Abstract
The methods used to assess cardiac parasympathetic (cardiovagal) activity and its effects on the heart in both humans and animal models are reviewed. Heart rate (HR)-based methods include measurements of the HR response to blockade of muscarinic cholinergic receptors (parasympathetic tone), beat-to-beat HR variability (HRV) (parasympathetic modulation), rate of post-exercise HR recovery (parasympathetic reactivation), and reflex-mediated changes in HR evoked by activation or inhibition of sensory (afferent) nerves. Sources of excitatory afferent input that increase cardiovagal activity and decrease HR include baroreceptors, chemoreceptors, trigeminal receptors, and subsets of cardiopulmonary receptors with vagal afferents. Sources of inhibitory afferent input include pulmonary stretch receptors with vagal afferents and subsets of visceral and somatic receptors with spinal afferents. The different methods used to assess cardiovagal control of the heart engage different mechanisms, and therefore provide unique and complementary insights into underlying physiology and pathophysiology. In addition, techniques for direct recording of cardiovagal nerve activity in animals; the use of decerebrate and in vitro preparations that avoid confounding effects of anesthesia; cardiovagal control of cardiac conduction, contractility, and refractoriness; and noncholinergic mechanisms are described. Advantages and limitations of the various methods are addressed, and future directions are proposed.
Keywords: Parasympathetic nervous system, Heart rate variability, Respiratory sinus arrhythmia, Spectral analysis, Post-exercise heart rate recovery, Cardiovascular reflexes
Introduction
The vagus nerves are a major conduit for efferent parasympathetic nerve activity targeted to multiple organ systems including cardiovascular, respiratory, gastrointestinal, and immune systems. The powerful effects of parasympathetic nerve activity on heart rate (HR), cardiac conduction, and smooth muscle tone are widely appreciated, and its anti-arrhythmic and anti-inflammatory actions are clinically relevant to a number of pathological states [1–3].
In this article, we provide an overview of the methods used to assess cardiac parasympathetic (cardiovagal) activity and its effects on cardiac function in humans and animals. We emphasize the strengths and limitations of different methods and experimental preparations and describe strategies to reveal underlying mechanisms of abnormal cardiovagal control of the heart in pathological states such as heart failure.
Cardiovagal tone and its modulation assessed through measurements of HR
Approaches commonly used to assess parasympathetic influences on HR include measuring: (1) the HR response to pharmacological blockade of muscarinic cholinergic receptors (cardiovagal tone), (2) the HR variability (HRV) attributed to parasympathetic modulation, (3) the rapid decline (recovery) of HR after termination of exercise (parasympathetic reactivation), and (4) reflex changes in HR elicited by activation or inhibition of specific sensory nerves. We begin by discussing the first three of these approaches.
Muscarinic cholinergic receptor blockade
Acetylcholine released from post-ganglionic cardiac parasympathetic nerves reduces HR by binding to muscarinic cholinergic receptors (primarily M2 subtype) on sinoatrial (S-A) nodal cells [4, 5]. Thus, the level of ongoing tonic parasympathetic activity can be estimated by measuring the increase in HR that occurs in response to acute administration of a muscarinic cholinergic receptor blocker such as methylatropine. The mean change in HR reflects the parasympathetic (vagal) tone. Although invasive, the method is easy to implement. Many anesthetic agents strongly inhibit cardiovagal tone [6–8]. Consequently, the majority of studies have utilized conscious human and animal subjects. When measuring resting vagal tone, it is obviously important to ensure a “resting” state before administration of the muscarinic receptor blocker and consider possible behavioral responses to drug administration that may alter sympathetic nerve activity (SNA). Stress and locomotor activity can occur in an unpredictable manner in conscious animals, particularly in freely behaving rodents. Furthermore, the dose-dependency and duration of peripheral muscarinic receptor blockade may vary depending on type of drug, route of administration, and species. If possible, the effectiveness of sustained muscarinic receptor blockade throughout the period studied should be confirmed.
The resting level of SNA and/or changes in SNA subsequent to injection of the muscarinic receptor antagonist may influence the HR response to muscarinic receptor blockade via well-known sympathetic-vagal interactions [9]. The influence of SNA on cardiovagal tone can be assessed by measuring HR responses to muscarinic receptor blockade before and during sustained pharmacological blockade of β-adrenergic receptors (Fig. 1) [10]. The determination of the intrinsic HR during double blockade and reversing the order of administration of the autonomic blockers allow one to estimate the cardiac parasympathetic and sympathetic tone in the absence and presence of the other (Fig. 1) [10]. Knowledge of the intrinsic HR is informative, particularly in pathological states that involve S-A node disease.
Fig. 1.
Determination of resting cardiac parasympathetic and sympathetic tone. The increase in mean HR after administering a muscarinic cholinergic receptor (M-ChR) blocker (e.g., atropine, tachycardia) reflects the cardiovagal tone present under baseline resting conditions. Conversely, the decrease in mean HR after β-adrenergic receptor (β-AdR) blockade (e.g., propranolol, bradycardia) reflects cardiac sympathetic tone. Subsequent administration of the second autonomic blocking agent during the peak HR response to the first agent identifies the intrinsic HR (double blockade) and enables calculation of residual sympathetic tone in absence of vagal tone (HR after M-ChR blockade—intrinsic HR) and residual vagal tone in absence of sympathetic tone (intrinsic HR–HR after β-AdR blockade)
HRV mediated by parasympathetic modulation
The increase in mean HR after muscarinic receptor blockade fails to capture the full importance of parasympathetic control. Fluctuations in parasympathetic nerve activity are a major source of HRV, particularly under resting conditions [11–13]. The rapidity of cholinergic transmission and muscarinic receptor signaling at the S-A node enables changes in parasympathetic activity to modulate pulse interval (PI, i.e., R-R interval) on a beat-to-beat basis. In contrast, the slower sympathetic noradrenergic transmission and β-receptor signaling cannot change HR so quickly [11–13]. Therefore, calculations of indices that reflect rapid beat-to-beat changes in PI provide measures of parasympathetic modulation.
Time-domain measurements
Parasympathetic indices measured in the time domain include the root mean square of successive R-R interval differences (rMSSD, ms) and the percentage of consecutive R-R intervals differing by >x ms (pNNx, %), where x =50 ms for humans [11]. These calculations have been adapted and normal values established for experimental animals including mice [12, 13]. HR varies with the respiratory cycle, increasing during inspiration and decreasing during expiration. This “respiratory sinus arrhythmia” results primarily from inhibition of cardiac parasympathetic activity during inspiration and return of vagal activity during expiration and can be quantified by measuring the maximum and minimum HR’s (PI’s) with each breath during either spontaneous or paced breathing [14–16]. The magnitude of the HR oscillation is increased at lower respiratory frequencies and higher tidal volumes [14–16]. Respiratory sinus arrhythmia is increased in young healthy subjects and is reduced with aging and in diseases associated with decreased parasympathetic nerve activity [14–17]. Although sinus arrhythmia (and parasympathetic activity in general) is a marker of good health, it is important to appreciate that excessive parasympathetic activation can have potentially deleterious consequences including syncope, pulmonary airway constriction, and increased gastric acid secretion.
Frequency-domain measurements
Spectral analysis of the R-R interval or PI time series provides a means of quantitating the variability of regular oscillations of PI over a range of frequencies (i.e., the frequency domain) [11–13]. The spectral power (variability) is distributed within three major frequency bands: very low frequency (VLF, ~0.04 Hz in humans), low frequency (LF, ~0.1 Hz in humans), and high frequency (HF,>0.15 Hz in humans). As expected, the absolute frequencies corresponding to these peaks vary between humans and different animal species [11–13, 18]. The HF-HRV reflects variations in PI synchronized to the respiratory cycle (breathing). As discussed earlier, this respiratory sinus arrhythmia is driven primarily by fluctuations in parasympathetic nerve activity.
Several factors limit the utility of HF-HRV (i.e., respiratory sinus arrhythmia) to estimate cardiovagal modulation. First, nonautonomic mechanisms such as stretch of the S-A node associated with respiratory-related changes in right atrial pressures may change HR and contribute to HF-HRV [19]. The nonautonomic contribution is usually small, but can be significant in some animal species. Repeat measurements of HRV after blockade of muscarinic receptors provide assurance that the results reflect cardiovagal modulation of HR. Second, HF-HRV is dependent on the depth and frequency of breathing, and fluctuations in SNA may potentially contribute to the respiratory sinus arrhythmia at very low breathing rates [14–16]. The degree of error introduced by changes in the depth and frequency of breathing, the need to correct for these differences, and the optimal method for making the corrections are controversial [14–16, 20]. Measurement of the transfer function between tidal volume or its correlates and HR and having subjects breathe at a paced frequency can control for variations in the depth and rate of breathing [14–16, 20, 21]. Third, the tonic level of SNA may indirectly influence HF-HRV via sympathetic-vagal interactions [9].
Given the significant contribution of sympathetic modulation to LF-HRV, the LF/HF ratio of HRV is commonly used as a measure of “sympathovagal balance” [11–13]. While the measurement is generally accepted, the interpretation of changes in the LF/HF ratio under various conditions has been the subject of much debate. Parasympathetic modulation contributes to LF-HRV, the extent of which depends on the physiologic state. Muscarinic receptor blockade can cause large reductions in LF-HRV in humans and animals [22, 23], even to the point of decreasing the LF/HF ratio. Clearly, in this situation, use of the LF/HF ratio as a measure of sympathovagal balance can be misleading. Attention to the absolute values of LF- and HF-HRV as well as their ratio and use of autonomic blockers to confirm parasympathetic and sympathetic contributions to HRV help ensure that results are properly interpreted.
HR recovery after exercise
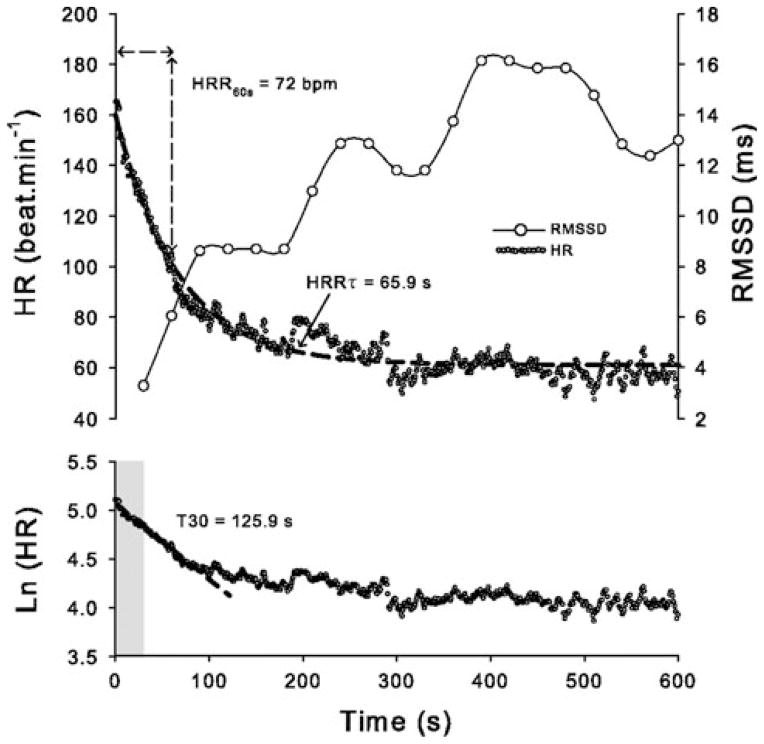
Exercise is accompanied by an increase in HR mediated by parasympathetic inhibition and sympathetic activation. Upon the termination of exercise, HR falls exponentially. The rapid early phase of this post-exercise HR recovery has been attributed to reactivation of parasympathetic neurons (Fig. 2) [24]. Like parasympathetic indices of HRV, post-exercise HR recovery is strongly attenuated after muscarinic receptor blockade and predicts morbidity and mortality in patients [24, 25].
