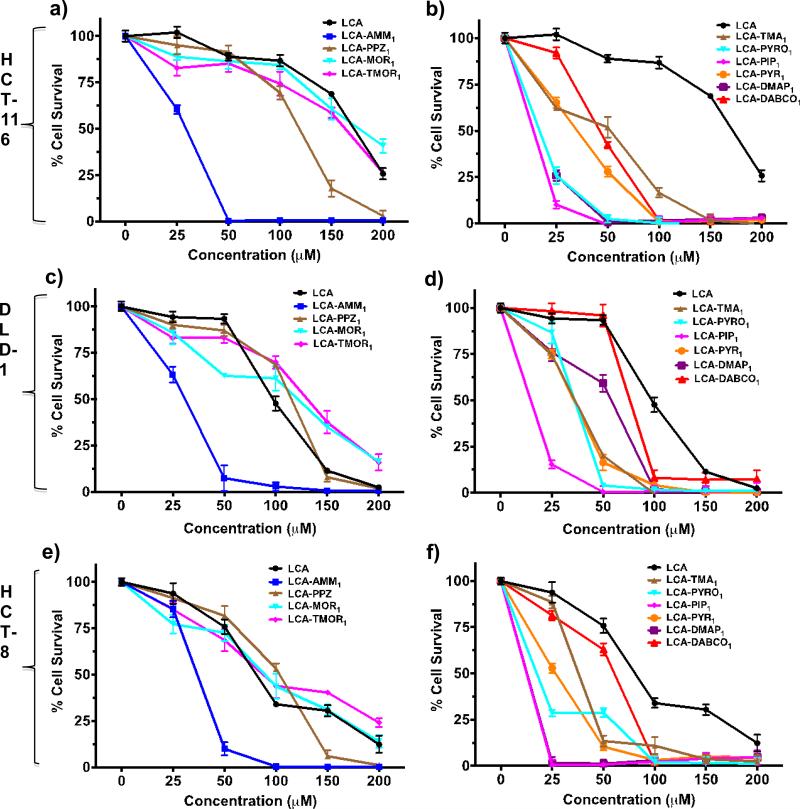
We examined the cytotoxic effect of these amphiphiles against three human colon cancer cell lines (HCT-8, HCT-116 and DLD-1) at 48h. We discovered that introduction of soft charge ammonium head group (LCA-AMM1) on lithocholic acid enhances its cytotoxicity by 3-6 fold (Fig. 2, Table 1). Other soft charge modifications on lithocholic acid like piperazine (LCA-PPZ1), morpholine (LCA-MOR1), thiomorpholine (LCATMOR1) are not effective in enhancing the cytotoxic potential of lithocholic acid (Fig. 2, Table 1). Further quaternization of lithocholic acid with trimethyl ammonium head group (LCA-TMA1) lowers its cytotoxicity as compared to ammonium head group amphiphiles. Amphiphile LCA-PIP1 bearing piperidine head group is most active with IC50 value of ~12-15 μM in HCT-116, HCT-8 cells and DLD-1 cells (Fig. 2). Amphiphile LCA-PIP1 is nearly 6-10 fold more cytotoxic as compared to lithocholic acid LCA, and 3-fold more effective than amphiphile LCA-AMM1 bearing ammonium head group (Table 1). Structure-activity studies revealed that aliphatic 6-membered head group based amphiphileLCA-PIP1 is 2-3 fold more cytotoxic as compared to aromatic head group based amphiphile LCAPYR1 and 5-membered aliphatic derived amphiphile LCA-PYROL1 as well. Introduction of a hydrophobic 6-membred piperidine head group allows its efficient interactions with intra-cellular targets making it more effective. Therefore, structure-activity studies conclude that anticancer activities of amphiphiles are strongly contingent on nature of the head group. As bile acids are known to bind with cell membrane TGR5 receptors, or nuclear FXR receptors; nature of the charged head group may help in preferential interactions of these amphiphiles with cell surface membrane receptors or intracellular receptors. We believe that nature of the head group may also help in preferential activation of death receptors, or intracellular proteins responsible for apoptosis; as these amphiphiles induce apoptosis by intrinsic and extrinsic pathways as described later.
Fig. 2.
a-f) Structure-activity investigation showing IC50 values of different amphiphiles in three colon cancer cell lines a-b) HCT-116, c-d) DLD-1, and e-f) HCT-8. All experiments have been performed at least two times in four replicates and IC50 values reported are mean ± SD.
Table 1.
