Abstract
Cholangiocarcinoma (CCA) is difficult to diagnose at an early stage and most tumors are detected at late stage where surgery or other therapy is ineffective. Many advanced techniques are applied to diagnose CCA; however, most are expensive and have varying degrees of accuracy. A less invasive and simpler procedure such as serum markers would be of substantial clinical benefit for diagnosis, monitoring, and predicting outcome for CCA patients. Recent advances in “Omics” technologies offer remarkable opportunities for establishment of biomarker-related to diseases. In this review, the potential biomarkers obtained from proteomics and glycomic studies are evaluated. Several protein markers were discovered from patient specimen, using two dimensional-differential gel electrophoresis couple with liquid chromatography tandem mass spectrometry (2D-DIGE/LC-MS-MS), matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS), surface enhanced laser desorption/ionization (SELDI)-TOF-MS and capillary electrophoresis (CE)-MS, etc. Newly reported CCA-associated glycobiomarkers were identified using lectin-assisted, monoclonal antibody-assisted or specific-target strategies. The combination between carbohydrate binding-lectin and core protein-binding mAb significantly increased the values for detection of the glyco-biomarkers for CCA. Searching for specific and sensitive molecular markers to be used for population screening is worth being evaluated. This could lead to earlier diagnosis and improve outcome. Further investigation of those biomarker functions is also of value in order to better understand the tumor biology and use them as targets for future therapeutic agents.
Keywords: Biomarkers, Gene profiles, Molecular signature, Proteomics
Cholangiocarcinoma (CCA) is difficult to diagnose at an early stage, hence most tumors are detected past the point where surgery or other therapy is effective. As a result, most patients have poor prognosis [1]. To detect CCA, several approaches have been tested such as: ultrasonography and CT scanning, percutaneous transhepatic cholangiography, endoscopic retrograde cholangiography or fine needle aspiration. However, these techniques are expensive and have a varying degree of accuracy and are available to only a limited number of patients. An improved detection of malignancy with a simpler and less invasive approach such as serum markers would be of substantial clinical benefit for diagnosis, monitoring, and predicting outcome for CCA patients.
Currently, there are only nine cancer serum biomarkers that have been approved by the FDA for clinical use [2] such as α-fetoprotein (AFP) for monitoring liver cancer; prostate specific antigen (PSA) for screening and monitoring of prostate cancer; CA19-9 for pancreatic cancer; CA15-3/CA27-29 and HER2/neu for monitoring of breast cancer, etc. At present, carcinoembryonic antigen (CEA) and CA19-9 are the clinically used serum markers for CCA. However, the low sensitivity/specificity and also inadequate early detection, limit the usage of these markers for diagnosis of CCA. New tumor markers with higher sensitivity and specificity are urgently needed.
Several single serum markers for diagnosis of CCA have been recently reviewed [3]. Recent advances in “Omics” technologies offer remarkable opportunities for establishment of biomarker-related diseases. A variety of technologies have been applied to biomarker discovery. In this review, the potential biomarkers obtained from proteomic and glycomic studies are evaluated.
Proteomic-based markers
Serum proteome
Proteomic and advanced proteomic analysis allow protein profiling in large numbers, instead of focusing on single protein and is a valuable discovery tool for biomarkers. Proteomic-based approaches are increasingly applied to cancer biomarker discovery as proteins can be qualitatively and quantitatively analyzed. The protein profiles of biological samples determined in a high-throughput fashion allow us to identify proteins that are differentially regulated in cancers and may possibly be novel biomarkers.
