Abstract
Astatine-211 is possibly the most promising radionuclide for targeted α-particle therapy when it comes to the treatment of occult disseminated cancer. Preclinical research has proven effective, and patient studies have been initiated based on these results. However, a lack of production capacity and the complex radiochemistry of 211At are major obstacles for research and prospective clinical applications. In the present study, astatination of immunoconjugates, already prepared well in advance before radiolabeling, was performed to investigate the possibility of formulating a kit-like reagent for the production of 211At radiopharmaceuticals. The shelf-life of ɛ-lysyl-3-(trimethylstannyl)benzamide immunoconjugates was evaluated, that is, the effect of different storage times on the quality of the immunoconjugates. The quality being referred to is the capacity to maintain a good radiochemical yield and good cell-binding property after labeling with 211At. The stability of the conjugates was found to be pH dependent with high stability at pH≥7 and less stability at pH≤5.5. The immunoconjugates (based on trastuzumab) could be kept for more than 3 months in a phosphate buffered saline solution (pH 7.4) at 4°C before labeling, without compromising the quality of the labeled product. The conjugates are also unaffected by storage at −20°C. Conjugates with a good shelf-life compatible with distant shipping as well as improved radiochemistry are important steps to facilitate further clinical progress with 211At.
Key words: : antibodies, astatine-211, immunoconjugate, labeling, shelf-life
Introduction
The α-emitting radionuclide 211At has frequently been recognized as one of the most promising candidates for endoradiotherapeutic treatment of disseminated microtumors. Several research and preclinical studies utilizing 211At for therapeutic nuclear medicine applications have been conducted with both the free halide1 and 211At-labeled tumor-specific carrier vectors.2 Many of these studies included tumor-specific monoclonal antibodies, as they have suitable binding properties for a number of different malignancies.3–5 Encouraging preclinical results have been obtained with astatinated antibodies and two phase I studies have emerged from these studies.6,7 However, 211At requires a medium energy cyclotron (28 MeV alpha) for its production, which is a major obstacle hampering clinical trials. Only a few cyclotrons in the world have the capacity to produce the nuclide, and at the production capacity, each facility is limited. In addition, the α-decay of astatine may result in a considerable absorbed dose to the reaction solvent during labeling, which can affect the chemistry (i.e., self-oxidation) of astatine, decomposition of the precursor, and/or alter the structural and biological integrity of the antibody. It has previously been reported that antibodies can be subjected to a maximum absorbed dose of ∼1000 Gy without affecting their immunoreactivity.8 Therefore, one of the most demanding challenges in 211At-radioimmunotherapy applications has been the development of adequate chemical labeling procedures for the production of 211At-labeled antibodies at clinical levels of activity. Unlike the direct iodination of proteins, astatine cannot be stably attached to unmodified antibodies.9 Therefore, a number of different bifunctional labeling reagents have been developed for the astatination of proteins.10–13 The radiochemistry is generally conducted in two steps: labeling of the reagent and conjugation of the labeled reagent to the antibody. However, when using this strategy, problems generally occur with yields and the final quality under high-activity conditions due to radiolytic effects in the reacting solvent.14,15 Recently, a different chemical route for producing astatinated antibodies was reported.16 In this method, the antibody is conjugated with the reagent before labeling, which means that only one radiochemical step is involved in the reaction. The procedure enables very fast production of astatinated antibodies; therefore, no detrimental absorbed doses arise in the reacting solvent even at high-activity levels. Yields and quality have been shown to be very good, and the labeling system has the potential to be used in the clinical preparation of astatinated antibodies. In addition, the conjugates have the potential to be produced in advance to labeling as kit-like reagents (Fig. 1). This enables distant shipping to hospitals, with or close to cyclotron facilities, with the capacity to produce astatine.
FIG. 1.