IC50 of lithocholic acid amphiphiles againt three colon cancer cell lines, HCT-116, DLD-1 and HCT-8.a
| Amphiphile | HCT-116 (μM) | DLD-1 (μM) | HCT-8 (μM) | HPLC, RTb (min) |
|---|---|---|---|---|
| LCA | 81.1 ± 3.8 | 173.1 ± 3.0 | 97.4 ± 2.5 | -c |
| LCA-AMM1 | 36.7 ± 3.4 | 29.4 ± 2.3 | 30.7 ± 6.9 | -d |
| LCA-PPZ1 | 102.0 ± 4.3 | 118.4 ± 4.5 | 114.6 ± 4.1 | -d |
| LCA-MOR1 | 89.0 ± 2.7 | 176.2 ± 3.8 | 120.9 ± 3.4 | 24.17 |
| LCA-TMOR1 | 87.3 ± 3.1 | 163.2 ± 2.7 | 130.5 ± 4.1 | 28.63 |
| LCA-TMA1 | 38.1 ± 2.9 | 53.0 ± 1.0 | 37.0 ± 2.3 | 7.94 |
| LCA-PYRO1 | 17.8 ± 2.4 | 17.0 ± 4.6 | 36.4 ± 2.9 | 8.23 |
| LCA-PIP1 | 11.7 ± 3.0 | 13.7 ± 1.0 | 14.7 ± 3.0 | 8.12 |
| LCA-PYR1 | 26.5 ± 2.0 | 35.4 ± 2.8 | 35.4 ± 4.3 | 7.84 |
| LCA-DMAP1 | 13.2 ± 3.0 | 17.0 ± 2.9 | 59.1 ± 4.5 | 7.90 |
| LCA-DABCO1 | 60.7 ± 1.2 | 46.4 ± 2.9 | 76.4 ± 5.9 | 7.98 |
IC50 values were calculated from MTT experiments
Mobile phase for all amphiphiles was AcCN:MeOH (95:5) except for LCA-MOR1 and LCA-TMOR1, where mobile phase was AcCN. LCA-MOR1 and LCA-TMOR1 stock solutions were made in 10% EtOAc in AcCN.
not determined
could not be determined as this amphiphile is soluble in DMSO only.
Selectivity over normal cells
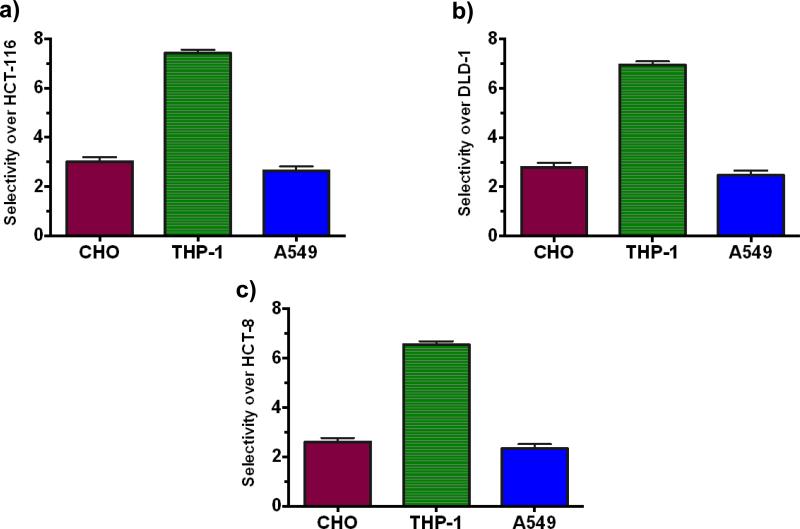
We then studied the selectivity of most potent amphiphile LCA-PIP1 over normal cells, and investigated the toxicity of amphiphile against macrophages (THP-1), CHO and A549, and Red blood cells. As shown in Fig. 3, amphiphile LCA-PIP1 is five times less toxic in normal macrophages as compared to colon cancer cells; and ~3-fold more selective for colon cancer cells over CHO cells. LCA-PIP1 is also ~2-fold more selective over lung cancer A549 cells (Fig. 3). As these are charged amphiphiles, we investigated the membrane disruptive character of amphiphile LCA-PIP1 and performed the hemolytic assay. Amphiphile LCA-PIP1 was found to be non-hemolytic against RBCs with MHC50 > 1mM concentration.20b These results suggest that lithocholic acid derived amphiphile LCA-PIP1 is inducing the apoptotic machinery in colon cancer cells for its activity, and does not disrupt the cellular membranes in a detergent manner for its activity. We therefore investigated the mechanism of cell death induced by amphiphile LCA-PIP1 against colon cancer cells.
Fig. 3.
Selectivity of LCA-PIP1 for different colon cancer cell lines a) HCT-116, b) DLD-1, c) HCT-8) over CHO, THP-1 and A549 cell lines.
Effect of Ester and Amide linkages
In LCA-PIP1 amphiphile, piperidine head group is conjugated to lithocholic acid through ester linkage. To explore the effect of linkage between piperidine head group and lithocholic acid, we synthesized an amide derivative of LCA-PIP1 and compared the cytotoxicity of ester and amide derivatives of LCA-PIP1 against three colon cancer cell lines (Fig. S1, ESI). MTT studies suggested that ester derivative of LCA-PIP1 is more effective than amide derivative of LCA-PIP1 (Fig. S1, ESI) that might be due to easy cleavage of piperidine head group for enhanced activity.