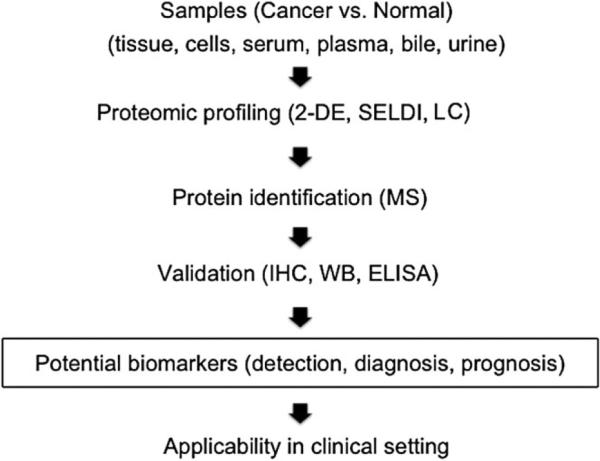
The process of evaluating a biomarker starts from discovery through validation, clinical trial and clinical application. The pipeline includes selection of biological samples of cancer and normal control, proteomic profiling by using alternative approaches (e.g. two dimensional gel electrophoresis, 2-DE; surface enhanced laser desorption/ionization, SELDI; and liquid chromatography, LC), data analysis to find the differentially expressed proteins, protein identification, validation of the proteins of interest by suitable methods, and evaluation of their applicability in a clinical setting as diagnostic and prognostic tools (Fig. 1).
Fig. 1.
Pipeline of molecular biomarker discovery by proteomic analysis
In Table 1, we review the results of proteomic profiling of CCA in various biological samples and the potential use of these biomarkers. Two proteomic profiles of CCA and normal liver tissues were analyzed by two dimensional differential gel electrophoresis (2D-DIGE) coupled with mass spectrometry [4, 5]. Immunohistochemistry (IHC) confirmed an increased expression of 14-3-3β/α protein in CCA cells while periostin and α-SMA (α-smooth muscle actin) were overexpressed in the stromal myofibroblasts surrounding CCA cells [4]. The detection of periostin or 14-3-3 proteins in serum of cancer patients suggested that these markers may be a useful marker for detection of malignancy [6, 7]. Periostin has been reported to be highly expressed in fibroblasts derived from CCAs and also to correlate with poor prognosis [8]. Another 2D-DIGE analysis and validated IHC data of CCA tissue microarrays showed elevated expression of S100A9 (S100 calcium-binding protein A9) and CCTγ (chaperonin-containing TCR1 subunit 3 (γ)) in CCA tissues. These data provided the novel diagnostic markers for CCA [5].
Table 1.
Potential cholangiocarcinoma (CCA) biomarkers discovered by proteomic analysis
| Sample | Proteomic approach | Validation method | Candidate biomarker | Applicability | References |
|---|---|---|---|---|---|
| Tissue | 2-D DIGE/LC-MS-MS | IHC | 14-3-3 β/α protein, periostin, α-SMA | Detection, prognosis | [4] |
| 2-D DIGE/MALDI-TOF-MS | IHC | S100A9, CCTγ | Diagnosis | [5] | |
| LC-MS-MS | IHC, WB | actinin-1, actinin-4, protein DJ-1, cathepsin B | Diagnosis | [9] | |
| LC-MS | IHC, WB | GOLM1, ANXA4, EPS8 | Early detection | [10] | |
| LM and AMT tag proteomics | IHC, WB | vimentin, profilin-1, CAII | Diagnosis | [11] | |
| Cell line | 2-DE/MALDI-TOF-MS | IHC | ANXA2, ENO1, PRX1, EBP50 | Prognosis | [12-14] |
| 2-DE/MALDI-TOF-MS/ESI-MS-MS | WB | CK7, CK19, galectin-3 | Diagnosis | [15] | |
| 2-DE/LC-MS-MS | WB | fascin, CK7, CK8, CK18, CK19 | Early detection | [16] | |
| Conditioned media | SDS-PAGE/LC-MS-MS | WB | lipocalin 2 | Diagnosis | [17] |
| Serum | SELDI-TOF-MS | WB, ELISA | TTR | Diagnosis | [18] |
| SELDI-TOF-MS | WB, ELISA | ApoA-I | Diagnosis | [19] | |
| 2-DE/LC-MS-MS | IHC, WB | A1BG, AFM | Diagnosis, prognosis | [20] | |
| Plasma | 2-DE/MALDI-TOF-MS | Nephelometry | AP1 | Diagnosis | [21] |
| SDS-PAGE/LC-MS-MS | IHC, WB | Orm2, KIF18A | Early diagnosis | [22] | |
| Bile | SDS-PAGE/LC-MS-MS | IHC, ELISA | Mac-2BP | Diagnosis | [24, 25] |
| SDS-PAGE/LC-MS-MS | ND | MUC 1, lipocalin 2, vimentin | Diagnosis | [26] | |
| 2DE/MS-MS | IHC, WB, ELISA | SSP411, PGAM-1, PDIA3, HSPD1 | Diagnosis | [30] | |
| Urine | CE-MS | CE-MS | fragments of interstitial collagens | Detection, diagnosis | [33] |