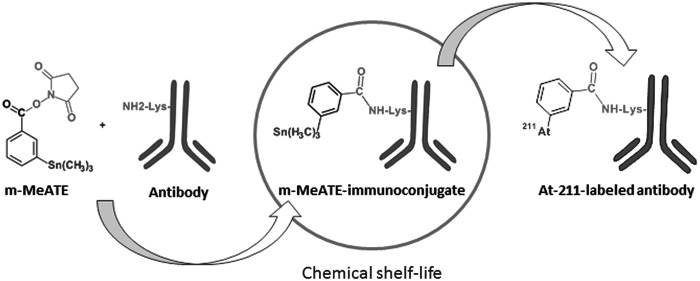
Conjugation of antibody and subsequent radiolabeling with 211At.
The subject of the present study was to investigate the shelf-life of ɛ-lysyl-3-(trimethylstannyl)benzamide immunoconjugates for subsequent astatination of antibodies. The immunoconjugates were evaluated regarding the chemical shelf-life before labeling and were analyzed for radiochemical yield (RCY), including radiochemical purity (RCP), structure integrity, and immunoreactive fractions after astatination.
Materials and Methods
General
Astatine-211 was obtained from the PET and Cyclotron Unit at Copenhagen University Hospital (Denmark). The nuclide was transformed into a chemically useful form by dry distillation at the Sahlgrenska Academy (Gothenburg, Sweden).17 The bifunctional labeling reagent N-succinimidyl-3-(trimethylstannyl)benzoate, (m-MeATE) of 97% purity was purchased from Toronto Research Chemicals, Inc. All other chemicals included in this study were obtained from Sigma-Aldrich, Inc. and were of at least analytical grade.
Antibody and cell line
The monoclonal antibody trastuzumab (Herceptin) was used in the study. Trastuzumab, which is specific for the human epidermal growth factor ErbB2 (Her2), was obtained from the Swedish Pharmacy, Sahlgrenska University Hospital. The human tumor cell line expressing Her2, SKOV3, was obtained from the American Type Culture Collection.
Antibody conjugation
ɛ-Lysyl-3-(trimethylstannyl)benzamide-trastuzumab conjugates were prepared in advance at different time points before radiolabeling, as described previously.16
Briefly, from a stock solution of 50 mg/mL m-MeATE in chloroform, 2 μL (100 μg, 0.26 μmol) was transferred to a reaction vial (1.1 STVG Chromacol) and the chloroform evaporated. The reagent was redissolved in 10 μL of dimethyl sulfoxide (DMSO). A 2-μL aliquot of the m-MeATE/DMSO solution was added to 1 mg of antibody at a concentration of 3–5 mg/mL in a 0.2 M carbonate buffer (pH 8.5), corresponding to a 7.5 times molar access of the m-MeATE reagent over antibody. After 30 minutes of reacting at room temperature under gentle agitation, the resulting ɛ-lysyl-3-(trimethylstannyl)benzamide-trastuzumab immunoconjugate was isolated by size exclusion chromatography on a NAP-5 (GE Healthcare; Sephadex G-25) column in 0.05 M citrate buffer (pH 5.5) or in a phosphate buffered saline (PBS) solution. In an average, 6.7±2.4 ATE reagents were attached to each antibody after the conjugations (determined by amine detection before and after conjugation with 2,4,6-trinitrobenzenesulfonate [TNBSA] using a standard protocol).18 The conjugates were vialed and sealed in air (atmospheric pressure) in conical plastic tubes (Eppendorf 1.7 mL) and then kept in a refrigerator (at 4°C) for up to 104 days before astatination. Immunoconjugates were also refrigerated at −20°C for 2 weeks before thawing to room temperature and labeling. The shelf-life of the immunoconjugates was determined as the capacity to maintain good labeling yield, good RCP, and good binding properties.
An immunoconjugate stock preparation was also synthesized for tin analysis. At regular time intervals after storage, samples were taken from the stock preparation and subjected to purification by size exclusion chromatography using NAP-5 columns to remove any small molecular entities, such as free trimethyltin, before measuring the remaining tin content.