Colony forming assay
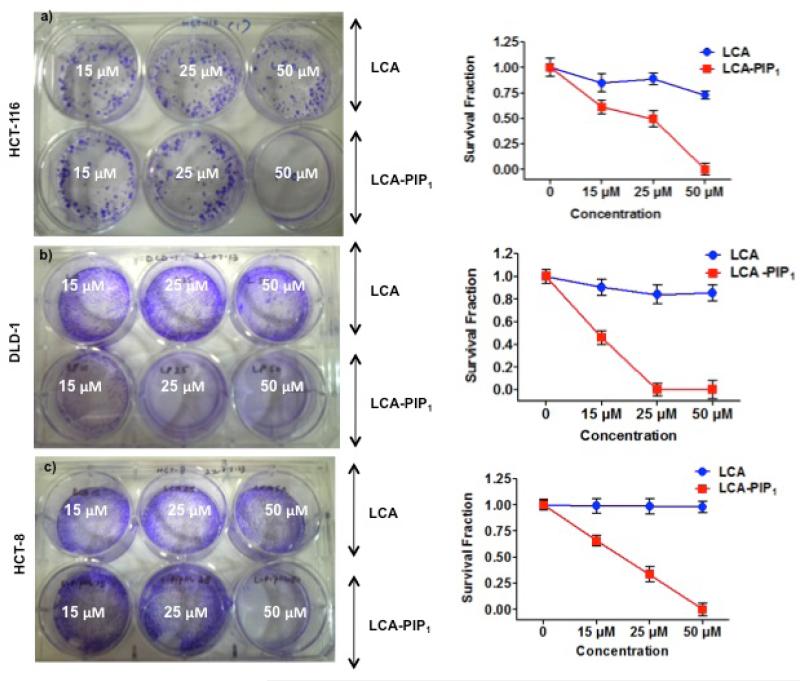
We then studied colony-forming ability of colon cancer cells on treatment with LCA and LCA-PIP1 at different concentrations. Treatment of LCA on these cells does not inhibit colony formation at 15, 25 and 50 μM conc. whereas LCA-PIP1 on treatment at 15μM induces ~50% inhibition in HCT-116 cells (Fig. 4a). We have not observed any colony on treatment with 50μM of LCA-PIP1. Similarly treatment of LCA-PIP1 inhibits colony formation in DLD-1 and HCT-8 colon cancer cells (Fig. 4b, c). These studies conclude that introduction of single piperidine head group on LCA makes it highly active and inhibit colony-forming properties of cancer cells.
Fig. 4.
Colony forming assay showing effect of LCA and LCA-PIP1 at different concentrations on colony forming abilities and survival fraction of a) HCT-116, b) DLD-1, and c) HCT-8 cells. All experiments were done two times in duplicates and survival fraction values are reported as mean ± SD.
Cell cycle and Annexin-FITC analysis
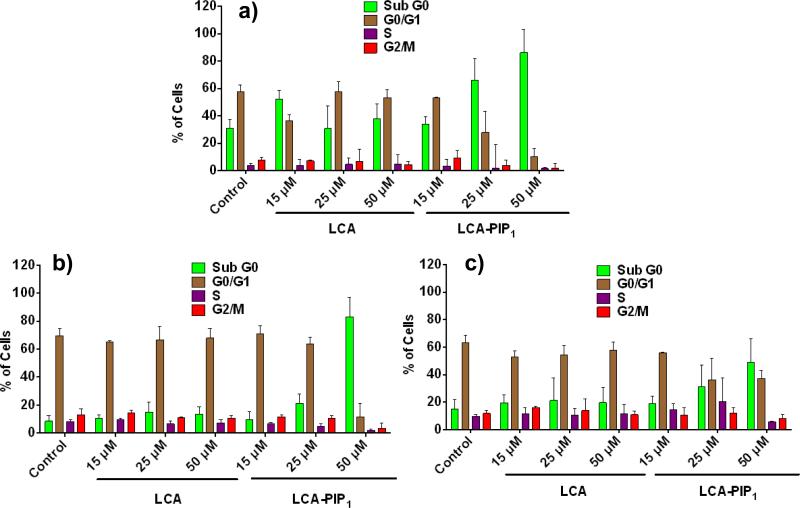
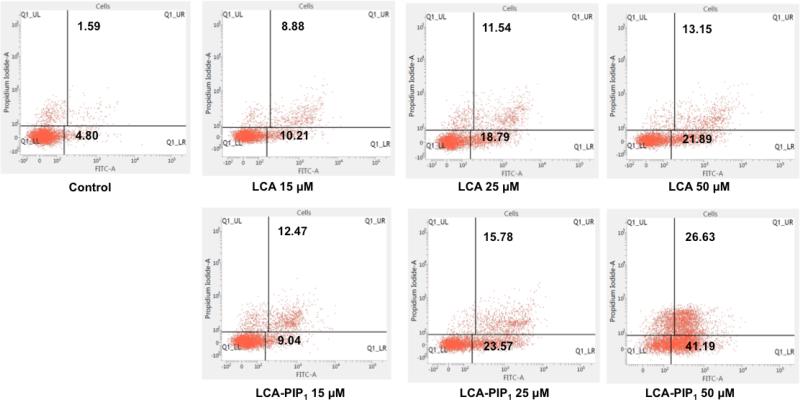
We then performed cell cycle analysis to know the fate of cells in different phases of cell cycle on treatment with LCA and LCA-PIP1 at different concentrations for 48h. Cell cycle analysis revealed that LCA alone does not induce any concentration dependent change in cell cycle in HCT-116, DLD-1 and HCT-8 cells, whereas treatment of LCA-PIP1 arrests cells in sub-G0 phase and does not allow them to enter in G0-G1 phase (Fig. 5). LCA-PIP1 at 50μM induces ~80% arrest in sub-G0 phase in HCT-116 and DLD-1 cells, and ~40% arrest in HCT-8 cells. To ensure whether arrest in cell cycle phase by LCA-PIP1 induces apoptosis, we probed LCA and LCA-PIP1treated HCT-116 cells with Annexin-V-FITC apoptosis assay. As shown in Fig. 6, treatment of LCA-PIP1 induces 1.3 and 2.0-fold increase in apoptotic cells at 25μM and 50μM conc. as compared to LCA. LCA-PIP1 at 50μM induces ~40% cells to early apoptosis and ~26% cells to late apoptosis. Therefore, cell cycle and Annexin-FITC studies conclude that introduction of a piperidine head group on LCA in case of LCA-PIP1 enhances sub-G0 arrest of cells and induces apoptosis.