2-DE two-dimensional gel electrophoresis, A1 α1-antitrypsin, A1BG α1β-glycoprotein, AFM afamin, ALB serum albumin, ANXA2 annexin A2, ANXA4 annexin IV, ApoA-I apolipoprotein A-I, CAII carbonic anhydrase II, CCTγchaperonin-containing, CE capillary electrophoresis, CEACAM6 carcinoembryonic antigen-related cell adhesion molecule 6, CK cytokeratin, DIGE differential gel electrophoresis, EBP50 ezrin-radixin-moesin-binding phosphoprotein 50, ENO1 α-enolase, EPS8 epidermal growth factor receptor pathway substrate, ESI electrospray ionization, GLUTI glucose transporter 1, GOLM1 golgi membrane protein 1, HK II hexokinase type II, HSPD1 heat shock 60 kDa protein 1 (chaperonin), ITIH4 inter-alpha-trypsin inhibitor heavy chain H4, KIF18A kinesin 18A, LC-MS-MS liquid chromatography tandem mass spectrometry, LM and AMT tag laser microdissection and accurate mass and time tag, Mac-2BP mac-2-binding protein, MALD1-TOF matrix-assisted laser desorption/ionization-time of flight, MUC 1 Mucin-1, ND No Data, Orm2 Orosomucoid 2, PDIA3 protein disulfide isomerase family A member 3, PGAM-1 phosphoglycerate mutase 1 (brain), PRX1 peroxiredoxin 1, S100A9 S100 calcium-binding protein A9, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, SELD1 surface enhanced laser desorption/ionization, SSP411 sperm-specific protein 411, TCR1 subunit 3(γ), TTR transthyretin, α-SMA α-smooth muscle actin
A comparison of CCA and normal bile duct tissues, using differential liquid chromatography-mass spectrometry (LC-MS) based proteomics, identified the upregulation of actinin-1, actinin-4, protein DJ-1 and cathepsin B in CCA tissues. These four proteins were overexpressed in 5/6 CCA cases as shown by IHC and western blotting (WB), and may be considered as candidates for CCA diagnosis [9].
Fractionation of protein samples to enrich cellular subproteomes prior to analysis can increase the coverage of certain classes of molecules. Several highly expressed proteins in CCA were identified using a membrane protein enrichment strategy coupled with 18O-labeling based quantitative proteomics. Golgi membrane protein 1 (GOLM1), annexin IV and epidermal growth factor receptor pathway substrate (EPS8) were validated using WB and IHC of tissue microarrays. GOLM1 was overexpressed in 89% of CCA cases, and detectable in serum. This may represent an early detection of CCA [10].
In order to obtain an accurate protein profile of more homogeneous tumor cell populations from bulk tissues, Dos Santos performed a combination of laser microdissection (LM) and accurate mass and time (AMT) tag proteomics. The differentially expressed proteins between four intrahepatic CCA (ICC) and five perihilar large bile duct sections obtained from control subjects without ICC were analyzed [11]. WB analysis verified that vimentin and profilin-1 were overexpressed while carbonic anhydrase II (CAII) was under-expressed. IHC showed that vimentin was overexpressed in 70% of ICC but none was detected in the controls. These findings suggested that vimentin could be a diagnostic marker for ICC.
Proteomic profiles of four Opisthorchis viverrini-induced CCA cell lines (M156, K100, M139 and M213) were compared with non-tumor H69 biliary cell line using 2-DE followed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOFMS) [12–14]. Annexin A2 (ANXA2), α-enolase (ENO1), peroxiredoxin 1 (PRX1), ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) were differentially expressed. Data verification by IHC analysis of 301 cases CCA in the tissue microarrays showed an association of these protein candidates with prognostic parameters. ANXA2 was associated with lymphatic invasion, metastasis and shorter survival time [14]; ENO1 was related with poor prognosis and tumor invasion [12]; low expression of PRX1 and high expression of EBP50 correlated with reduced survival [13]. Collectively, these proteins could be the potential prognostic markers in CCA.