Fig. 2.
Indices that reflect HR recovery (HRR) after submaximal exercise. Shown are HR, Ln (HR), and rMSSD measured over time after termination of submaximal exercise in an individual subject. HRR indexes include the: (i) decrease in HR over the first 30 s of HRR obtained by semi-logarithmic regression analysis (T30), (ii) absolute difference between HR at completion of exercise and after 60 s of recovery (HRR60s), and (iii) time constant of HR decay obtained by fitting HRR data to a first-order exponential decay curve (HRRτ). Short-term HRR indexes (T30 and HRR60s) are considered as relatively specific markers of parasympathetic reactivation, while the slower HRRτ may involve a combination of parasympathetic reactivation and withdrawal of sympathetic activity. The root mean square of successive R-R interval differences (rMSDD) calculated over consecutive 30 s periods confirms parasympathetic reactivation. Reproduced with permission from Buchheit et al. [34]
Comparisons between methods and measurements
Are different methods and measurements equivalent in terms of quantitating parasympathetic influences on the heart? Although HF-HRV indices reflecting parasympathetic modulation generally correlate with HR and parasympathetic tone measured in the resting state, there is considerable variability in the measurements and their correlation between subjects [15, 16, 26, 27]. Within subjects, a positive linear relationship between the parasympathetic contribution to mean HR and respiratory sinus arrhythmia (HF-HRV) over a range of parasympathetic activity has been demonstrated in anesthetized dogs [28] and awake humans [29]. The positive correlation relates to the complete (or nearly complete) inhibition of parasympathetic activity during inspiration that usually persists as parasympathetic tone is increased, resulting in high HF-HRV. These results support the measurement of HF-HRV as an estimate of parasympathetic tone. In contrast, several studies in humans, some of which incorporated autonomic blocking agents and paced breathing in the protocols, have revealed nonlinear relationships between parasympathetic tone and modulation (HRV) [16, 20, 26, 27, 30–32]. At relatively high levels of parasympathetic activity, HF-HRV may fail to increase and may actually decrease during progressive lengthening of the mean PI in humans (Fig. 3) [27, 30, 31]. Thus, HF-HRV may “saturate” at high levels of parasympathetic activity, presumably due to overwhelming of the inspiratory inhibition or a change in respiratory pattern/drive. Interestingly, parasympathetic blockade with atropine has been reported to reduce the R-R interval to a level significantly shorter than the minimum R-R interval measured during inspiration before parasympathetic blockade [26]. This result which is also evident after blocking β-receptors, suggests that there is significant cardiovagal activity during inspiration in healthy humans. Consistent with this view, changes in respiratory sinus arrhythmia are dissociated from changes in mean R-R interval during paced slow breathing in some subjects (Fig. 4) [26]. The correlation between HRV and post-exercise HR recovery is also variable with discordant values within and between subjects under a number of conditions [33–35].
Fig. 3.
Relationships between mean R-R interval and 3 indices of HRV in 3 subjects. Nitroprusside and phenylephrine were infused to change blood pressure and evoke baroreflex-mediated changes in parasympathetic activity. Initially, HRV and R-R interval both increased markedly with increases in parasympathetic activity in the young subject (24-year-old woman, squares), but HRV declined as mean R-R interval continued to increase at higher levels of parasympathetic activity. The nonlinear relationship between HRV and mean R-R interval was also apparent although less pronounced in the middle-aged (47-year-old woman, circles) and older (60-year-old man, triangles) subjects who exhibited low HRV. Indices of HRV included the standard deviation of R-R intervals (SD), the root mean square of successive R-R interval differences (MSSD), and high frequency (HF) HRV. Reproduced with permission from Goldberger et al. [30]
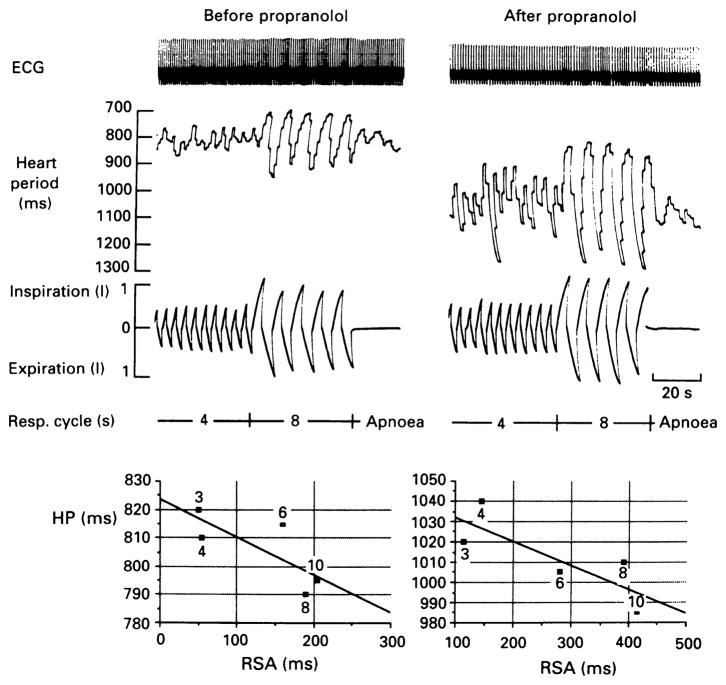
Fig. 4.
Example of increases in respiratory sinus arrhythmia during paced breathing at lower frequencies with decrease or no change in mean R-R interval. Upper panel: shown are ECG, heart period (R-R interval), and respiration during normal (4 s respiratory cycle length, 15 breaths/min) and slow (8 s respiratory cycle length, 7.5 breaths/min) breathing in a subject before (left panel) and after (right panel) administration of the β-adrenergic receptor blocker propranolol (0.2 mg/kg, IV). Respiratory sinus arrhythmia is markedly increased at lower breathing frequency while mean heart period remains relatively unchanged. Lower panel: Shown are values of mean heart period (HP) plotted against respiratory sinus arrhythmia (RSA, max–min HP) with respiratory cycle lengths of 3, 4, 6, 8, and 10 s as indicated on figure. Linear regression analysis shows inverse relationship between HP and RSA before and after propranolol. Reproduced with permission from Kollai and Mizsei [26]
The discordance between different measures of parasympathetic control likely relate to differences in parasympathetic tone versus modulation, the level of sympathetic tone, and different underlying mechanisms driving changes in cardiovagal activity under various conditions. For example, resting HF-HRV reflects inspiratory inhibition of basal parasympathetic activity mediated by oscillations in central respiratory drive and afferent input from cardiopulmonary receptors [14–16], and is generally accompanied by low sympathetic tone. In contrast, post-exercise HR recovery reflects reactivation of parasympathetic neurons mediated by sudden removal of vagolytic effects of skeletal muscle mechanoreceptor afferent activity and central drive, and persistent vagotonic input from arterial baroreceptor afferents and is accompanied by high sympathetic tone [24].
The results suggest that different methods of assessing parasympathetic control of the heart provide complementary information. How can we use this information to better predict cardiac risk in patients and develop new therapeutic approaches? A better understanding of the reflexes that modulate cardiovagal activity and HR are needed. The methods used to evaluate these reflexes in humans and animals are summarized in the next section.
Reflex control of cardiovagal activity and HR
Parasympathetic-mediated changes in HR are initiated primarily in the central nervous system or from activation or inhibition of sensory nerves (Fig. 5). Stimulation of arterial baroreceptors, peripheral chemoreceptors, trigeminal receptors, and subsets of cardiopulmonary receptors with vagal afferents reflexively increase cardiovagal activity and decrease HR. In contrast, stimulation of pulmonary stretch receptors with vagal afferents and subsets of visceral and somatic receptors with spinal afferents reflexively decrease cardiovagal activity and increase HR. A wide range of methods have been devised for reflex testing in the research laboratory and clinic. Several techniques used for assessing baroreflex control of cardiovagal activity and HR are reviewed below followed by brief descriptions of methods used to evaluate other reflexes that powerfully influence cardiovagal nerve activity and HR.
Fig. 5.
Overview of reflex control of cardiovagal (parasympathetic) nerve activity and HR. Excitatory (+) and inhibitory (−) afferent inputs to the central nervous system trigger reflex decreases and increases in HR, respectively
Arterial baroreceptor reflex
Baroreceptor afferents innervate large arteries including carotid sinuses, aortic arch, and right subclavian artery [36, 37]. Baroreceptor activity provides the major excitatory drive to cardiovagal efferent neurons under normal resting conditions [36, 38]. An increase in blood pressure evokes a further reflex increase in cardiovagal activity and a corresponding decrease in HR [36]. Conversely, cardiovagal activity is inhibited and HR increases when blood pressure is reduced. The baroreflex is typically assessed by measuring reflex changes in HR (or PI) during experimentally induced changes in baroreceptor afferent activity.
Drug-induced changes in blood pressure
Intravenous injections of vasoconstrictor (e.g., phenylephrine) and vasodilator (e.g., nitroprusside, nitroglycerin) drugs change blood pressure and elicit a baroreflex response in humans and animals. The cardiovagal baroreflex can be specifically evaluated by beat-by-beat measurements of systolic arterial pressure and PI after injection of phenylephrine (Oxford technique) [39–42]. The slope of the linear systolic arterial pressure—PI relation (ms/mmHg) provides a measure of cardiovagal baroreflex sensitivity (BRS). The method selectively interrogates the vagal limb of the reflex due to the rapidity of the reflex response. The method may underestimate BRS if blood pressure increases beyond the linear range of the sigmoidal arterial baroreflex function curve [43–45]. Conversely, the method may overestimate BRS if blood pressure and cardiac afterload increase sufficiently to increase intracardiac pressures with subsequent activation of cardiopulmonary vagal afferents and reflex bradycardia.