Fig. 5.
Cell cycle analysis of a) HCT-116, b) DLD-1, and c) HCT-8 cells treated with LCA and LCAPIP1 at different concentrations of 15, 25 and 50μM for 48h showing enhanced sub-G0 arrest on treatment with LCA-PIP1. All experiments were performed in triplicates, and values are reported as mean ± SD.
Fig. 6.
Annexin-V/FITC apoptosis assay graphs in HCT-116 cells treated with LCA and LCA-PIP1 at different concentrations of 15μM, 25μM, 50μM for 48h showing enhanced apoptosis by LCA-PIP1.
In vivo anticancer activities
HCT-116 Nude mice colon tumor model
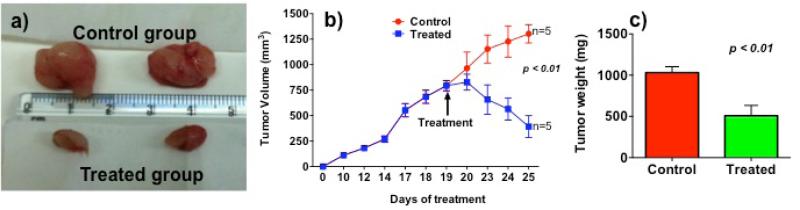
To explore in vivo potential of LCA-PIP1 in xenograft tumor model, we studied anticancer activity of LCA-PIP1 in xenograft tumor model in NUDE mice. We used HCT-116 cancer cells in NUDE mice to develop tumors on flank. To test the potential of LCAPIP1 in larger tumor volumes, we developed tumors of ~750 mm3 as compared to ~100-200 mm3 tumors usually reported in the studies. Single injection of 20 mg/kg body weight is able to reduce tumor volume by 75% as shown in Fig. 7. We observed more than 50% decrease in tumor weight after 6-days of treatment. These studies conclude that introduction of a single piperidine head group on LCA make it highly potent anticancer agent.
Fig. 7.
In vivo anticancer effect of LCA-PIP1 in HCT-116 tumor models in NUDE mice, a) representative excised tumors from control and treated group; b) change in tumor volume on treatment with LCA-PIP1; c) change in tumor weight after treatment with LCA-PIP1.
In vitro and in vivo mechanism
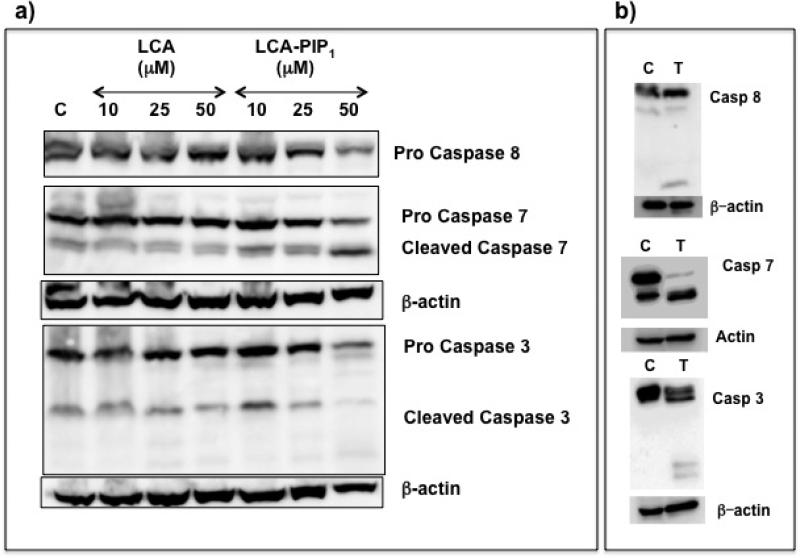
To unravel the mechanism of cell death, we performed western studies in HCT-116 cells on treatment with LCA and LCA-PIP1 to see the expression of caspases required for apoptosis. Western studies showed increased expression of pro-caspase 8 on LCA-PIP1 treatment at low concentrations, which gets decreased at high concentration (Fig. 8a). We observed increased levels of cleaved casapase-7 and caspase-3 on LCA-PIP1 treatment. To determine the mechanism involved in tumor regression upon treatment with LCA-PIP1, we harvested tumors from mice and subjected them to western blot analysis for expression of caspases (Fig. 8b). We observed the elevated expression of activated (cleaved) caspases 3, 7 and 8 in LCA-PIP1 treated tumors as compared to vehicle control group (Fig. 8b). Induction of apoptotic pathway leads to cascade of events, releasing various apoptotic mediators from mitochondria, and activation of caspase-3 and caspase-7 required for apoptosis.21,22 Caspase 3 that is critical for apoptosis by intrinsic apoptotic pathway and activation of caspase 8 critical for extrinsic apoptotic pathway.23 Therefore these studies conclude that amphiphile LCA-PIP1 induces tumor regression through activation of intrinsic and extrinsic pathways.