The markers for distinguishing CCA from hepatocellular carcinoma (HCC) were also identified by proteomic approaches. The proteomic pattern of bile duct epithelial carcinoma cell line (HuCCA-1) was compared to HCC cell lines (HepG2 and HCC-S102) [15]. The 2D gel electrophoresis (2DE) analysis and WB results demonstrated the exclusive expression of cytokeratin 7 (CK7), cytokeratin 19 (CK19) and galectin-3 in HuCCA-1. Additionally, the unknown protein U2/2 with MW/pI 42.2/6.20 was also highly expressed in HuCCA-1 but not in HCC cell lines. These proteins may serve as the useful biomarkers for differential diagnosis of CCA from HCC. Subproteomes of membrane and cytosolic proteins from the HuCCA-1 and the HCC-S102 cell lines could be enriched to improve the detection of low abundance or low-solubility proteins [16]. Comparative analysis using 2-DE and LC-MS-MS, some differentially expressed proteins, fascin, CK7, CK8, CK18, and CK19, were identified and confirmed by WB. Moreover, 10 membrane proteins were found in HuCCA-1 but not in HCC-S102, namely calgizzarin (S100A11), ezrin, moesin, radixin, immunoglobulin kappa chain variable region, inte-grin alpha-6 precursor (ITGA6), cytochrome c oxidase poly-peptide VIb isoform 1 (COX6B1), glycerol-3-phosphate dehydrogenase(GPD),hippocalcin-likeprotein 1(HPCAL1), mitogen-activated protein kinase 1 (MAPK1). Distinctive proteins found between CCA versus HCC cell lines may be possible biomarkers for distinguish CCA from HCC.
Comparison of secretomes from CCA (HuCCA-1) and four HCC cell lines (HCC-S102, HepG2, SK-Hep-1, and Alexander) was carried out by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) combined with LC-MS-MS approach [17]. Neutrophil gelatinase-associated lipocalin (lipocalin 2), laminin 5β3, cathepsin D precursor, desmoplakin, annexin IV variant, and annexin A5 were overexpressed in HuCCA-1. WB validation showed that lipocalin 2 was detected only in the conditioned media (CM) of HuCCA-1 but not in those of the HCC cell lines. Lipocalin 2 was also detected in CCA tissues but not in the normal adjacent tissues. This finding suggested that lipocalin 2 may be a possible diagnostic marker for discrimination of CCA from HCC.
Molecular markers in biological fluids, for example, serum, plasma, bile and urine allow us to detect or diagnose the patients in a non-invasive fashion. Serum secretomes of CCA analyzed by SELDI-TOF-MS, were reported from two studies. In the first study, sera profiles from 427 serum samples including 56 CCAs, 49 hepatobiliary diseases, 269 other cancer controls, and 53 healthy individuals were compared [18]. Downexpression of 13.76, 13.88, and 14.04k mass-to-charge (m/z) peaks were detected in sera of CCA patients compared to those from healthy controls and benign hepatobiliary diseases. These peptides were identified and confirmed by WB to be native transthyretin (TTR) and its two variants. The enzyme-linked immunosorbent assay (ELISA) indicated that TTR levels were consistent with SELDI analysis in CCA compared with control groups. Combination of TTR with carbohydrate antigen 19-9 (CA19-9) yielded 98.2% sensitivity and 100% specificity in differentiating CCA patients from benign hepatobiliary disease patients. This indicated that TTR may be a complementary marker of CA19-9 for CCA diagnosis.
The SELDI profiling of patients with 60 CCAs, 60 benign hepatobiliary diseases and 53 normal individuals was analyzed [19]. The 28k m/z peak was decreased in CCA patients’ sera and identified as apolipoprotein A-I (ApoA-I). WB and ELISA results validated the lower expression level of ApoA-I in CCA sera than in normal individuals, suggesting ApoA-I to be a potential diagnostic marker for CCA.