211At labeling of immunoconjugates
The immunoconjugates were labeled with 211At, as described previously.16
Astatine-211 (10–200 MBq) was prepared as a dry residue and dissolved in 15 μL (20 μμg/mL) of N-iodosuccinimide (NIS)/methanol/1% acetic acid. Trastuzumab-lysyl-3-(trimethylstannyl)benzoate (100–200 μg at a concentration of 0.5–1 mg/mL in a buffer of pH 5.5; either 0.05 M citrate buffer or PBS acidified using 0.1 M citric acid) was then added to the 211At/NIS solution. After a 1-min reaction time, 3 μL of NIS (1 mg/mL) in methanol/1% HAc was added to the reaction mixture to iodo-substitute the remaining stannyl groups on the antibody. After an additional 1-min reaction time, the reaction was stopped with an excess of sodium ascorbate. The antibody fraction was then isolated by size exclusion chromatography on a NAP-5 column.
The RCY was determined as the antibody-bound fraction of the eluted nondecay corrected activity.
Radiochemical purity
The RCP was determined by methanol precipitation. For each labeled product, analytical samples were made in triplicates. A 1–15 kBq aliquot of the labeled product was added to 0.2 mL of PBS containing 1% bovine serum albumin and 0.05% sodium azide. The proteins were then precipitated by the addition of 0.5 mL of methanol. The total activity in each tube was measured before centrifugation. The supernatants were then withdrawn and the activity of the remaining cell pellets measured, rendering the protein-bound radioactivity. The RCP was calculated as the fraction protein-bound radioactivity of the total applied activity.
Immunoreactive fractions
The immunoreactive fraction was determined essentially according to the method described by Lindmo et al.19 A single-cell suspension of SKOV3 cells was prepared at a concentration of 5×106 cells/mL. The cells were serially diluted (1:2) six times and a constant amount of 211At-labeled trastuzumab (5–10 ng) added to each dilution. The total radioactivity (T) added to each dilution, that is, the 211At-trastuzumab, was determined in a γ-counter. The astatinated antibodies were then reacted with the cell antigens for 3 h at room temperature during gentle agitation. After cell pelleting through centrifugation and washing, the bound fraction of each dilution was determined by measuring the activity associated with the cells (B). The immunoreactive fraction was determined by nonlinear regression analysis. The maximum bound fraction over total applied labeled antibodies (Bmax/T) was calculated by fitting the average data points from the binding assay using the least square method.
Analyses
Radioactivity measurements were conducted at high activities >1 MBq using an ionization chamber (Capintec; CRC-15 dose calibrator) and at low activities (<15 kBq) using a NaI(Tl) γ-counter (Wizard 1480, Wallac). The two radioactive detector instruments were cross-calibrated for the 70–90 keV photons emitted in the 211Po branch of the decay of 211At.
FPLC chromatography was performed on a Superdex-200 (10/300) column using an Äkta-FPLC system (GE Healthcare Bio-Sciences AB). In each run, 0.2–0.3 mg/mL of antibody was analyzed, isocratically, using PBS or the citrate buffer (pH 5.5) as the mobile phase with a flow rate of 0.5 mL/min.
Tin analyses were conducted on isotopes 118Sn and 120Sn using a Perkin Elmer (Elan 6000) ICP-MS with a sample dilution using 0.5 M HNO3 (suprapure) with an internal standard of 2.5 ppb In. Protein samples were diluted to ∼1–2 μg/mL.
Spectrophotometric analyses of chromogenic derivatives of TNBSA were performed using an Ultrospec 3300 pro at 335 nm.