Fig. 8.
a) Representative western blots of lysates of LCA and LCA-PIP1 treated HCT-116 cells, b) Representative western blots of lysates of control and LCA-PIP1 treated tumors showing the activation of caspases.
Conclusions
In summary, we have shown that conjugation of charged head groups on lithocholic acid modulates their interactions with cancer cells. Anticancer potential of these amphiphiles is contingent on nature of the charged head groups. Piperidine head group based amphiphile LCA-PIP1 is most active in as compared to LCA suggesting the effective intracellular interactions with targets responsible for inducing apoptosis. Annexin-FITC studies and cell cycle studies suggest induction of apoptosis and sub-G0 cell cycle arrest by LCA-PIP1. Single dose of LCA-PIP1 could induce ~75% reduction in tumor volume and ~50% reduction in tumor weight for high aggressive HCT-116 tumor models. Western studies on tumors indicated the activation of caspases for inducing apoptosis. As these molecules are based on based on bile acids, further modification of these molecules would have future applications for treatment of different gastrointestinal cancers. Amphiphilic character of these molecules can further be explored to form nanoparticles and its delivery. Therefore, engineering of these lithocholic acid amphiphiles open up a new class of molecules as therapy for colon cancer.
Experimental Section
Materials and methods
All the chemicals and solvents used are of ACS grade. Lithocholic acid (LCA), Propidium iodide, Annexin-FITC kit was purchased from Sigma-Aldrich. All antibodies were purchased from Cell Signaling, except β-actin that was purchased from Sigma. MTT and ECL kit was purchased from Amersham. Cell culture media, trypsin, and antibiotics were purchased from Hyclone, USA or Sigma-Aldrich. Plasmocin prophylactic and plasmocin treatment was purchased from invivogen. All synthesized compounds were purified using Combi-flash or glass chromatography using 230-400 mesh size silica gel. 1H and 13C NMR spectra were recorded using Bruker 400 MHz spectrometer. Chemical shifts (δ) are reported in ppm with tetramethyl silane as internal standard. High-resolution mass spectra were measured on LC-MS/MS (AB-SCIEX TRIPLE TOF-5600) and MALDI (AB SCIEX TOF/TOF 5800) mass spectrometer.
General procedure for the synthesis of cationic amphiphiles
Lithocholic acid amphiphiles were synthesized and characterized (except LCA-PPZ1, LCA-MOR1, LCA-TMOR1) from chloroacetyl derivative of lithocholic acid methyl ester as described previously.20 Amphiphiles LCA-PPZ1, LCAMOR1, LCA-TMOR1 were synthesized from chloroacetyl derivative of lithocholic acid methyl ester20 as described below.
Amphiphile LCA-PPZ1
Piperazine (86 mg, 1 mmol) was added to a solution of chloroacetyl derivative of lithocholic acid methyl ester (200 mg, 0.42 mmol) in ethyl acetate (5 mL) and K2CO3 in a sealed tube and reaction mixture was refluxed for 36 h. After completion of reaction, reaction mixture was filtered to remove K2CO3. Solvent was removed by evaporation, and the product was purified by column chromatography using EtOAc : Pet Ether (20:50) as a colorless solid (185 mg, 85%; Rf = 0.40, EtOAc : Pet Ether, 50: 50); 1H-NMR (CDCl3, 400 MHz) δ: 0.63 (s, 3H, -CH3), 0.91 (s, 6H, 2 × CH3), 0.99-2.23 (steroid), 2.67(s, 4H, -N-(CH2)2), 3.19 (m, 2H, -CO-CH2-N-), 3.66 (s, 3H, -CO-OCH3), 4.78 (s, 1H, -O-CH); 13C-NMR (CDCl3, 100 MHz) δ: 12.04, 18.28, 20.84, 23.30, 24.19, 26.32, 26.66, 27.01, 28.19, 29.70, 31.23, 34.60, 35.01, 35.79, 40.13, 40.41, 42.74, 51.49, 52.68, 55.99, 56.48, 74.92, 169.59, 174.78; MALDI Mass: m/z (C31H52N2O4) calculated 516.39; found (M)+ 517.402.