Our group recently reported the two dimensional gel electrophoresis profiles of low abundant proteins in the sera of CCA patients in comparison to healthy subjects [20]. Of the 36 differentially expressed proteins, α1β-glycoprotein (A1BG) and afamin (AFM) were detected consistently at different degrees in CCA sera compared to those of healthy controls. Serum A1BG/AFM ratio evaluated by a single WB analysis could detect CCA cases with 87.5% specificity and 84.4% sensitivity. The levels of A1BG/AFM ratio were significantly higher in CCA cases compared to controls. IHC staining of A1BG and AFM in CCA tissues confirmed the expressions of A1BG and AFM in serum. In addition, a high level of A1BG/AFM ratio in postoperative serum was associated with worse outcomes and the infiltration of resection margins. The A1BG/AFM ratio may constitute a candidate marker for diagnosis, prognosis and monitoring of CCA.
An assessment of low abundance plasma proteins of CCA patients and control subjects using 2-DE and MALDITOF-MS showed upregulation of α1-antitrypsin (AP1) in CCA patients [21]. Higher level of AP1 in CCA patients’ sera than those of healthy controls was verified by nephelometry (NPL). Using binary logistic regression analysis of three serum biomarkers (CA19-9, AP1 and α-fetoprotein: AFP), serum levels of at least CA19-9 together with AP1 were the minimum requirement to obtain prediction accuracy of greater than 80% for diagnosis of CCA. However, to obtain high predictability of 100% or approaching, an addition of at least one of the three liver enzymes (alkaline phosphatase: ALP; aspartate aminotransferase: AST; alanine aminotransferase: ALT) was required.
The early stages of CCA development can be studied using the hamster model. CCA were induced by the combination of N-nitrosodimethylamine (NDMA) treatment and Opisthorchis viverrini (OV) infection. Proteomic profiles of pooled plasma from normal control, NDMA-treated, OV-infected and OV+NDMA treated groups were generated by SDS-PAGE coupled with LC-MS-MS [22]. Two over-expressed molecules, orosomucoid-2 (Orm2) and kinesin-18A (KIF18A) were verified by WB in the hamster liver and plasma. The expression levels of these proteins were increased significantly at 21 days post-treatment, which was defined as the precancerous stage. IHC staining revealed that KIF18A was expressed in the epithelial cells of newly formed small bile ducts, some inflammatory cells and hepatocytes. Determination of these proteins in early stage CCA patients’ sera is needed to verify the possibility of using these proteins as early diagnosis of CCA.
Bile proteome
Instead of serum markers, bile is also a logical attractive alternative fluid for identifying CCA associated/specific biomarkers. Bile is more proximal than serum to the tumor and its flow through the biliary tree should favor the enrichment of CCA-derived products. Unfortunately, bile samples can only be obtained by invasive techniques and therefore the use for screening purposes or surveillance of population at risk is limited [23].
The catalog of human bile protein components of CCA patients was established and identified by SDS-PAGE/LCMS-MS approach [24]. Among malignancy-related proteins, Mac-2-binding protein (Mac-2BP) was of interest [25]. Level of Mac-2BP in bile samples was measured using ELISA. Biliary Mac-2BP levels were approximately threefold higher in the CCA patients compared with those from benign biliary conditions and primary sclerosing cholangitis (PSC). Mac-2BP was expressed in 94.4% (34/36 cases) of CCA tissue microarrays. Mac-2BP levels were as accurate as biliary CA19-9 levels, with an area under the curve (AUC) of 0.70 on receiver operator characteristic analysis. Combining both markers improved their diagnostic utility, yielding an AUC of 0.75 which is higher than that of using CA19-9 alone.