The injection of the vasodilator nitroprusside before phenylephrine enables assessment of the baroreflex over a wider pressure range (modified Oxford) [44, 45]. This approach may enable characterization of the entire sigmoidal baroreflex function curve including calculations of threshold, midpoint and saturation pressures, and maximum slope (gain) [43–45]. Similarly, steady state changes in HR during sustained infusions of phenylephrine and nitroprusside at multiple doses enable construction of the sigmoidal baroreflex function curve [43]. In this case, inhibition of SNA may contribute to reflex decreases in HR during phenylephrine infusion [43]. Therefore, β-adrenergic receptor blockade is required to selectively interrogate the parasympathetic limb of the reflex when using the steady state infusion protocol.
Valsalva maneuver
The Valsalva maneuver involves performing a forced expiration against a closed glottis or elevated airway pressure for a sustained period of time followed by a resumption of breathing [39, 40, 46, 47]. The forced expiration increases intrathoracic and intra-abdominal pressures that lead to decreases in venous return of blood to the heart, cardiac output, and arterial pressure. Release of straining causes a transient fall in blood pressure followed by increases in pressure and PI. Four phases of the response to the maneuver are described with the baroreflex being engaged during phase 2 when blood pressure falls and HR increases and during phase 4 when blood pressure rises and HR decreases [39, 46, 47]. Baroreflex control of HR is often quantified as the ratio of maximum to minimum HR during the maneuver (Valsalva Ratio). The slope of the relationship between increasing systolic pressure and increasing PI during phase 4 may also be taken as a measure of BRS.
The Valsalva maneuver is used clinically to assess autonomic function. The test is relatively simple to perform, does not require extensive equipment, and results are reproducible within a given subject [39, 46, 47]. The test has several disadvantages that limit its usefulness including considerable variability in how the test is carried out among clinicians and laboratories, engagement of cardiopulmonary reflexes in addition to the arterial baroreflex during the maneuver, and effects of disease on the blood pressure responses that confound interpretation of the changes in PI [39, 46, 47].
Neck suction/pressure
In humans, application of external negative and positive pressures to the cervical region via a neck chamber device evokes carotid sinus baroreflexes by changing the transmural pressure across the carotid sinus wall [39, 40, 48–50]. The magnitude, duration, and frequency of the pressure changes can be rigorously controlled. The ability to rapidly apply multiple, step changes in carotid transmural pressure enables assessment of cardiovagal BRS and construction of the sigmoidal baroreflex function curve without significant distortion by changes in SNA. Advantages of this method over the Oxford technique include the avoidance of possible effects of phenylephrine and nitro-vasodilators on afferent, central, and efferent components of the baroreflex and the ability to assess baroreflex control of vascular resistance and blood pressure in addition to HR. On the other hand, baroreflex-mediated changes in blood pressure will alter the activity of aortic baroreceptors leading to reflex buffering of the initial carotid baroreflex response. By measuring rapid PI responses to brief applications of neck suction, one can avoid the opposing action of the aortic baroreflex. The neck chamber and application of pressure changes may cause discomfort or anxiety that can potentially influence PI.
Spontaneous BRS
The methods described earlier require invasive procedures (e.g., intravenous drug administration) and specialized laboratory equipment (e.g., neck suction/pressure chamber). Computer-based analysis of spontaneous fluctuations in arterial blood pressure and HR enables calculation of spontaneous BRS in humans and animals [40, 51–54]. Two general methods are used: the sequence technique and spectral methods.
The sequence method involves detection of baroreflex sequences of 3 or more consecutive arterial pulses where systolic blood pressure and corresponding PI are positively correlated [40, 51–55]. Sequences in which systolic pressure and PI increase are referred to as “up” sequences, while sequences in which pressure and PI decrease are referred to as “down” sequences. The average slope of the linear systolic pressure–PI relations for all baroreflex sequences detected typically denotes the BRS. The ratio of the number of baroreflex sequences to the total number of linear systolic pressure sequences provides a measure of the extent of engagement of the reflex and is referred to as the baroreflex effectiveness index [55]. The sequence method also provides a means of evaluating the latency of cardiovagal baroreflex control of HR which can vary under physiologic and pathological states [53].
Criteria for defining a baroreflex sequence (e.g., correlation coefficient, minimum changes in systolic pressure and PI per beat, and the delay between systolic pressure and corresponding PI) can be adjusted by the investigator. Consistent with measurements of baroreflex latency in humans (time required for pressure change to elicit a reflex change in PI), PI’s are typically paired with systolic pressures immediately preceding each PI (0 beat delay) or systolic pressures from one beat earlier (1 beat delay) [40]. In contrast, a longer delay is often used in analysis of data from experimental animals with higher HR (1–4 beat delay) [54]. Spontaneous BRS calculated using the sequence technique reflects primarily reflex changes in cardiovagal activity (not SNA). The software may also be capable of detecting non-baroreflex sequences where systolic pressure and PI change in opposite directions; i.e., in a feed-forward manner as occurs during parallel, centrally induced changes in autonomic activity to the heart and peripheral vasculature [56].
Spontaneous BRS can also be measured using spectral methods. Oscillations in blood pressure and HR are measured in the LF and HF ranges by spectral analysis. The alpha index provides an estimate of BRS [40, 52]. It is calculated as the square root of the ratio between PI and systolic pressure spectral powers within the LF and/or the HF bands. An additional method involves calculation of the transfer function between the blood pressure and HR oscillations where the gain of the transfer function equates to BRS [40]. Both methods assume that the oscillations in blood pressure cause baroreflex-mediated oscillations in HR. Along this line, most investigators require that the oscillations in pressure and HR are coherent (coherence2 >0.5). Some investigators also require that the blood pressure oscillations lead the HR oscillations as one would expect if the HR changes are driven by the baroreflex. These criteria are usually satisfied when using the LF spectral powers in healthy control subjects. Cross-spectral analysis within the HF band may be questioned due to lack of a phase difference between systolic blood pressure and PI and possible baroreflex-independent effects of respiration on PI.
The sequence and spectral methods described earlier greatly expand the opportunities to measure BRS under various behavioral and environmental conditions, outside as well as inside the laboratory (Fig. 6). The methods are simple, inexpensive, and noninvasive. When combined with telemetric recordings of blood pressure and PI in mice and rats, the methods enable repeated measurements of cardiovagal BRS over prolonged periods without the disturbance and stress so easily imposed by injection of vasoactive drugs via external catheters.
Fig. 6.
Spontaneous baroreflex sensitivity (BRS) and number of baroreflex sequences measured over 24-h day/night cycle in young and old mild hypertensive subjects. Shown are the average hourly values of the number of baroreflex sequences (left) and BRS (average slope of systolic pressure-PI relations) (right) measured over a 24-h period using the sequence method in young (24 ±6 years, n =8, solid circles) and old (64 ±3 years, n =8, open circles) subjects. Data for “up” and “down” baroreflex sequences are shown in the upper and lower panels, respectively. Young subjects showed a striking diurnal variation in both the number of sequences and BRS with a decrease in number of sequences and increase in BRS at night. The elderly subjects exhibited low numbers of sequences, decreased BRS, and loss of diurnal variability. Reproduced with permission from Parati et al. [52]
HR turbulence
A premature ventricular contraction (PVC) triggers a fluctuation in PI over several heart beats, a phenomenon referred to as HR turbulence [57–59]. In normal subjects, PI decreases as blood pressure falls and then increases as blood pressure rises after the compensatory pause. The pattern of response suggests that the baroreflex mediates the changes in PI, and elimination of the changes in PI after blockade of muscarinic receptors indicates that the response is mediated primarily by parasympathetic modulation. HR turbulence, like other measures of cardiovagal BRS, predicts cardiovascular risk post-myocardial infarction [58]. Although a promising noninvasive test, the use of this method is limited in most subjects due to the low number of PVC’s. In the clinical electrophysiology laboratory, the frequency of PVC’s can be increased by programed stimulation thereby enhancing the utility of the method [59].
Isolated carotid sinus preparation (Moisejeff preparation)
Animal studies allow more control of the stimulus applied to the baroreceptors. For example, vascular isolation of the carotid sinuses enables precise control of the pressure stimulus (i.e., mean pressure, rate of change in pressure, and frequency, amplitude, and shape of pulsatile waveforms) [36, 60, 61]. The presence or absence of intact aortic baroreceptor afferents, cardiac afferents, and anesthesia are important determinants of the reflex response to changes in carotid sinus pressure. An intact aortic baroreflex will buffer responses to carotid baroreflex activation. Input from cardiac afferents reduces arterial BRS. The isolated carotid sinus preparation generally requires anesthesia, but has been used in conscious animals [60].
Electrical stimulation of baroreceptor afferents
Electrical stimulation of carotid sinus or aortic depressor nerves enables exquisite control of the magnitude and pattern of baroreceptor input to the central nervous system [62–69]. An additional advantage of direct stimulation of the afferents is that it bypasses the mechano-viscoelastic coupling between changes in pressure, blood vessel distension, and distortion of the sensory nerve endings. Furthermore, by varying the stimulus parameters, one can characterize and contrast reflex responses to activation of myelinated A-fiber versus nonmyelinated C-fiber afferents, which are differentially affected in hypertension. The method does not reproduce the natural recruitment pattern of afferent fibers with different pressure thresholds that occurs with increases in blood pressure. This difference and the presence of chemoreceptor afferents in carotid sinus nerves (and aortic depressor nerves in some species) are limitations. Fortunately, the aortic depressor nerves of rabbits, rats, and mice contain few chemoreceptor fibers [70], encouraging the use of this preparation to study baroreflex function in these species.
Electrical stimulation of baroreceptor afferents has most commonly been performed in anesthetized animals. Adaptation of the method for use in conscious animals [66, 67] and humans [68, 69] has provided new insights into baroreflex function and has led to clinical trials demonstrating sustained reductions in blood pressure in patients with drug-resistant hypertension [69].
Peripheral chemoreceptor reflex
Chemoreceptor stimulation during hypoxia, hypercapnia, and/or acidosis triggers reflex increases in both parasympathetic and sympathetic nerve activities; responses that are mitigated by hyperventilation and enhanced by apnea [71–73]. In the absence of hyperventilation, the reflex increase in cardiovagal activity predominates resulting in significant bradycardia [71, 72]. A standard method to measure chemoreceptor reflex sensitivity in humans and animals involves measuring ventilatory and cardiovascular responses to controlled decreases in inspired oxygen (O2) concentration [72, 73]. Carbon dioxide (CO2) is often added to the inspired air to prevent arterial pCO2 (estimated from measurements of end-tidal CO2) from falling. Inhalation of 5–10% O2 which results in arterial pO2 of ~35–40 mmHg causes near maximum activation of arterial chemoreceptors. During hypoxia, the addition of apnea enables the full expression of the chemoreceptor reflex-mediated increase in cardiovagal activity and bradycardia [71, 72]. Inhalation of 100% O2 inhibits chemoreceptor activity and has been used to assess the contribution of tonic chemoreceptor activity to ventilation, blood pressure, and HR in pathological states, including heart failure [74].