Results and Discussion
Initially, the immunoconjugates were stored in a citrate buffer at pH 5.5. This is the reaction pH for astatination allowing for direct introduction of the prefabricated immunoconjugate during astatination. Labeling yields for the astatination of the immunoconjugates were in the range of 75%±5.5%, and no sign of decline was observed in yield during storage in the citrate buffer (pH 5.5) for the first 5 days before labeling. However, with longer storage, the labeling efficiency decreased to ∼ 20% at day 35. The RCP, as determined by methanol precipitation, was in the range of 99%±0.8% for all conjugates during the first 9 days of storage. After this time, however, a gradual decrease in RCP down to ∼80% was observed at day 35. The RCY (combining labeling yield and RCP) for immunoconjugates stored in the citrate buffer exhibited an exponential decrease from 74%±7.4% starting after ∼5 days of storage down below 21%±2.2% at day 29 (Fig. 2).
FIG. 2.
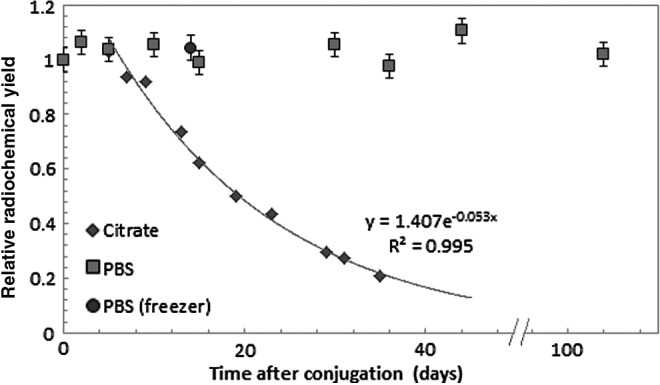
Radiochemical yield of 211At-labeled immunoconjugates after different times between conjugation and labeling. Storage in citrate (pH 5.5) (diamonds) and PBS (pH 7.4) (squares) buffer at 4°C or in PBS at −20°C (circles). Relative to a fresh reference solution. PBS, phosphate buffered saline.
The tin content of the immunoconjugates stored in citrate was investigated to determine whether detachment of the trimethylstannyl group caused the decrease in RCY. The number of tin groups per antibody on an aged series of immunoconjugates was measured using ICP-MS and compared to the amount on day 1 (6.7±2.4). As seen in Figure 3, the number of tin groups per antibody exponentially decreased starting from day 1. However, this initial decrease did not seem to influence the RCY until reaching a level of 50%–60% (after 5 days). The delay in the decrease in RCY could be explained by the low amount of astatine atoms applied during radiolabeling, ∼0.6 Pmol (10 MBq) 211At. This is several orders of magnitude lower than the number of antibody molecules and, therefore, available tin leaving groups for the astatodestannylation reaction. Therefore, if trying to achieve a very high-specific activity astatinated product using an immunoconjugate aged in the citrate buffer, a decrease in RCY would likely correspond to the loss of trimethyltin during these first days. In this case, the initial amount of m-MeATE groups and hence tin residues on each antibody could increase the shelf-life of the immunoconjugate even when stored in the citrate buffer. However, to maintain binding properties, the modifications to the antibody should be as gentle as possible, which is why increasing the shelf-life of the immunoconjugate by increasing the initial amount of conjugated m-MeATE groups per antibody is not a good option.
FIG. 3.
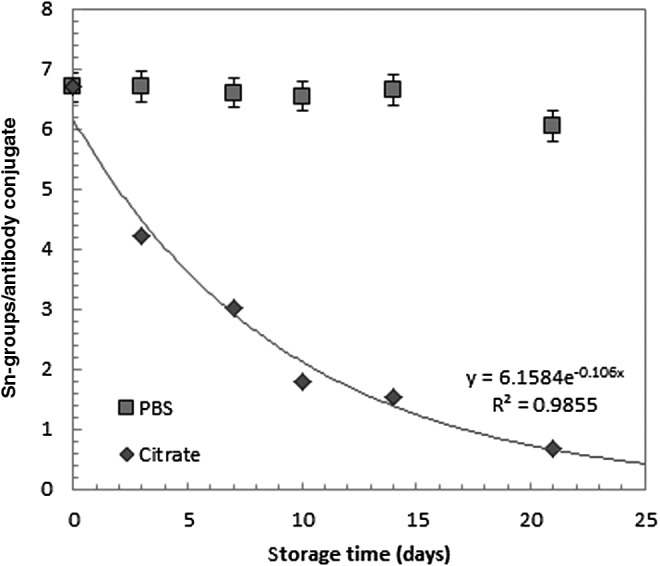
Remaining tin fraction in immunoconjugates at different times after conjugation. Storage in citrate (pH 5.5) and PBS (pH 7.4) buffer.