Another comprehensive catalog of bile proteins in four hilar CCA patients was also constructed by SDS-PAGE/LCMS-MS [26]. The products of 813 unique genes were identified. Of these, 268 were present in at least 3 of 4 patients. This dataset represented the largest catalog of bile proteins to date. A significant proportion of bile proteins were similar to major abundant proteins found in plasma [27] but included known CCA-associated proteins, for example, MUC 1 [28], lipocalin 2 [17] and vimentin [11, 29]. Comparative proteomic profiling of pooled bile samples from 15 CCA patients and 10 cholangitis patients were analyzed by a classic 2D-MS-MS strategy [30]. Thirty-eight proteins were upregulated in bile from CCA patients. WB confirmed that phosphoglycerate mutase 1 (brain) (PGAM-1), protein disulfide isomerase family A, member 3 (PDIA3), heat shock 60 kDa protein 1 (chaperonin) (HSPD1) and sperm-specific protein 411 (SSP411; also named SPATA20) were significantly upregulated in individual bile samples from CCA patients. These proteins were also overexpressed in CCA tissues relatively to normal tissues. Among these, SSP411 displayed a potential serum diagnostic biomarker for CCA, with a sensitivity of 90.0% and specificity of 83.3%.
Urine proteome
Although bile is the biofluid that would be most concentrated for CCA proteins, the collection of bile is invasive and time consuming. Urine appears to be an attractive diagnostic biofluid in many aspects: (1) it harbors lower numbers of proteins and lipids; (2) urine proteins remain relatively stable due to minimal proteolysis after sampling; and (3) it is non-invasive and frequently accessible [31, 32].
Urine proteomes of 14 CCA, 13 PSC and 14 benign biliary disorder (BBD) patients were established by capillary electrophoresis mass spectrometry (CE-MS) [33]. Of 42 CCA-specific peptide markers, the majority of sequence-identified peptides were fragments of interstitial collagens. In cross-sectional validation of 123 patients, the urine peptide marker model correctly classified 35 of 42 CCA patients and 64 of 81 PSC and BBD patients with AUC of 0.87, 83% sensitivity, and 79% specificity. Evaluation of 101 normal controls resulted in 86% specificity. All 10 patients with CCA on top of PSC were correctly classified, indicated its potential for PSC surveillance. IHC staining of matrix metallopeptidase 1 (MMP-1 also known as interstitial collagenase) noted the increased expression of MMP-1 in CCA tissues compared with PSC controls. This attributed increasing fragments of interstitial collagens in CCA urine. The urine test could differentiate CCA from PSC and other BBD and may provide an alternative diagnostic noninvasive tool for PSC surveillance and CCA detection.
Glyco-biomarkers
Aberrant in synthesis of glycans and glycoproteins in association with tumor development and/or progression have been demonstrated in many types of cancer [34–36]. Many cell surface glycans, such as CA19-9 (also known as sialyl-Lewis A, sLea) and sLex, were identified and used as biomarkers for diagnosis, monitoring, and prognostic prediction of many diseases including cancer. Roles of specific glycans and glycoproteins on cancer development and metastasis are progressively reported. For example, CA19-9 and sLex were identified as E-selectin adhesion moieties mediating extravasation during metastasis of cancer cells [37, 38]. CA-S27 (sLea associated glycan) involved in cell adhesion, migration and invasion of CCA cells [39].
During the past decades, many glycomics and glycolproteomics studies revealed a number of glyco-biomarkers for CCA. The values of some glyco-biomakers, such as CEA, CA19-9, mucin 5AC (MUC5AC), MUC1, total sialic acids, have been summarized in the previous review [3]. This review is aimed to summarize the glyco-biomarkers for CCA and also updating the values of newly reported CCA-associated glyco-biomarkers based on the strategies for discovery (Table 2): (1) lectin-assisted strategy; (2) monoclonal antibody (mAb)-assisted strategy; and (3) specific-target strategy.
Table 2.