In anesthetized animals, mechanical ventilation and administration of neuromuscular blocking agents enable responses to hypoxia to be measured in the absence of hyperventilation. In this preparation, phrenic nerve activity can provide a measure of respiratory drive. Chemoreceptors can be activated by perfusing the isolated carotid sinus region with hypoxic blood or buffer [71]. This preparation eliminates confounding effects of systemic hypoxemia on peripheral vascular resistance, cardiac function, and the central nervous system and can be implemented in conscious as well as anesthetized animals [71, 75]. Another often used method to stimulate chemoreceptors in animals (anesthetized or awake) is to inject cyanide (bolus) either intravenously or into a common carotid artery proximal to the carotid chemoreceptors [76]. Although a powerful stimulant to chemoreceptors, cyanide also is a vasodilator and can have central nervous system effects. The chemoreflex/parasympathetic-mediated bradycardia occurs rapidly, thereby enabling its measurement before delayed vasodilator and depressor responses to cyanide confound the interpretation.
Diving reflex
Stimulation of trigeminal afferents produces apnea and strong reflex activation of cardiovagal activity and bradycardia [77–80]. The reflex is activated by exposure of the face to cold water during underwater diving, hence the term “diving reflex.” Like the chemoreceptor reflex, the increased parasympathetic activity is accompanied by increased SNA. Co-activation of trigeminal and chemoreceptor afferents, as may occur when submerged underwater for prolonged periods, markedly enhance the reflex bradycardia while the apnea persists [71]. The diving reflex can be simulated in the laboratory by applying cold compresses to the face of human subjects, i.e., the “cold face test” [78, 79]. In animal studies, the diving reflex can be evoked by selective, local stimulation of trigeminal afferents or by submerging the animal in water [80].
Cardiopulmonary reflexes involving activation of vagal afferents
Stimulation of nonmyelinated vagal afferent C-fibers innervating the heart and cardiopulmonary region produces strong reflex activation of efferent cardiovagal activity, bradycardia, inhibition of SNA, and vasodilation [81–84]. When triggered by activation of cardiac sensory receptors, the reflex is referred to as the “Bezold-Jarisch reflex.” Mechanical stimulation of afferents by strong contractions of an under-filled left ventricle and/or endogenous chemical factors produced during myocardial ischemia and reperfusion contribute to activation of this reflex.
The Bezold-Jarisch reflex has been studied extensively in experimental animals, most commonly by measuring reflex responses to intravenous, or preferably, intracoronary injection of chemicals (e.g., veratridine, prostanoids including prostacyclin, reactive oxygen species, serotonin, and the serotonin 5-HT3 receptor agonist phenylbiguanide). Similar autonomic responses are evoked by injection of chemicals into the pulmonary circulation (pulmonary chemoreflex). The Bezold-Jarisch reflex may occur during spontaneous myocardial ischemia and can be triggered by clinical interventions (e.g., intracoronary injection of contrast agent, reperfusion). Severe orthostatic stress as achieved by prolonged upright tilt or upright tilt combined with lower body negative pressure can evoke the Bezold-Jarisch reflex (i.e., vasovagal response) and is used to assess orthostatic tolerance [84].
Lung inflation reflex
Activation of pulmonary and thoracic stretch receptors during lung inflation has been reported to inhibit efferent cardiovagal activity, increase HR, and thereby contribute to respiratory sinus arrhythmia [71, 85, 86]. The evidence suggests that the contribution of the reflex to respiratory sinus arrhythmia in humans is relatively small compared with central mechanisms [86].
Cardiac and visceral reflexes involving activation of spinal (sympathetic) afferents
Stimulation of spinal afferents innervating the heart and visceral organs (e.g., intestine, bladder) causes reflex inhibition of cardiovagal activity and increases in SNA, HR, and blood pressure [87–89]. A variety of mechanical and chemical stimuli have been used to activate these sensory nerves in experimental animals. Chemicals known to stimulate cardiac sympathetic afferents include prostanoids, reactive oxygen species, serotonin, low pH, bradykinin, and adenosine. Note that many of these factors also stimulate cardiac vagal afferents that evoke directionally opposite autonomic responses. Consequently, reflex responses to stimulation of cardiac sympathetic afferents are often meager in the intact healthy animal but are robust after bilateral vagotomy or in disease states associated with decreased sensitivity of arterial baroreceptor and cardiopulmonary vagal afferent reflexes.
Somatic afferent reflexes
The activity of sensory nerves innervating skin and skeletal muscle modulate autonomic tone. For example, activation of group III (thinly myelinated) and group IV (nonmyelinated) afferents contributes to increases in HR and blood pressure during exercise [79, 90–92]. While activation of chemosensitive group IV afferents (metaboreceptors) are mainly responsible for the slowly developing increases in SNA during muscle contraction, activation of mechano-sensitive group III afferents (mechanoreceptors) contributes—along with signals originating in the central nervous system (central command)—to rapid reflex inhibition of efferent cardiovagal activity and the nearly immediate increase in HR with the onset of exercise.
Muscle contraction is usually induced by electrical stimulation of efferent motor nerves in experimental animals under anesthesia, while muscle contraction (exercise) is usually voluntary in human studies (although electrically induced contractions are feasible). The role of skeletal muscle afferents in mediating cardiac responses to exercise has also been studied in conscious and decerebrate animals [93–95]. The absence of anesthesia and its untoward effects on cardiovascular and autonomic state in these preparations provides a major advantage.
Direct assessment of cardiovagal activity and mechanisms of altered reflex control
HR and HRV are indirect measures of cardiovagal nerve activity and its reflex modulation. Several factors may dissociate measurements of PI from cardiovagal nerve activity including local modulation of neurotransmitter release from post-ganglionic nerve terminals, changes in neurotransmitter degradation or reuptake, and altered chronotropic responsiveness of S-A nodal cells (e.g., changes in muscarinic receptor signaling).
Measurement of efferent cardiovagal neuronal activity
Parasympathetic activity can be measured directly from somata of preganglionic parasympathetic neurons in the medulla oblongata of brain stem [96–100] or from efferent nerve fibers in the vagus nerve or its branches in experimental animals [94, 100–107].
Recording from somata of preganglionic neurons in brain stem
Somata of preganglionic cardiovagal neurons are located primarily in the nucleus ambiguus (NA) and the dorsal motor nucleus of the vagus (DMNX). Action potential discharge is recorded from these neurons using micro-electrodes placed extracellularly by stereotaxic guidance. The method requires histologic confirmation of proper electrode placement and evidence that the neuron projects to the heart. The latter is critical in that regulation of firing patterns differ for parasympathetic neurons innervating different target tissues. Antidromic activation of the neuron by electrical stimulation of cardiac branches of the vagus nerve provides good evidence that the neuron innervates the heart. A decrease in HR in response to iontophoretic application of a glutamate receptor agonist onto the NA or DMNX neuron confirms a cardioinhibitory function.
Cardiovagal neurons in NA and DMNX differ in morphology, axonal conduction velocity, pattern of action potential firing, innervation of cardiac ganglia, and effects on cardiac function. For example, NA neurons have thinly myelinated axons, exhibit strong respiratory- and cardiac-modulation, and exert more powerful chronotropic effects (decreases in HR) [96–99, 108]. In contrast, DMNX neurons have nonmyelinated axons, show little or no respiratory- and cardiac-modulation, have smaller effects on HR, and possibly stronger dromotropic (decreases in A-V nodal conduction) and inotropic (decreases in contractility) effects [99, 100, 109]. NA and DMNX neurons have been reported to innervate distinct populations of neurons within cardiac ganglia, consistent with different functional roles [110].
Recording from efferent fibers in vagus nerve
Nerve fibers are split from strands of nerve dissected from either a cervical vagus nerve or from cardiac branches of the vagus [94, 100–107]. Typically, the right vagus is the subject of study reflecting its prominent role in control of HR. Most commonly, nerve fibers are draped over bipolar or unipolar electrodes, although activity can also be recorded using a suction microelectrode. As with NA/DMNX recordings, it is essential to ensure that the activity being recorded arises from efferent cardioinhibitory parasympathetic nerves projecting to the heart. Consequently, repeated splitting of nerve fibers is typically required to obtain single-fiber or few-fiber preparations where the activity can be shown to be cardiovagal. Section of the vagus nerve distal to the recording site eliminates the activity of sensory nerves from the recording.
While the search for cardiovagal fibers within the cervical vagus nerve can be painstaking, this approach avoids the need to open the chest and preserves the option to record activity during spontaneous breathing. In contrast, recording from an anatomically identified cardiac branch of the vagus involves less fiber splitting but requires thoracotomy and mechanical ventilation. Furthermore, these nerves also contain sensory and sympathetic nerve fibers. As a result, regional sympathetic denervation and demonstration thatelectrical stimulation of the vagal nerve decreases HR should be performed before sectioning the nerve distally and recording efferent nerve traffic. Other criteria used for identifying fibers isolated from cervical and intrathoracic vagus nerves as cardiovagal include the presence of cardiac and respiratory rhythmicity, inhibition of activity during inspiration, and increases in activity during experimentally induced increases in arterial blood pressure or electrical stimulation of baroreceptor afferents. The absent or weak respiratory-modulation of vagal efferent activity originating in the DMNX raise the intriguing possibility that these nerves may mediate the apparent persistence of some parasympathetic activity (based on R-R intervals) during inspiration in conscious humans [26]. Typically, single-fiber or few-fiber activity is counted electronically using a window discriminator. Simultaneous counting of activity from multiple fibers can be accomplished by discriminating spikes using waveform recognition programs and spike-triggered averaging [107].
Defining afferent, central, and efferent sites of reflex modulation
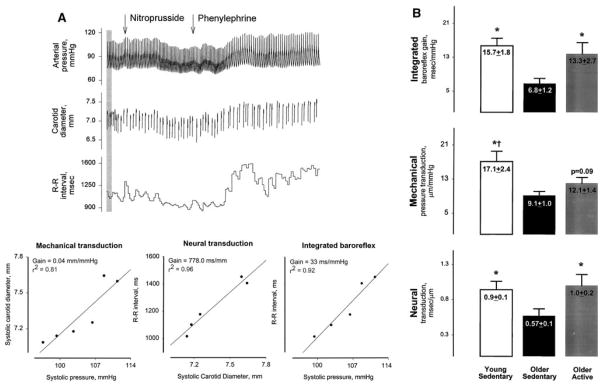
Abnormalities in reflex control of HR may be reflex-specific or widespread and may reflect alterations in sensory transduction (afferent), central mediation of the reflex, efferent neurotransmission and transmitter release, and/or chronotropic responsiveness of the S-A node. Comparisons of reflex bradycardia during activation of baroreceptor, chemoreceptor, and diving reflexes provide insight into site of impairment in disease. A variety of methods can be used to further localize the site(s) responsible for changes in overall reflex function in animals and humans. For example, measurements of arterial blood pressure, carotid artery diameter (ultrasonography), and PI during drug-induced or spontaneous changes in blood pressure enable assessment of the mechanical (vascular compliance) and the neural-SA nodal components of the cardiovagal baroreflex in humans (Fig. 7) [111–117]. Significant impairment of both neural and mechanical components of the reflex with aging has been reported [113].