The decrease in residual tin most likely depends on a protodestannylation reaction of the immunoconjugate related to the pH of the storage and reaction buffer (pH 5.5).20 If this is true, it would mean that, by keeping the conjugates in a buffer with higher pH, the stability of the immunoconjugate should increase, and consequently, the RCY of the final product could be maintained for a longer time. This was investigated by keeping immunoconjugates in PBS of pH 7.4 and performing radiolabeling with 211At after different times of refrigerated storage. The results show that the RCY in this case is maintained after 36 days storage at 4°C, compared to freshly made immunoconjugates (Fig. 2). To enable the radiochemical reaction, the immunoconjugate storage solution was acidified just before labeling by addition of 0.1 M citric acid (3% V:V) to reach a final pH of 5.5. With regard to kit formulation, the used method of acidification before radio labeling is generally much simpler than changing the buffer of the immunoconjugate solution. Therefore, as proven effective, this would be the method of choice and all subsequently produced conjugated antibodies should be kept refrigerated in PBS, and the pH adjusted with acidification just before labeling. The initial results suggested that the storage time in PBS at 4°C could be significantly longer than 36 days. Immunoconjugates were therefore stored for 104 days in a refrigerator at 4°C before labeling with astatine. This resulted in an average labeling yield of 77.2%±1.2% with a RCP of 97.7%±1.1% (specific activity 310–350 MBq/mg) rendering a possible storage time for the prefabricated immunoconjuates of more than 3 months.
Besides storing at a standard refrigerator temperature of 4°C, conjugates were also kept refrigerated at −20°C. After 14 days at −20°C, the conjugates were thawed and subjected to astatine labeling. The results showed that yields and quality after radiolabeling were unaffected by the freezer storage and thawing process and a RCY of >80% and RCP >99% was obtained (Fig. 2) Although the stability at 4°C is more than 3 months, the stability upon freezing also opens for lyophilization, which may increase the shelf-life further.
Tin analyses of immunoconjugates stored for more than 20 days in PBS (pH 7.4) at 4°C also revealed that only minor protodestannylation takes place in this buffer, as confirmed by the high RCY (Fig. 3).
The structural integrity of the aged immunoconjugates, both in citrate and in PBS, was also investigated by FPLC and compared to the unmodified antibody to confirm that the storage did not cause any decomposition of the antibodies. The results from the chromatography did not show any signs of adverse structural changes in the different immunoconjugates, not even after being stored for over 40 days. The retention time for both conjugates (stored in citrate or PBS) and the unmodified antibody was 26.5 minutes using the conditions described above.
As the structural integrity of the antibody was maintained, even after aging, it was hypothesized that the immunoreactivity of the labeled conjugate would also be preserved. The immunoreactivity of conjugates stored for over 1 month was found stable for both PBS and citrate media, when taking the decrease in RCP into account for the citrate case (Fig. 4). An immunoreactive fraction value of 0.72 was obtained for the average data points from Figure 4.
FIG. 4.