Amphiphile LCA-MOR1
To a solution of chloroacetyl derivative of lithocholic acid methyl ester (250 mg, 0.53 mmol) in ethyl acetate (5 mL) was added K2CO3 and Morpholine (95 mg , 1.1 mmol) in a sealed tube and the mixture was refluxed for 48h. After completion of reaction, K2CO3 was removed by filtration and solvent was removed by evaporation. Final product was purified by column chromatography using EtOAc : Pet Ether (20:50) as a colorless solid (246 mg, 90%; Rf = 0.35, EtOAc : Pet Ether, 50: 50); 1H-NMR (CDCl3, 400 MHz) δ: 0.63 (s, 3H, -CH3), 0.92 (s, 6H, 2 × CH3), 0.99-2.38 (steroid), 2.59 (s, 4H, -N-(CH2)2), 3.18 (m, 2H, -CO-CH2-N-), 3.66 (s, 3H, -CO-OCH3), 3.76 (t, J = 4.4, 4H, -N-(CH2)2), 4.78 (s, 1H, -O-CH); 13C-NMR (CDCl3, 100 MHz) δ: 12.04, 18.28, 20.84, 23.31, 24.19, 26.32, 26.67, 27.01, 28.19, 29.70, 31.01, 31.07, 32.25, 34.60, 35.00, 35.38, 35.79, 40.14, 40.43, 41.91, 42.74 51.50, 53.25, 56.00, 56.50, 59.97, 66.75, 74.97, 74.92, 169.50, 174.78; MALDI Mass: m/z (C31H51NO5) calculated 517.38; found (M)+ 518.377.
Amphiphile LCA-TMOR1
Reaction mixture of chloroacetyl derivative of lithocholic acid methyl ester (300 mg, 0.64 mmol) in ethyl acetate (6 mL), K2CO3 (300 mg) and thiomorpholine (124 mg , 1.2 mmol) was refluxed in a sealed tube for 40 h. Solid K2CO3 was removed from reaction mixture and solvent was removed by evaporation. The product was purified by column chromatography using EtOAc : Pet Ether (20:50) as a colorless solid (310 mg, 91%; Rf = 0.62, EtOAc : Pet Ether, 50: 50); 1H-NMR (CDCl3, 400 MHz) δ: 0.63 (s, 3H, -CH3), 0.91 (s, 6H, 2 × CH3), 0.99-2.33 (steroid), 2.72 (s, 4H, -S-(CH2)2), 2.84 (d, J = 4.4, 4H, -N-(CH2)2) 3.20 (s, 2H, -CO-CH2-N-), 3.66 (s, 3H, -CO-OCH3), 4.77 (s, 1H, -O-CH); 13 CNMR (CDCl3, 100 MHz) δ: 12.04, 18.27, 20.84, 23.31, 24.18, 26.32, 26.71, 27.00, 27.81, 28.18, 29.69, 31.01, 31.07,3 32.28, 34.59, 35.00, 35.37, 35.79, 40.13, 40.44, 41.92, 42.74, 51.48, 54.53, 56.00, 56.49, 60.51, 74.94, 169.75, 174.74; MALDI Mass: m/z (C31H51NO4) calculated 533.35; found (M)+ 534.284.
Amide derivative of LCA-PIP1
Amino derivative of lithocholic acid was synthesized as described previously.24 Amide derivative of LCA-PIP1 was synthesized using amino derivative of lithocholic acid (LCA-NH2) with similar procedures.20 Yield 80% (solid). 1H-NMR (CDCl3, 400 MHz) δ: 0.62 (d, J=5.6, 3H, -CH3), 0.90 (s, 6H, -2 × CH3), 0.99-2.37 (steroid), 3.39 (s, 3H, -N-CH3); 2H, -N-CH2), 3.65 (s, 3H, -CO-OCH3), 3.91 (m, 2H, -N-CH2), 4.69 (m, 2H, -CO-CH2-N-); 13C-NMR (CDCl3, 100 MHz) δ: 12.03, 18.27, 20.16, 20.87 23.38, 23.64, 24.18, 26.27, 27.07, 28.16, 30.56, 32.75, 34.58, 35.37, 35.64, 40.00, 40.17, 42.73, 50.92, 51.46, 55.97, 56.62, 62.69, 71.84, 162.86, 174.86; MALDI Mass: m/z (C33H57N2O3) calculated 529.44; found (M)+ 529.431.
Cell cultures
HCT-116, DLD-1, HCT-8, A549 and CHO cell lines were maintained as monolayers. HCT-116 cells in McCoy`s medium, DLD-1 and HCT-8 cells in RPMI-1640 medium, and A549 and CHO cells in DMEM media with 10% (v/v) fetal bovine serum, and antibiotics were maintained at 37°C in a humidified atmosphere with 5% CO2. THP-1 cells were grown in DMEM media and differentiated to adherent macrophages by adding Phorbol myristic acid (PMA).
Cytotoxicity Assay25
MTT studies were performed for anticancer activities of all amphiphiles in three different colon cancer cell lines. Cells were plated in 96 well plates at a density of 3-4 × 103 cells per well for 24h for their adherence. Cells were treated at different concentrations of 25, 50, 100, 150 and 200μM of synthetic lithocholic acid amphiphiles for 48h. MTT solution (25μL of 5mg/mL) was added to cells for incubation to get formazan crystals. After 3h of incubation, media was replaced with 150μL of DMSO to lyse the cells. Absorbance of formazen crystals was recorded at 555nm using spectramax M5 (Molecular devices). Cell viability was then calculated using equation [{A555 (treated cells) - background]/[A555 (untreated cells) - background}] 100.