Fig. 7.
Analysis of vascular and neural components of cardiovagal baroreflex sensitivity in humans. Panel A shown are measurements of arterial pressure, carotid artery diameter, and R-R interval from one subject during nitroprusside- and phenylephrine-induced decreases and increases in arterial pressure, respectively. The calculated slopes of relationships between systolic carotid diameter and systolic pressure (left), R-R interval and systolic diameter (middle), and R-R interval and systolic pressure (right) denote the sensitivity of the mechanical component, neural component, and integrated baroreflex, respectively. Panel B shown are data from young sedentary, older sedentary, and older active subjects. Both mechanical and neural components of the reflex were significantly decreased in the older sedentary group. Baroreflex sensitivity was preserved in active older individuals, an effect attributed primarily to a positive effect of activity on the neural component of the reflex. Reproduced with permission from Hunt et al. 2001 [112, 113]
Animal studies enable more precise dissection of the reflex pathway. Measurements of carotid sinus or aortic arch pressure-diameter-baroreceptor activity relationships define baroreceptor sensory transduction [36]. Changes in efferent vagus nerve activity and/or HR during graded electrical stimulation of baroreceptor afferents define the central mediation of the reflex [36, 63–66]. The same approaches can be used to dissect components of other reflexes. HR responses to chemical activation of NA and DMVX neurons, electrical stimulation of cervical vagus nerve(s), and stimulation of cardiac ganglia define efferent vagal control of HR [65, 96, 108, 109, 118, 119]. Release of the primary neurotransmitter acetylcholine from parasympathetic nerves can be measured [120, 121]. In animal models of disease, characterization of multiple components of multiple reflexes identifies not only sites of impairment but often reveals compensatory changes in other reflex components. In addition to disease models, these experimental approaches are applicable to most experimental questions (e.g., determining consequences of denervations, lesions, therapies, and genetic manipulation).
Limitations of in vivo studies and need for decerebrate and in vitro preparations
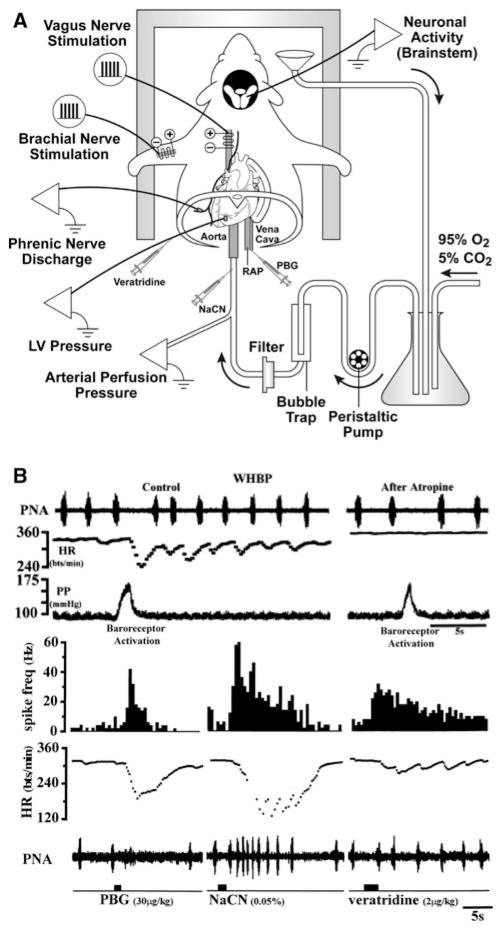
The majority of studies of reflex control of the circulation have involved use of in vivo preparations. While essential for understanding integrative cardiovascular control, human and intact animal studies often do not allow sufficient control of physiologic variables that influence the mechanism under investigation and/or do not provide adequate access to brain nuclei, ganglia, or cells for experimental recordings or manipulation. Furthermore, the anesthetic agents often needed for animal studies alter autonomic and cardiovascular functions with parasympathetic nerve activity being notably susceptible. Examples of decerebrate and in vitro preparations that overcome these limitations include decerebrate animals [94, 95], the working heart-brain stem preparation [122–124], innervated heart-ganglia preparations [125, 126], medullary brain slice preparations (e.g., NA) [127, 128], and intact whole-mount cardiac ganglia preparations and neurons dissociated from ganglia [129]. The working heart-brain stem preparation is illustrated in Fig. 8.
Fig. 8.
Working heart-brain stem preparation (WHBP). Rats or mice are subjected to subdiaphragmatic transection and decerebration under inhalation anesthesia. The WHBP is placed in a recording chamber, perfused with buffer, and instrumented. Panel A depicted are measurements of arterial perfusion pressure, left ventricular (LV) pressure, right atrial pressure (RAP), phrenic nerve activity, and neuronal activity in brain stem (e.g., nucleus tractus solitarius). The arterial baroreflex is activated by ramp increases in perfusion pressure. Chemical activation left ventricle, pulmonary, and arterial chemoreceptor afferents is achieved by injections of veratridine into the left ventricle, phenylbiguanide into the right atrium, and sodium cyanide into the aorta. The somatic afferent reflex is activated by electrical stimulation of the brachial nerve in the forelimb. HR responses to electrical stimulation of the vagus nerve can also be measured. Panel B shown are HR and phrenic nerve activity (PNA) responses to baroreceptor activation (before and after atropine); and chemical activation of pulmonary C-fibers (phenylbiguanide, PBG), arterial chemoreceptors (sodium cyanide, NaCN), and ventricular receptors (veratridine). Modified from Nalivaiko et al. [124] and Paton [122] and reproduced with permission
Beyond control of HR and cholinergic neurotransmission
The effects of cardiovagal activity on cardiac function extend beyond control of HR. The S-A node, A-V node, and atrial myocytes are densely innervated by post-ganglionic parasympathetic nerves [130–133]. In addition to HR, vagal stimulation also slows A-V nodal conduction, shortens atrial refractoriness, and reduces contractility of atrial myocytes. The vagal innervation of ventricles is relatively sparse compared with atria, but present albeit variable between species [134–136]. Furthermore, vagal stimulation can decrease left ventricular contractility, prolong refractoriness of ventricular myocytes, and prevent ventricular arrhythmias, particularly when sympathetic tone is high [126, 135]. These decreases in contractility and arrhythmias are mediated primarily through restraint of the sympathetic nervous system with little direct effect of the parasympathetic neurotransmitter acetylcholine on ventricular myocytes [126, 135].
Parasympathetic influences on cardiac conduction, refractoriness, and contractility can be assessed using standard techniques. Exciting methodology takes advantage of the location of multiple cardiac ganglia in anatomically distinct fat pads on the surface of the heart, and the exquisite, differential control of cardiac functions (HR vs. A-V conduction) attained by selective stimulation of different ganglia [130–133]. A variety of in vivo and in vitro methods are being used to explore the functional significance and potential clinical implications of differential control of cardiac functions by ganglia in animal models and humans [126, 135–138].
Treatment of chronic diseases with drugs and devices designed to enhance parasympathetic effects (e.g., acetyl-cholinesterase inhibitors and vagus nerve stimulation) has revealed novel beneficial downstream consequences of increased parasympathetic activity including anti-inflammatory, anti-apoptotic, and pro-angiogenic effects [2, 139–143]. These observations have greatly expanded the types of methods used in studies of parasympathetic regulation with more emphasis on chronic adaptations, measurements of gene expression and pro-inflammatory mediators, and genetic manipulation through gene transfer and gene targeting.
While acetylcholine and muscarinic receptors are central to cardiovagal control of the heart, noncholinergic mechanisms are emerging as important and are being targeted for experimental and therapeutic manipulation. Examples of mediators that significantly modulate parasympathetic control at the level of cardiac ganglia and the nerve–heart interface include endogenous nitric oxide, enkephalins, and numerous peptides [125, 144–148]. Pharmacological and genetic approaches to manipulate these systems complement the traditional use of drugs that block or facilitate cholinergic neurotransmission [125, 148].
Summary and future directions
The importance of cardiac parasympathetic (cardiovagal) activity on cardiac function is well established and widely appreciated. Advancement of our understanding in this area is dependent on the methods we use to study the system. Approaches commonly used to assess parasympathetic control of the heart include measuring the HR response to pharmacological blockade of muscarinic cholinergic receptors (cardiovagal tone), beat-to-beat HRV (parasympathetic modulation), the rate of post-exercise recovery of HR (parasympathetic reactivation), and reflex changes in HR. Clearly, the different methods engage different mechanisms and therefore provide complementary, not redundant information. Use of multiple methods within both research and clinical laboratories are therefore encouraged. The many methods for interrogating cardiovascular reflexes in patient populations have unique strengths and limitations. Some are limited by poor control of the sensory stimulus, the involvement of multiple reflexes in mediating autonomic and HR responses, and deficiencies in standardization of the method. Standardization of methods between laboratories will be particularly important for reaching the full potential of autonomic testing to predict cardiovascular risk in patients.
In many respects, progress toward understanding fundamental mechanisms of parasympathetic regulation lags behind our knowledge of sympathetic control. Reasons for this discrepancy include the potent inhibition of parasympathetic activity by most anesthetics, the consequent need for studies in conscious animals, the complex neurochemistry and circuitry of intrinsic cardiac ganglia, and the technical challenges of recording activity from cardiovagal efferent fibers in animal models. The increasing use of radiotelemetry to record ECG, blood pressure, and PI in unstressed freely behaving mice, and the development of decerebrate and in vitro methods that eliminate the need for anesthesia is reducing these barriers to progress.
The rapid pace of gene discovery and availability of methods for site-specific and temporally regulated gene targeting encourage future studies of molecular determinants of parasympathetic regulation. HRV, post-exercise HR recovery, and BRS are heritable traits in humans [149–155], with significant variations associated with several polymorphisms [156–162]. Striking changes in HRV and BRS in mouse models [163–168] suggest new candidate genes worthy of future investigation. Opportunities are abundant for translational research in parasympathetic regulation!
Acknowledgments
The authors acknowledge the numerous contributions madeby investigators worldwide to development of methods to assess cardiovagal nerve activity and its reflex control, which formed the basis of this review article. Unfortunately, due to space limitations, many important publications on this subject could not be cited, for which we apologize. The authors thank Harald M. Stauss, MD, PhD at the University of Iowa for his helpful comments on the manuscript. The author’s research into mechanisms of autonomic regulation has been funded by the National Institutes of Health (HL14388), the Department of Veterans Affairs, and the American Heart Association.