Potential glyco-biomarkers for cholangiocarcinoma (CCA)
| Strategy for marker discovery | Potential lectins or mAbs | Screening |
Identification of carrier proteins |
Clinical applications |
References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Methods | Samples | Methods | Carriers/targets | Samples | Methods | Purpose | |||
| Lectin-assist | WFA | Tissue | LM | Tissue | LC-MS/MS | MUC1 | Bile | ELISA | Diagnosis | [40] |
| WFA | Tissue | LM | Bile | LC-MS/MS | L1CAM | Bile | ELISA | Diagnosis | [41] | |
| sWGA | Tissue | LHC | NA | NA | NA | Tissue | LHC | Diagnosis | [42] | |
| Prognosis | ||||||||||
| SBA | Serum | LB | NA | NA | NA | Tissue | LB | Diagnosis | [44] | |
| mAb-assist | CA-S27 | Serum | ELISA | Serum | LC-MS/MS | MUC5AC | Serum | ELISA | Diagnosis | [36] |
| Cell lines | Tissue | Prognosis | ||||||||
| KKU-S121 | Serum | ELISA | Serum | LC-MS/MS | MUC5AC | Serum | ELISA | Diagnosis | [46] | |
| Cell lines | Tissue | Prognosis | ||||||||
| Specific target | NA | NA | NA | NA | NA | GAG | Tissue | CAE, HPLC | Diagnosis | [47] |
| NA | NA | NA | NA | NA | perlecan | Tissue | IHC | Diagnosis | [50] | |
| NA | NA | NA | NA | NA | syndecan-1 | Tissue | IHC | Prognosis | [49] | |
| NA | NA | NA | NA | NA | agrin | Tissue | IHC | Diagnosis | [48] | |
| Prognosis | ||||||||||
CAE cellulose acetate electrophoresis, ELISA enzyme-linked immunosorbent assay, GAG glycosaminoglycans, HPLC high performance liquid chromatography, L1CAM LI cell adhesion molecule, LB lectin blotting, LC-MS/MS liquid chromatography tandem mass spectrometry, LHC lectin histochemistry, LM lectin microarray, mAbs monoclonal antibodies, MUC1 mucin 1, MUC5AC mucin 5AC, SBA soy bean agglutinin, sWGA succinylated wheat germ agglutinin, WFA Wisteria floribunda agglutinin
Lectin-assisted strategy
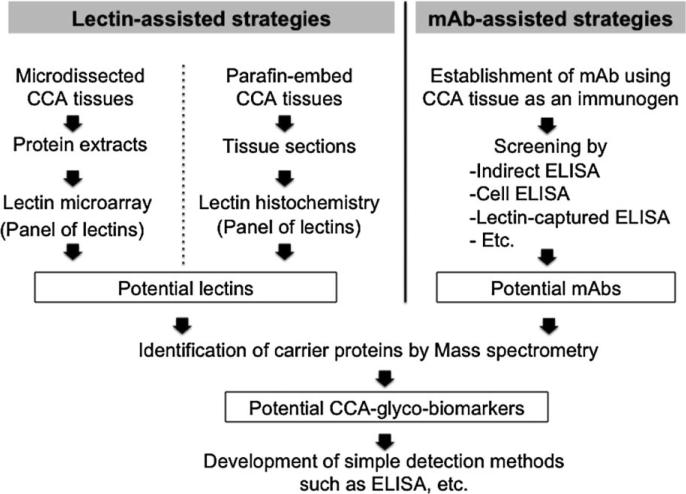
Lectin-assisted strategy for discovery of glyco-biomarkers was used based on the sugar specificity of the lectins. Many glyco-biomarkers were successfully identified using lectin approach. The lectin-assisted strategy for detection of CCA-associated glyco-biomarkers started by screening the potential-lectins, which could differentiate CCA from normal bile duct epithelium or sera of CCA patients from normal subjects as summarized in Figure 2. Lectinmicroarray consisting of 43 lectins has been used to identify the CCA-associated glyco-biomarkers in Japanese CCA patients’ tissues [39]. Wisteria floribunda agglutinin (WFA) was the most effective lectin to differentiate CCA from normal bile duct. WFA-binding glycoconjugates was purified using lectin-affinity chromatography and identified by LC-MS/MS. Mucin (MUC)-1 was identified as a major carrier of WFA-binding glycans in bile of CCA patients [40]. However, the identification of core-protein for WFA in microdissected-CCA tissues revealed the L1 cell adhesion molecule (L1CAM) as the main core-protein [41]. The ELISA was then developed using the combination of WFA and specific-mAbs against MUC1 or L1CAM. The ELISA was consequently used in the detection of WFA-positive MUC1 and WFA-positive L1CAM in bile and serum samples. The sensitivity and specificity for CCA diagnosis were 90% and 72% for WFA-positive MUC1 and 66% and 93% for WFA-positive L1CAM, respectively. Combination of WFA-positive MUC1 and WFA-positive L1CAM increased the sensitivity and specificity for diagnosis of CCA up to 90% and 79%, respectively.