Colony formation assay26
Colony formation studies were performed with colon cancer cells on treatment with LCA and LCA-PIP1 at different concentrations. 200 cancer cells per well were plated in a 6-well plate. After adherence (24h), cells were treated with different conc. of LCA and LCA-PIP1. After 2-weeks, cells were stained with crystal violet and colonies were counted. From the number of colonies, we first calculated the plating efficacy (PE) from untreated samples as
The number of colonies after treatment of cells, called survival fraction, is calculated using following equation:
Cell cycle and Annexin-FITC analysis
In a 6-well plate, colon cancer cells at a density of ~2 × 105 cells/well were seeded. After 24h, cells were treated with amphiphiles for 48h. Cells were trypsinized and collected by centrifugation after treatment. For cell cycle analysis, cells were washed twice with cold PBS and fixed in 70% ice-cold ethanol. Ethanol was removed by washing the cells again with PBS and cells were treated with RNase (10μL of 20 mg/ml) at 37°C for 1h. Cells were stained with propidium iodide (50 μg/ml) at room temperature for 20 min and counted on a FACS (Becton Dickinson, Mountain View, CA). Annexin-FITC studies were performed using kit from Sigma-Aldrich according to manufacturer's instructions. Cells were stained simultaneously with FITC labeled Annexin V (50μg/mL) and propidium iodide (100μg/mL) after their re-suspension in binding buffer and analyzed using a flow cytometer (Becton Dickinson).
In vivo experiments
6 weeks old female NUDE mice were received from Institutional Animal Facility and were allowed to acclimatize to facility conditions before any experimental procedure. All the protocols for animal experiments were approved from Institutional Animal Ethical Committee of National Institute of Immunology. Mycoplasma testing was performed in the cells to make sure that cells were free of any sort of contamination. We injected 1.5 × 106 HCT-116 cells/mice in right flank of each mice to generate tumor models. Tumors became palpable after 7 days and we started measuring tumor sizes after 10th day. Digital calipers were used for measuring tumor sizes throughout the study. Tumor volume was calculated using the formula: Volume = 0.5 L × W2, where W = width of tumor, L = length of tumor. Once the tumors attained an average volume of ~750 mm3, we divided mice into two groups of 5 mice each. On the 19th day, we started treatment of mice with either vehicle control or amphiphileLCA-PIP1 at dose of 20 mg/kg near tumor sites. Tumors measurements were performed regularly after giving treatment and eventually mice were sacrificed on 25th day.
Western blot studies in cell lysates and tumor samples
Tumors were harvested from control and treated NUDE mice. These tumors were then subjected to homogenization using RIPA lysis buffer. Tumor lysates were loaded in equal concentration and then electro-transferred onto polyvinylidene fluoride membranes. Blots were then blocked with 5% non-fat dry milk (NFDM) dissolved in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2h at room temperature. Blots were washed three times with TBST and incubated overnight at 4°C, with specific primary antibodies in TBST containing 2% NFDM. Next day membranes were washed three times with TBST and incubated for 2h at room temperature with secondary horseradish peroxidase conjugated anti-mouse or anti-rabbit IgG in TBST containing 2% NFDM. Detection was performed using enhanced chemiluminescence reagent (ECL) and a bioluminescent image analyzer LAS-4000. Western blots were performed from lysates originating from at least two different tumors. Similarly, western blot studies were performed using cell lysates.
Supplementary Material
Acknowledgements
We thank RCB for intramural funding, and Department of Biotechnology for funding. AB thanks Department of Science and Technology for Ramanujan Fellowship. SK, VS thanks RCB; AS and SB thank DBT for research fellowships. SS would like to acknowledge National Institute of Immunology core funds, Department of Biotechnology (DBT), India (BT/PR3148/AGR/36/706/2011); Department of Science and Technology (DST), India (SR/SO/BB-08/2010); Indo-French Centre for the Promotion of Advanced Research (IFCPAR) (IFC/4603-A/2011/1250) and Council of Scientific and Industrial Research (CSIR), India [37(1541)/12/EMR-II] for financial assistance. We thank Dr. Nirpender Singh for helping in recording Mass spectra, and AIRF, JNU for recording NMR spectra.
Footnotes
Supporting Information: Comparison of cytotoxicity of ester and amide derivatives of LCA-PIP1. Characterization of final compounds LCA-PPZ1, LCA-MOR1, LCA-TMOR1. HPLC profiles of all amphiphiles.