References
- 1.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Katona PG, Lipson D, Dauchot PJ. Opposing central and peripheral effects of atropine on parasympathetic cardiac control. Am J Physiol Heart. 1977;232:H146–H151. doi: 10.1152/ajpheart.1977.232.2.H146. [DOI] [PubMed] [Google Scholar]
- 5.Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol. 2001;96:528–538. doi: 10.1007/s003950170003. [DOI] [PubMed] [Google Scholar]
- 6.Halliwill JR, Billman GE. Effect of general anesthesia on cardiac vagal tone. Am J Physiol. 1992;262(Heart Circ Physiol 31):H1719–H1724. doi: 10.1152/ajpheart.1992.262.6.H1719. [DOI] [PubMed] [Google Scholar]
- 7.Bouairi E, Neff R, Evans C, Gold A, Andresen MC, Mendelowitz D. Respiratory sinus arrhythmia in freely moving and anesthetized rats. J Appl Physiol. 2004;97:1431–1436. doi: 10.1152/japplphysiol.00277.2004. [DOI] [PubMed] [Google Scholar]
- 8.Tzeng Y-C, Galletly DC, Larsen PD. Paradoxical respiratory sinus arrhythmia in the anesthetized rat. Auton Neurosci. 2005;118:25–31. doi: 10.1016/j.autneu.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 10.De Angeles K, Wichi RB, Jesus WRA, Moreira ED, Morris M, Krieger EM, Irigoyen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol. 2004;96:2174–2178. doi: 10.1152/japplphysiol.00870.2003. [DOI] [PubMed] [Google Scholar]
- 11.Task Force of the European Society of Cardiology, the North American Society of Pacing, Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 12.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol. 2007;93(1):83–94. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 13.Laude D, Baudrie V, Elghozi J-L. Effects of atropine on the time and frequency domain estimates of blood pressure and heart rate variability in mice. Clin Exp Pharmacol Physiol. 2008;35:454–457. doi: 10.1111/j.1440-1681.2008.04895.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30:486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 16.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther. 2002;27:85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 18.Baudrie V, Laude D, Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R904–R912. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- 19.Perlini S, Giangregorio F, Coco M, Radaelli A, Solda PL, Bernardi L, Ferrari AU. Autonomic and ventilatory components of heart rate and blood pressure variability in freely behaving rats. Am J Physiol. 1995;269(Heart Circ Physiol 38):H1729–H1734. doi: 10.1152/ajpheart.1995.269.5.H1729. [DOI] [PubMed] [Google Scholar]
- 20.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi L, Rossi M, Soffiantino F, Marti G, Ricordi L, Finardi G, Fratino P. Cross correlation of heart rate and the respiration versus deep breathing. Assessment of new test of cardiac autonomic function in diabetes. Diabetes. 1989;38:589–596. doi: 10.2337/diab.38.5.589. [DOI] [PubMed] [Google Scholar]
- 22.Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. J Auton Nerv Sys. 1995;51:85–89. doi: 10.1016/0165-1838(95)80010-8. [DOI] [PubMed] [Google Scholar]
- 23.Fazan R, Jr, de Oliveira M, Dias da Silva J, Joaquim LF, Montano N, Porta A, Chapleau MW, Salgado HC. Frequency-dependent baroreflex modulation of blood pressure and heart rate variability in conscious mice. Am J Physiol Heart Circ Physiol. 2005;289:H1968–H1975. doi: 10.1152/ajpheart.01224.2004. [DOI] [PubMed] [Google Scholar]
- 24.Coote JH, Bothams VF. Cardiac vagal control before, during and after exercise. Exp Physiol. 2001;86(6):811–815. doi: 10.1111/j.1469-445x.2001.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y-D, Dewland TA, Wencker D, Katz SD. Post-exercise heart rate recovery independently predicts mortality risk in patients with chronic heart failure. J Cardiac Fail. 2009;15:850–855. doi: 10.1016/j.cardfail.2009.06.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollai M, Mizsei G. Respiratory sinus arrhythmia is a limited measure of cardiac parasympathetic control in man. J Physiol. 1990;424:329–342. doi: 10.1113/jphysiol.1990.sp018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiviniemi AM, Hautala AJ, Seppanen T, Makikallio TH, Huikuri HV, Tulppo MP. Saturation of high frequency oscillations of R-R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Am J Physiol Heart. 2004;287:H1921–H1927. doi: 10.1152/ajpheart.00433.2004. [DOI] [PubMed] [Google Scholar]
- 28.Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- 29.Bloomfield DM, Sweibel S, Bigger JT, Jr, Steinman RC. R-R variability detects increases in vagal modulation with phenylephrine infusion. Am J Physiol. 1998;274:H1761–H1766. doi: 10.1152/ajpheart.1998.274.5.H1761. [DOI] [PubMed] [Google Scholar]
- 30.Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Relationship of heart rate variability to parasympathetic effect. Circulation. 2001;103:1977–1983. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]
- 31.Goldberger JJ, Ahmed MW, Parker MA, Kadish AH. Dissociation of heart rate variability from parasympathetic tone. Am J Physiol Heart. 1994;266:H2152–H2157. doi: 10.1152/ajpheart.1994.266.5.H2152. [DOI] [PubMed] [Google Scholar]
- 32.Medigue C, Girard A, Laude D, Monti A, Wargon M, Elghozi J-L. Relationship between pulse interval and respiratory sinus arrhythmia: a time- and frequency-domain analysis of the effects of atropine. Pflugers Arch Eur J Physiol. 2001;441:650–655. doi: 10.1007/s004240000486. [DOI] [PubMed] [Google Scholar]
- 33.Buchheit M, Gindre C. Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart. 2006;291:H451–H458. doi: 10.1152/ajpheart.00008.2006. [DOI] [PubMed] [Google Scholar]
- 34.Buchheit M, Papelier Y, Laursen PB, Ahmaidi S. Non-invasive assessment of cardiac parasympathetic function: post-exercise heart rate recovery or heart rate variability? Am J Physiol Heart. 2007;293:H8–H10. doi: 10.1152/ajpheart.00335.2007. [DOI] [PubMed] [Google Scholar]
- 35.Katz SD. In search of the optimal measure for assessment of parasympathetic control of heart rate. Clin Auton Res. 2010;20:1–2. doi: 10.1007/s10286-010-0055-9. [DOI] [PubMed] [Google Scholar]
- 36.Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev. 1976;56:100–176. doi: 10.1152/physrev.1976.56.1.100. [DOI] [PubMed] [Google Scholar]
- 37.Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci. 2001;940:1–19. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 38.Kollai M, Jokkel G, Bonyhay I, Tomcsanyi J, Naszlady A. Relation between baroreflex sensitivity and cardiac vagal tone in humans. Am J Physiol. 1994;266(Heart Circ Physiol 35):H21–H27. doi: 10.1152/ajpheart.1994.266.1.H21. [DOI] [PubMed] [Google Scholar]
- 39.Eckberg DL. Parasympathetic cardiovascular control in human disease: a critical review of methods and results. Am J Physiol. 1980;239(Heart Circ Physiol 8):H581–H593. doi: 10.1152/ajpheart.1980.239.5.H581. [DOI] [PubMed] [Google Scholar]
- 40.Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- 41.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- 42.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 43.Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst. 1987;21:203–213. doi: 10.1016/0165-1838(87)90023-3. [DOI] [PubMed] [Google Scholar]
- 44.Ebert TJ, Cowley AW. Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol. 1992;262:H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- 45.Hunt BE, Farquhar WB. Nonlinearities and asymmetries of the human cardiovagal baroreflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1339–R1346. doi: 10.1152/ajpregu.00038.2004. [DOI] [PubMed] [Google Scholar]
- 46.Korner PI, Tonkin AM, Uther JB. Valsalva constrictor and heart rate reflexes in subjects with essential hypertension and with normal blood pressure. Clin Exp Pharmacol Physiol. 1979;6:97–110. doi: 10.1111/j.1440-1681.1979.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 47.Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva’s maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Physiol. 1996;271(Heart Circ Physiol 40):H1240–H1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- 48.Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- 49.Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88(6):671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- 50.Cooper VL, Hainsworth R. Carotid baroreflex testing using the neck collar device. Clin Auton Res. 2009;19:102–112. doi: 10.1007/s10286-009-0518-z. [DOI] [PubMed] [Google Scholar]
- 51.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to the analysis of the arterial baroreflex. J Hypertens. 1985;3(Suppl 3):579–581. [PubMed] [Google Scholar]
- 52.Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268(Heart Circ Physiol 37):H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 53.Gulli G, Claydon VE, Cooper VL, Hainsworth R. R-R interval-blood pressure interaction in subjects with different tolerances to orthostatic stress. Exp Physiol. 2005;90(3):367–375. doi: 10.1113/expphysiol.2004.029496. [DOI] [PubMed] [Google Scholar]
- 54.Laude D, Baudrie V, Elghozi J-L. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R142–R150. doi: 10.1152/ajpregu.00319.2007. [DOI] [PubMed] [Google Scholar]
- 55.Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, Pedotti A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol. 2001;280:R744–R751. doi: 10.1152/ajpregu.2001.280.3.R744. [DOI] [PubMed] [Google Scholar]
- 56.Legramante JM, Raimondi G, Massaro M, Cassarino S, Peruzzi G, Iellamo F. Investigating feed-forward neural regulation of circulation from analysis of spontaneous arterial pressure and heart rate fluctuations. Circulation. 1999;99:1760–1766. doi: 10.1161/01.cir.99.13.1760. [DOI] [PubMed] [Google Scholar]
- 57.Wichterle D, Melenovsky V, Malik M. Mechanisms involved in heart rate turbulence. Cardiac Electrophysiol Rev. 2002;6:262–266. doi: 10.1023/a:1016385126668. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe MA, Marine JE, Sheldon R, Josephson ME. Effects of ventricular premature stimulus coupling interval on blood pressure and heart rate turbulence. Circulation. 2002;106:325–330. doi: 10.1161/01.cir.0000022163.24831.b5. [DOI] [PubMed] [Google Scholar]
- 60.Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol. 1981;241(Heart Circ Physiol 10):H838–H849. doi: 10.1152/ajpheart.1981.241.6.H838. [DOI] [PubMed] [Google Scholar]
- 61.Chapleau MW, Hajduczok G, Abboud FM. Pulsatile activation of baroreceptors causes central facilitation of baroreflex. Am J Physiol. 1989;256(Heart Circ Physiol 25):H1735–H1741. doi: 10.1152/ajpheart.1989.256.6.H1735. [DOI] [PubMed] [Google Scholar]
- 62.Richter DW, Keck W, Seller H. The course of inhibition of sympathetic activity during various patterns of carotid sinus nerve stimulation. Pflugers Arch. 1970;317:110–123. doi: 10.1007/BF00592496. [DOI] [PubMed] [Google Scholar]