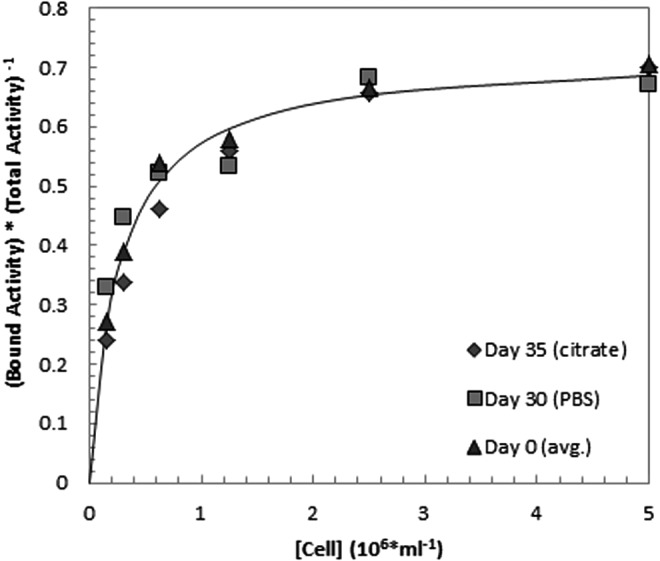
Immunoreactivity of 211At-labeled immunoconjugates of trastuzumab (freshly made, stored in citrate, or stored in PBS) toward Skov3-cells expressed as the bound activity fraction over the total amount applied.
A high-activity radiolabeling, employing 200 MBq of 211At, was also performed with an immunoconjugate aged in PBS for 29 days to indicate if the method would be useful for production of a clinical kit-like reagent. The RCY of the reaction was 71.8% resulting in a labeled product with a specific activity of 718 MBq/mg. The absorbed dose to the reaction volume was ∼165 Gy, indicating that much higher activity conditions may be used for subsequent clinical validations of the method.
Conclusions
The results indicate that immunoconjugates prepared by conjugating an antibody (e.g., Traztuzumab) with N-succinimidyl 3-(trimethylstannyl)benzoate can be stored in a neutral buffer (pH 7.4) for more than 3 months at 4°C and stored at −20°C without compromising the quality of the labeled product. When stored in PBS, acidification of the immunoconjugate using citric acid before labeling enabled high efficiency and high specific activity astatination of the immunoconjugate, making the buffer change obsolete. However, it should be noted that the conjugates should not be stored in acidic pH conditions as this causes extensive decomposition due to protodestannylation.
The chemical stability in PBS is more than sufficient to allow for worldwide shipment of prefabricated immunoconjugates. The shelf-life of the conjugates for a clinical kit-like formulation will, however, ultimately rely on aseptic conditions but there will be no restrictions on the shelf-life due to chemical stability, if stored in a buffer slightly above neutral pH.
Acknowledgments
The authors thank Helena Kahu, Department of Oncology, Sahlgrenska Academy at the University of Gothenburg, for help with culturing the tumor cell lines. This work was supported by the King Gustaf V Jubilee Clinic Cancer Research Foundation in Gothenburgh, Sweden, grant no. 234/14 and the Swedish Cancer Society, grant no. 120765.
Disclosure Statement
The authors hereby declare that there is no existing conflict of interest, financial or otherwise, concerning the presented data and material of this article.