References
- 1.a Schneikert J, Behrens J. Gut. 2007;56:417. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shnyder SD, Fu Y, Habtemariam A, van Rijt SH, Cooper PA, Loadman PM, Sadler PJ. Med. Chem. Commun. 2011;2:666. [Google Scholar]
- 2.a Wu Q, Bai Z, Ma Q, Fan W, Guo L, Zhang G, Qiu L, Yu H, Shao G, Cao R. Med. Chem. Commun. 2014;5:953. [Google Scholar]; b Kumar D, Raj KK, Malhotra SV, Rawat DS. Med. Chem. Commun. 2014;5:528. [Google Scholar]; c Hess C, Ventez D, Neri D. Med. Chem. Commun. 2014;5:408. [Google Scholar]; d Penthala NR, Sonar VN, Horn J, Laggas M, Yadlapalli JSKB, Crooks PA. Med. Chem. Commun. 2013;4:1073. doi: 10.1039/C3MD00130J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Baptissart M, Vega A, Maqdasy S, Caira F, Baron S, Lobaccaro JA. Biochimie. 2013;95:504. doi: 10.1016/j.biochi.2012.06.022. [DOI] [PubMed] [Google Scholar]; b Pearson JR, Gill CI, Rowland IR. Nat. Rev. 2009;67:509. doi: 10.1111/j.1753-4887.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 4.a Mukhopadhyay S, Maitra U. Curr. Sci. 2004;87:1666. [Google Scholar]; b Gioiello A, Venturoni F, Ramimi S, Custodi C, Pellicciari R, Macciarulo A. Med. Chem. Commun. 2014;5:750. [Google Scholar]
- 5.Chiang JY. J. Lipid Res. 2009:1955. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallimore AM, Godkin A. N. Engl. J. Med. 2013;368:282. doi: 10.1056/NEJMcibr1212341. [DOI] [PubMed] [Google Scholar]
- 7.Schlottmann K, Wachs F, Krieg RC, Kullmann F, Scholmerich J, Rogler G. Cancer Res. 2000;60:4270. [PubMed] [Google Scholar]
- 8.Bernstein H, Bernstein C, Payne CM, Dvorak K. World J. Gastroenterol. 2009;15:3329. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akare S, Martinez JD. Biochim. Biophys. Acta. 2005;1735:59. doi: 10.1016/j.bbalip.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Powell AA, Larue JM, Batta AK, Martinez JD. Biochem. J. 2001;356:481. doi: 10.1042/0264-6021:3560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degirolamo C, Modica S, Palasciano G, Moschetta A. Trends Mol. Med. 2011;17:564. doi: 10.1016/j.molmed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Katona BW, Anant S, Covey DF, Stenson WF. J. Biol. Chem. 2009;284:3354. doi: 10.1074/jbc.M805804200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao D, Stratagouleas ED, Martinez JD. Carcinogenesis. 2001;22:35. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- 14.a Bajaj SO, Shu P, Beuning PJ, O'Doherty GA. Med. Chem. Commun. 2014;5:1138. doi: 10.1039/C4MD00095A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Singh M, Singh A, Kundu S, Bansal S, Bajaj A. Biochim. Biophys. Acta Biomembr. 2013;1828:1926. doi: 10.1016/j.bbamem.2013.04.003. [DOI] [PubMed] [Google Scholar]; c Herzog IM, Fridman M. Med. Chem. Commun. 2014;5:1014. [Google Scholar]; d Herzog IM, Feldman M, Eldar-Boock A, Satchi-Fianaro R, Fridman M. Med. Chem. Commun. 2013;4:120. [Google Scholar]
- 15.Chhikara BS, Mandal D, Parang K. J. Med. Chem. 2012;55:1500. doi: 10.1021/jm201653u. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal HK, Chhikara BS, Bhavaraju S, Mandal D, Doncel GF, Parang K. Mol. Pharm. 2013;10:467. doi: 10.1021/mp300361a. [DOI] [PubMed] [Google Scholar]
- 17.Pal K, Pore SK, Sinha S, Janardhanan R, Mukhopadhyay D, Banerjee R. J. Med. Chem. 2011;54:2378. doi: 10.1021/jm101530j. [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Roy S, Reddy BS, Pal K, Sudhakar G, Iyer S. Mol. Cancer Res. 2011;9:364. doi: 10.1158/1541-7786.MCR-10-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sreekanth V, Bansal S, Motiani RK, Kundu S, Muppu SK, Majumdar TD, Sengupta S, Bajaj A. Bioconjugate Chem. 2013;18:1468. doi: 10.1021/bc300664k. [DOI] [PubMed] [Google Scholar]
- 20.a Singh M, Singh A, Kundu S, Bansal S, Bajaj A. Biochim. Biophys. Acta. 2013;1828:1926. doi: 10.1016/j.bbamem.2013.04.003. [DOI] [PubMed] [Google Scholar]; b Bansal S, Singh M, Kidwai S, Bhargava P, Singh A, Sreekanth V, Singh R, Bajaj A. Med. Chem. Commun. 2014 DOI:101039/c4md00303a. [Google Scholar]
- 21.Kamal A, Ponnampalli S, Vishnuvardhan MVPS, Rao MPN, Mullagiri K, Nayak VL, Chandrakant Med B. Chem. Commun. 2014 DOI: 10.1039/c4md00219a. [Google Scholar]
- 22.Fulda S, Debatin KM. Oncogene. 2006;25:4798. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Zhou L, Liu J, Xiao J, Gao H, Yang K. New J. Chem. 2013;37:575. [Google Scholar]
- 24.Li C, Rehman A, Dalley K, Savage PB. Tetrahedron Lett. 1999;40:1861. [Google Scholar]
- 25.Sharma A, Kundu S, Reddy AM, Bajaj A, Srivastava A. Macromol. Biosci. 2013;13:927. doi: 10.1002/mabi.201300018. [DOI] [PubMed] [Google Scholar]
- 26.Franke NAP, Rodermond HP, Stap J, Haveman J, Van Bree Nat C. Protocols. 2006;1:2315. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.