- 63.Oberg B, Kendrick ED, Thoren P, Wennergren G. Reflex cardiovascular responses to graded stimulations of low- and high-threshold afferents in the carotid sinus and aortic nerves in the cat. Acta Physiol Scand. 1981;113:129–137. doi: 10.1111/j.1748-1716.1981.tb06873.x. [DOI] [PubMed] [Google Scholar]
- 64.Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol. 1999;277(Regulatory Integrative Comp Physiol 46):R748–R756. doi: 10.1152/ajpregu.1999.277.3.R748. [DOI] [PubMed] [Google Scholar]
- 65.Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1033–R1040. doi: 10.1152/ajpregu.00768.2001. [DOI] [PubMed] [Google Scholar]
- 66.Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R., Jr Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol Heart Circ Physiol. 2007;292:H593–H600. doi: 10.1152/ajpheart.00181.2006. [DOI] [PubMed] [Google Scholar]
- 67.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 68.Braunwald E, Epstein SE, Glick G, Wechsler AS, Braunwald NS. Relief of angina pectoris by electrical stimulation of the carotid-sinus nerves. N Engl J Med. 1967;277:1278–1283. doi: 10.1056/NEJM196712142772402. [DOI] [PubMed] [Google Scholar]
- 69.Doumas M, Guo D, Papademetriou V. Carotid baroreceptor stimulation as a therapeutic target in hypertension and other cardiovascular conditions. Expert Opin Ther Targets. 2009;13:413–425. doi: 10.1517/14728220902780185. [DOI] [PubMed] [Google Scholar]
- 70.Sapru HN, Gonzalez E, Krieger AJ. Aortic nerve stimulation in the rat: cardiovascular and respiratory responses. Brain Res Bull. 1981;6:393–398. doi: 10.1016/s0361-9230(81)80009-3. [DOI] [PubMed] [Google Scholar]
- 71.de Burgh Daly M. Monographs of the Physiological Society. Vol. 46. Oxford Medical Publications; Oxford: 1997. Peripheral arterial chemoreceptors and respiratory-cardiovascular regulation. [Google Scholar]
- 72.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 73.Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J, Shoemaker JK. Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Regul Integr Comp Physiol. 2009;296:R402–R410. doi: 10.1152/ajpregu.90772.2008. [DOI] [PubMed] [Google Scholar]
- 74.Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJS. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart. 1996;76:483–489. doi: 10.1136/hrt.76.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barros RCH, Bonagamba LGH, Okamoto-Canesin R, de Oliveira M, Branco LGS, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci. 2002;97:110–115. doi: 10.1016/s1566-0702(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 77.Schaller B, Cornelius JF, Prabhakar H, Koerbel A, Gnanaling-ham K, Sandu N, Ottaviani G, Filis A, Buchfelder M. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009;21:187–195. doi: 10.1097/ANA.0b013e3181a2bf22. [DOI] [PubMed] [Google Scholar]
- 78.Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16:202–207. doi: 10.1007/s10286-006-0332-9. [DOI] [PubMed] [Google Scholar]
- 79.Al-Ani M, Powell L, West J, Townend J, Coote JH. Exercise and diving, two conflicting stimuli influencing cardiac vagal tone in man. J Physiol. 1995;489(2):603–612. doi: 10.1113/jphysiol.1995.sp021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCulloch PF, DiNovo KM, Connolly TM. The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R224–R234. doi: 10.1152/ajpregu.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aviado DM, Aviado DG. The Bezold-Jarisch reflex: a historical perspective of cardiopulmonary reflexes. Ann NY Acad Sci. 2001;940:48–58. [PubMed] [Google Scholar]
- 82.Ustinova EE, Schultz HD. Activation of cardiac vagal afferents in ischemia and reperfusion: prostaglandins versus oxygen-derived free radicals. Circ Res. 1994;74:904–911. doi: 10.1161/01.res.74.5.904. [DOI] [PubMed] [Google Scholar]
- 83.Mark A. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1:90–102. doi: 10.1016/s0735-1097(83)80014-x. [DOI] [PubMed] [Google Scholar]
- 84.Cooper VL, Hainsworth R. Head-up sleeping improves orthostatic tolerance in patients with syncope. Clin Auton Res. 2008;18:318–324. doi: 10.1007/s10286-008-0494-8. [DOI] [PubMed] [Google Scholar]
- 85.Anrep GV, Pascual W, Rossler R. Respiratory variations of the heart rate. I. The reflex mechanism of the respiratory arrhythmia. Proc R Soc Lond Ser B. 1936;119B:191–217. [Google Scholar]
- 86.Koh J, Brown TE, Beightol LA, Eckberg DL. Contributions of tidal lung inflation to human R-R interval and arterial pressure fluctuations. J Auton Nerv Syst. 1998;68:89–95. doi: 10.1016/s0165-1838(97)00114-8. [DOI] [PubMed] [Google Scholar]
- 87.Longhurst JC, Tjen-A-Looi SC, Fu L-W. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion: mechanisms and reflexes. Ann N Y Acad Sci. 2001;940:74–95. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- 88.Malliani A. Cardiovascular sympathetic afferent fibers. Rev Physiol Biochem Pharmacol. 1982;94:11–74. [Google Scholar]
- 89.Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol. 1996;271(Regulatory Integrative Comp Physiol 40):R751–R756. doi: 10.1152/ajpregu.1996.271.3.R751. [DOI] [PubMed] [Google Scholar]
- 90.McWilliam PN, Yang T. Inhibition of cardiac vagal component of baroreflex by group III and IV afferents. Am J Physiol. 1991;260(Heart Circ Physiol 29):H730–H734. doi: 10.1152/ajpheart.1991.260.3.H730. [DOI] [PubMed] [Google Scholar]
- 91.Al-Ani M, Robins K, Al-Khalidi AH, Vaile J, Townend J, Coote JH. Isometric contraction of arm flexor muscles as a method of evaluating cardiac vagal tone in man. Clin Sci. 1997;92:175–180. doi: 10.1042/cs0920175. [DOI] [PubMed] [Google Scholar]
- 92.Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567(2):713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 94.Murata J, Matsukawa K. Cardiac vagal and sympathetic efferent discharges are differentially modified by stretch of skeletal muscle. Am J Physiol Heart Circ Physiol. 2001;280:H237–H245. doi: 10.1152/ajpheart.2001.280.1.H237. [DOI] [PubMed] [Google Scholar]
- 95.Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fiber mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol. 2007;292:H866–H873. doi: 10.1152/ajpheart.00771.2006. [DOI] [PubMed] [Google Scholar]
- 96.McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol. 1978;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McAllen RM, Spyer KM. The baroreceptor input to cardiac vagal motoneurons. J Physiol. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rentero N, Cividjian A, Trevaks D, Pequignot JM, Quintin L, McAllen RM. Activity patterns of cardiac vagal motoneurons in rat nucleus ambiguus. Am J Physiol Regul. 2002;283:R1327–R1334. doi: 10.1152/ajpregu.00271.2002. [DOI] [PubMed] [Google Scholar]
- 99.Nosaka S, Yasunaga K, Tamai S. Vagal cardiac preganglionic neurons: distribution, cell types, and reflex discharges. Am J Physiol Regul. 1982;243:R92–R98. doi: 10.1152/ajpregu.1982.243.1.R92. [DOI] [PubMed] [Google Scholar]
- 100.Jones JFX, Wang Y, Jordan D. Activity of C fibre cardiac vagal efferents in anaesthetized cats and rats. J Physiol. 1998;507(3):869–880. doi: 10.1111/j.1469-7793.1998.869bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iriuchijima J, Kumada M. Activity of single vagal fibers efferent to the heart. Jpn J Physiol. 1964;14:479–487. doi: 10.2170/jjphysiol.14.479. [DOI] [PubMed] [Google Scholar]
- 102.Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- 103.Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol. 1972;222:1–15. doi: 10.1113/jphysiol.1972.sp009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davidson NS, Goldner S, McCloskey DI. Respiratory modulation of baroreceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent nerve activity. J Physiol. 1976;259:523–530. doi: 10.1113/jphysiol.1976.sp011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramadan MRM, Drinkhill MJ, Mary DASG. The effect of distension of the urinary bladder on activity in efferent vagal fibres in anesthetized dogs. Q J Exp Physiol. 1989;74:493–501. doi: 10.1113/expphysiol.1989.sp003296. [DOI] [PubMed] [Google Scholar]
- 106.Cerati D, Schwartz PJ. Single cardiac vagal fiber activity, acute myocardial ischemia, and risk for sudden death. Circ Res. 1991;69:1389–1401. doi: 10.1161/01.res.69.5.1389. [DOI] [PubMed] [Google Scholar]
- 107.O’Leary DM, Jones JFX. Discharge patterns of preganglionic neurons with axons in a cardiac vagal branch in the rat. Exp Physiol. 2003;88(6):711–723. doi: 10.1113/eph8802590. [DOI] [PubMed] [Google Scholar]
- 108.Geis GS, Kozelka JW, Wurster RD. Organization and reflex control of vagal cardiomotor neurons. J Auton Nerv Syst. 1981;3:437–450. doi: 10.1016/0165-1838(81)90080-1. [DOI] [PubMed] [Google Scholar]
- 109.Jones JFX, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats, and rabbits. J Physiol. 1995;489(1):203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over postganglionic neurons in rat cardiac ganglia by NA and DmnX neurons: anatomical evidence. Am J Physiol Regul Integr Comp Physiol. 2004;286:625–633. doi: 10.1152/ajpregu.00143.2003. [DOI] [PubMed] [Google Scholar]
- 111.Bonyhay I, Jokkel G, Karlocal K, Reneman R, Kollai M. Effect of vasoactive drugs on carotid diameter in humans. Am J Physiol. 1997;273(Heart Circ Physiol 42):H1629–H1636. doi: 10.1152/ajpheart.1997.273.4.H1629. [DOI] [PubMed] [Google Scholar]
- 112.Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension. 2001;37:1362–1368. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- 113.Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001;103:2424–2427. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- 114.Kornet L, Hoeks AP, Janssen BJ, Willigers JM, Reneman RS. Carotid diameter variations as a non-invasive tool to examine carotid baroreceptor sensitivity. J Hypertens. 2002;20:1165–1173. doi: 10.1097/00004872-200206000-00029. [DOI] [PubMed] [Google Scholar]
- 115.Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–2312. doi: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- 116.Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583(3):1041–1048. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deley G, Picard G, Taylor JA. Arterial baroreflex control of cardiac vagal outflow in older individuals can be enhanced by aerobic exercise training. Hypertension. 2009;53:826–832. doi: 10.1161/HYPERTENSIONAHA.109.130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma X, Abboud FM, Chapleau MW. Neurocardiovascular regulation in mice: experimental approaches and novel findings. Clin Exp Pharmacol Physiol. 2003;30:885–893. doi: 10.1046/j.1440-1681.2003.03927.x. [DOI] [PubMed] [Google Scholar]
- 119.Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng Z. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol. 2007;293:H997–H1006. doi: 10.1152/ajpheart.01124.2006. [DOI] [PubMed] [Google Scholar]