References
- 1.Hamilton JG, Durbin PW, Parrott M. The accumulation and destructive action of astatine 211 (eka-iodine) in the thyroid gland of rats and monkeys. J Clin Endocrinol Metab 1954;14:1161. [DOI] [PubMed] [Google Scholar]
- 2.Zalutsky MR, Vaidyanathan G. Astatine-211-labeled radiotherapeutics: An emerging approach to targeted alpha-particle radiotherapy. Curr Pharmaceut Des 2000;6:1433. [DOI] [PubMed] [Google Scholar]
- 3.Andersson H, Elgqvist J, Horvath G, et al. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: An overview of results in an ovarian tumor model. Clin Cancer Res 2003;9(10 Pt 2):3914S. [PubMed] [Google Scholar]
- 4.Cheng J, Ekberg T, Engstrom M, et al. Radioimmunotherapy with astatine-211 using chimeric monoclonal antibody U36 in head and neck squamous cell carcinoma. Laryngoscope 2007;117:1013. [DOI] [PubMed] [Google Scholar]
- 5.Zalutsky MR, Stabin MG, Larsen RH, et al. Tissue distribution and radiation dosimetry of astatine-211-labeled chimeric 81C6, an alpha-particle-emitting immunoconjugate. Nucl Med Biol 1997;24:255. [DOI] [PubMed] [Google Scholar]
- 6.Andersson H, Cederkrantz E, Back T, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: Pharmacokinetics and dosimetry of (211)At-MX35 F(ab′)2—a phase I study. J Nucl Med 2009;50:1153. [DOI] [PubMed] [Google Scholar]
- 7.Zalutsky MR. Current status of therapy of solid tumors: Brain tumor therapy. J Nucl Med 2005;46:151s. [PubMed] [Google Scholar]
- 8.Larsen RH, Bruland OS. Radiolysis of radioimmunoconjugates - reduction in antigen-binding ability by alpha-particle radiation. J Labelled Compd Radiopharm 1995;36:1009 [Google Scholar]
- 9.Visser GWM, Diemer EL, Kaspersen FM. The nature of the astatine-protein bond. Int J Appl Radiat Isot 1981;32:905. [DOI] [PubMed] [Google Scholar]
- 10.Garg PK, Archer GE, Bigner DD, et al. Synthesis of radioiodinated N-succinimidyl iodobenzoate - optimization for use in antibody labeling. Appl Radiat Isot 1989;40:485. [DOI] [PubMed] [Google Scholar]
- 11.Hadley SW, Wilbur DS, Gray MA, et al. Astatine-211 labeling of an antimelanoma antibody and its Fab fragment using N-succinimidyl para-astatobenzoate - comparisons invivo with the para-[125i]iodobenzoyl conjugate. Bioconjug Chem 1991;2:171. [DOI] [PubMed] [Google Scholar]
- 12.Reist CJ, Foulon CF, Alston K, et al. Astatine-211 labeling of internalizing anti-EGFRvIII monoclonal antibody using N-succinimidyl 5-[At-211]astato-3-pyridinecarboxylate. Nucl Med Biol 1999;26:405. [DOI] [PubMed] [Google Scholar]
- 13.Wilbur DS, Vessella RL, Stray JE, et al. Preparation and evaluation of para-[at-211]astatobenzoyl labeled antirenal cell-carcinoma antibody A6h F(Ab′)2- in-vivo distribution comparison with para-[I-125]iodobenzoyl labeled A6h F(Ab′)2. Nucl Med Biol 1993;20:917. [DOI] [PubMed] [Google Scholar]
- 14.Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutic's, part 1: Effects of solvent on the degradation of radiohalogenation precursors by At-211 alpha-particles. J Nucl Med 2005;46:700. [PubMed] [Google Scholar]
- 15.Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 2: Radiolytic effects of At-211 alpha-particles influence N-succinimidyl 3-At-211-astatobenzoate synthesis. J Nucl Med 2005;46:1393. [PubMed] [Google Scholar]
- 16.Lindegren S, Frost S, Baeck T, et al. Direct procedure for the production of At-211-labeled antibodies with an epsilon-lysyl-3-(trimethylstannyl)benzamide immunoconjugate. J Nucl Med 2008;49:1537. [DOI] [PubMed] [Google Scholar]
- 17.Lindegren S, Back T, Jensen HJ. Dry-distillation of astatine-211 from irradiated bismuth targets: A time-saving procedure with high recovery yields. Appl Radiat Isot 2001;55:157. [DOI] [PubMed] [Google Scholar]
- 18.Hermanson GT. Bioconjugate Techniques, 2nd edition. London: Academic Press, 2008;1202 [Google Scholar]
- 19.Lindmo T, Boven E, Cuttitta F, et al. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77. [DOI] [PubMed] [Google Scholar]
- 20.Bamford CH, Tipper CFH. Reactions of Aromatic Compounds. Amsterdam, NY: Elsevier Pub. Co., 1972. [Google Scholar]