- 120.Abramochkin DV, Nurullin LF, Borodinova AA, Tarasova NV, Sukhova GS, Nikolsky EE, Rosenshtraukh LV. Non-quantal release of acetylcholine from parasympathetic nerve terminals in the right atrium of rats. Exp Physiol. 2009;95(2):265–273. doi: 10.1113/expphysiol.2009.050302. [DOI] [PubMed] [Google Scholar]
- 121.Shimizu S, Akiyama T, Kawada T, Shishido T, Yamazaki T, Kamiya A, Mizuno M, Sano S, Sugimachi M. In vivo direct monitoring of vagal acetylcholine release to the sinoatrial node. Auton Neurosci. 2009;148:44–49. doi: 10.1016/j.autneu.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 122.Paton JFR. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol. 1998;79:2365–2373. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- 123.Potts JT, Spyer KM, Paton JFR. Somatosympathetic reflex in a working heart-brainstem preparation of the rat. Brain Res Bull. 2000;53:59–67. doi: 10.1016/s0361-9230(00)00309-9. [DOI] [PubMed] [Google Scholar]
- 124.Nalivaiko E, Antunes VR, Paton JFR. Control of cardiac contractility in the rat working heart-brainstem preparation. Exp Physiol. 2009;95(1):107–119. doi: 10.1113/expphysiol.2009.048710. [DOI] [PubMed] [Google Scholar]
- 125.Mohan RM, Heaton DA, Danson EJF, Krishnan SPR, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- 126.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2 + transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone—epicardial Ca2 + fluorescence studiers using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol. 2009;95(1):80–92. doi: 10.1113/expphysiol.2009.048215. [DOI] [PubMed] [Google Scholar]
- 127.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 128.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardio-inhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- 129.Adams DJ, Harper AA. Electrophysiological properties of autonomic ganglion neurons. In: McLachlan EM, editor. Autonomic ganglia. Harwood Academic Publishers; Reading: 1995. pp. 153–212. [Google Scholar]
- 130.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251(Heart Circ Physiol 20):H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- 131.Sampaio KN, Mauad H, Spyer KM, Ford TW. Differential chronotropic and dromotropic response to focal stimulation of cardiac vagal ganglia in the rat. Exp Physiol. 2003;88(3):315–327. doi: 10.1113/eph8802525. [DOI] [PubMed] [Google Scholar]
- 132.Quan KJ, Lee JH, Van Hare GF, Biblo LA, Mackall JA, Carlson MD. Identification and characterization of atrioventricular parasympathetic innervation in humans. J Cardiovasc Electrophysiol. 2002;13:735–739. doi: 10.1046/j.1540-8167.2002.00735.x. [DOI] [PubMed] [Google Scholar]
- 133.Gray AL, Johnson TA, Ardell JL, Massari VJ. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol. 2004;96:2273–2278. doi: 10.1152/japplphysiol.00616.2003. [DOI] [PubMed] [Google Scholar]
- 134.Johnson TA, Gray AL, Lauenstein J-M, Newton SS, Massari VJ. Parasympathetic control of the heart. I. An interventriculo-septal ganglion is the major source of the vagal intracardiac innervation of the ventricles. J Appl Physiol. 2004;96:2265–2272. doi: 10.1152/japplphysiol.00620.2003. [DOI] [PubMed] [Google Scholar]
- 135.Casadei B. Vagal control of myocardial contractility in humans. Exp Physiol. 2001;86(6):817–823. doi: 10.1111/j.1469-445x.2001.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 136.Chiou C-W, Zipes DP. Selective vagal denervation of the atria eliminates heart rate variability and baroreflex sensitivity while preserving ventricular innervation. Circulation. 1998;98:360–368. doi: 10.1161/01.cir.98.4.360. [DOI] [PubMed] [Google Scholar]
- 137.Inoue H, Zipes DP. Changes in atrial and ventricular refractoriness and in atrioventricular nodal conduction produced by combinations of vagal and sympathetic stimulation that result in a constant spontaneous sinus cycle length. Circ Res. 1987;60:942–951. doi: 10.1161/01.res.60.6.942. [DOI] [PubMed] [Google Scholar]
- 138.Scanavacca M, Hachul D, Pisani C, Sosa E. Selective vagal denervation of the sinus and atrioventricular nodes, guided by vagal reflexes induced by high frequency stimulation, to treat refractory neurally mediated syncope. J Cardiovasc Electrophysiol. 2009;20:558–563. doi: 10.1111/j.1540-8167.2008.01385.x. [DOI] [PubMed] [Google Scholar]
- 139.Tsutsumi T, Ide T, Yamato M, Kudou W, Andou M, Hirooka Y, Utsumi H, Tsutsui H, Sunagawa K. Modulation of the myocardial redox state by vagal nerve stimulation after experimental myocardial infarction. Cardiovasc Res. 2008;77:713–721. doi: 10.1093/cvr/cvm092. [DOI] [PubMed] [Google Scholar]
- 140.Freeling J, Wattier K, LaCroix C, Li Y-F. Neostigmine and pilocarpine attenuated tumour necrosis factor α expression and cardiac hypertrophy in the heart with pressure overload. Exp Physiol. 2007;93(1):75–82. doi: 10.1113/expphysiol.2007.039784. [DOI] [PubMed] [Google Scholar]
- 141.Handa T, Katare RG, Kakinuma Y, Arikawa M, Ando M, Sasaguri S, Yamasaki Y, Sato T. Anti-Alzheimer’s drug, donepezil, markedly improves long-term survival after chronic heart failure in mice. J Cardiac Fail. 2009;15:805–811. doi: 10.1016/j.cardfail.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 142.Okazaki Y, Zheng C, Li M, Sugimachi M. Effect of the cholinesterase inhibitor donepezil on cardiac remodeling and autonomic balance in rats with heart failure. J Physiol Sci. 2010;60:67–74. doi: 10.1007/s12576-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kakinuma Y, Akiyama T, Sato T. Cholinoceptive and cholinergic properties of cardiomyocytes involving an amplification mechanism for vagal efferent effects in sparsely innervated ventricular myocardium. FEBS J. 2009;276:5111–5125. doi: 10.1111/j.1742-4658.2009.07208.x. [DOI] [PubMed] [Google Scholar]
- 144.Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009;164:1170–1179. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 145.Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2008;94(1):46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- 146.Caffrey JL. Enkephalin inhibits vagal control of heart rate, contractile force and coronary blood flow in the canine heart in vivo. J Auton Nervous Sys. 1999;76:75–82. doi: 10.1016/s0165-1838(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 147.Farias M, III, Jackson K, Johnson M, Caffrey JL. Cardiac enkephalins attenuate vagal bradycardia: Interactions with NOS-1-cGMP systems in canine sinoatrial node. Am J Physiol Heart Circ Physiol. 2003;285:H2001–H2012. doi: 10.1152/ajpheart.00275.2003. [DOI] [PubMed] [Google Scholar]
- 148.Danson EJ, Li D, Wang L, Dawson TA, Paterson DJ. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J Mol Cell Cardiol. 2009;46:482–489. doi: 10.1016/j.yjmcc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 149.Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
- 150.Sinnreich R, Friedlander Y, Sapoznikov D, Kark JD. Familial aggregation of heart rate variability based on short recordings–the kibbutzim family study. Hum Genet. 1998;103:34–40. doi: 10.1007/s004390050779. [DOI] [PubMed] [Google Scholar]
- 151.Singh JP, Larson MG, O’Donnell CJ, et al. Heritability of heart rate variability: the Framingham Heart Study. Circulation. 1999;99:2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- 152.Singh JP, Larson MG, O’Donnell CJ, et al. Genome scan linkage results for heart rate variability (the Framingham Heart Study) Am J Cardiol. 2002;90:1290–1293. doi: 10.1016/s0002-9149(02)02865-5. [DOI] [PubMed] [Google Scholar]
- 153.Tank J, Jordan J, Diedrich A, Stoffels M, Franke G, Faulhaber H-D, Luft FC, Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- 154.Maver J, Strucl M, Accetto R. Autonomic nervous system activity in normotensive subjects with a family history of hypertension. Clin Auton Res. 2004;14:369–375. doi: 10.1007/s10286-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 155.Uusitalo ALT, Vanninen E, Levalahti E, Battie MC, Videman T, Kaprio J. Role of genetic and environmental influences on heart rate variability in middle-aged men. Am J Physiol Heart Circ Physiol. 2007;293:H1013–H1022. doi: 10.1152/ajpheart.00475.2006. [DOI] [PubMed] [Google Scholar]
- 156.Ylitalo A, Airaksinen KE, Hautanen A, Kupari M, Carson M, Virolainen J, Savolainen M, Kauma H, Kesaniemi YA, White PC, Huikuri HV. Baroreflex sensitivity and variants of the renin angiotensin system genes. J Am Coll Cardiol. 2000;35:194–200. doi: 10.1016/s0735-1097(99)00506-9. [DOI] [PubMed] [Google Scholar]
- 157.Gollasch M, Tank J, Luft FC, Jordan J, Maass P, Krasko C, Sharma AM, Busjahn A, Bahring S. The BK channel β1 subunit gene is associated with human baroreflex and blood pressure regulation. J Hypertens. 2002;20:927–933. doi: 10.1097/00004872-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 158.Girard A, Sidi D, Aggoun Y, Laude D, Bonnet D, Elghozi JL. Elastin mutation is associated with a reduced gain of the baroreceptor-heart rate reflex in patients with Williams syndrome. Clin Auton Res. 2002;12:72–77. doi: 10.1007/s102860200023. [DOI] [PubMed] [Google Scholar]
- 159.Hautala AJ, Rankinen T, Kiviniemi AM, Makikallio TH, Huikuri HV, Bouchard C, Tulppo MP. Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. Am J Physiol Heart Circ Physiol. 2006;291:H459–H466. doi: 10.1152/ajpheart.01193.2005. [DOI] [PubMed] [Google Scholar]
- 160.Hautala AJ, Tulppo MP, Kiviniemi AM, Rankinen T, Bouchard C, Makikallio TH, Huikuri HV. Acetylcholine receptor M2 gene variants, heart rate recovery, and risk of cardiac death after an acute myocardial infarction. Ann Med. 2009;41:197–207. doi: 10.1080/07853890802477866. [DOI] [PubMed] [Google Scholar]
- 161.Probst-Hensch NM, Imboden M, Dietrich DF, Barthelemy J-C, Ackermann-Liebrich U, Berger W, Gaspoz J-M, Schwartz J. Glutathione S-transferase polymorphisms, passive smoking, obesity, and heart rate variability in nonsmokers. Environ Health Perspect. 2008;116:1494–1499. doi: 10.1289/ehp.11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matsunaga T, Gu N, Yamazaki H, Tsuda M, Adachi T, Yasuda K, Moritani T, Tsuda K, Nonaka M, Nishiyama T. Association of UCP2 and UCP3 polymorphisms with heart rate variability in Japanese men. J Hypertens. 2009;27:305–313. doi: 10.1097/HJH.0b013e32831ac967. [DOI] [PubMed] [Google Scholar]
- 163.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 164.Choate JK, Danson EJF, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol Heart Circ Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- 165.Cogliati T, Good DJ, Haigney M, Delgado-Romero P, Eckhaus MA, Koch WJ, Kirsch IR. Predisposition to arrhythmia and autonomic dysfunction in Nhlh1-deficient mice. Mol Cell Biol. 2002;22:4977–4983. doi: 10.1128/MCB.22.14.4977-4983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW. Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension. 2010;55:627–635. doi: 10.1161/HYPERTENSIONAHA.109.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]