Fig. 2.
Lectin- and monoclonal antibody assisted strategies for discovery of glyco-biomarkers
Lectin histochemistry of tumor tissues from Thai CCA patients emphasized the alteration of glycan synthesis in CCA, when comparing CCA with the adjacent normal bile ducts [42]. The study revealed that succinylated-wheat germ agglutinin (sWGA), Ulex europaeus agglutinin-I (UEA-I), and Sophora japonica agglutinin (SJA) could distinguish CCA from normal bile ducts. The lectins showed less reactivity with normal bile ducts, hepatocyte, and stromal cells in the liver, while it strongly reacted with neoplastic bile ducts. The lectin profiles of tumor tissues from Thai and Japanese CCA patients were different. This discrepancy may be due to the different etiology of CCA of these two subject sets. This postulation is supported by the fact that tumor tissues from Thai- and Japanese-CCA exhibited different expression profiles [43].
Glycan profiles of CCA patients’ sera using lectin blotting revealed soybean agglutinin (SBA) is the most effective lectin to detect CCA-associated glyco-biomarkers [44]. From this basis, SBA was continuously used for development of lectin-based ELISA to detect markers in CCA patients’ sera. The SBA sandwich ELISA systems were developed to detect serum mucin MUC5AC [45], CCA-CA [46] and CA-S27 [36]. The ELISA systems offered a strong power for diagnosis and prognostic prediction of glycolbiomarkers for CCA.
Monoclonal antibody-assisted strategy
Monoclonal antibody (mAb)-assisted strategy is another successful approach to identify CCA-associated glycobiomarkers. Using tumor homogenate as the source of antigens, two monoclonal antibodies (KKU-S121 mAb and carbohydrate antigen S27; CA-S27 mAb) recognizing CCA-associated glyco-biomarkers were successfully established [36, 46]. Lea associated glycan epitope was identified as a specific antigen of CA-S27 mAb (so called CA-S27 antigen) while the epitope recognized by KKU-S121 mAb is still unknown and was designated as CCA-associated carbohydrate antigen (CCA-CA). Although a high level of MUC5AC showed the association with poor survival of CCA patients, high levels of both CA-S27 and MUC5AC in serum indicated worse survival of the patients compared with those exhibiting high MUC5AC alone.
Specific target strategy
Determination of glycosaminoglycans (GAG) by cellulose acetate electrophoresis and high-performance liquid chromatography (HPLC) revealed the elevation of hyarulinic acid in liver tissues obtained from CCA patients, when compared to those from normal liver and HCC [47]. Heparan sulfate proteoglycans such as perlecan (basement-membrane-type heparan sulfate proteoglycan), syndecan-1, and agrin were elevated in CCA and played important roles in CCA progression [48–50]. Perlecan was highly produced in CCA-associated fibro-myxoid stroma [50]. Syndecan-1 translocation was found in CCA as membranous and diffuse cytoplasmic expressions, whereas those found in normal biliary cells exhibited basolateral expression [49]. Lymph node metastasis and poor prognosis of CCA patients was associated with low expression of sydecan-1 in tumor tissues, suggesting the possibility of sydecan-1 as prognostic marker for CCA patients [49]. High level of agrin, and being a member of heparan sulfate proteoglycan super family, were detected in CCA tissues compared with normal livers. Moreover, agrin was speculated to play an important role in revascularization and progression of CCA [48].
Conclusion and prospective
In summary, “proteomic and glycomic” technology facilitates measurement and analysis of a number of protein and glycoprotein markers simultaneously. These approaches produce a protein or glyco-biomarker profile signature that can identify or diagnose CCA from the appropriated biological samples. Although different approaches have been used for searching for glyco-biomarkers, the combination of lectin and mAb is a powerful strategy to enhance the efficiency of glyco-biomarkers detection. Many biomarkers are discovered and useful for distinguishing CCA from normal and non-malignant biliary tract pathologies. The incorporation of these new promising markers with existing markers or liver function enzymes should provide greater diagnostic value than single markers alone. Most importantly, researchers are still searching for specific and sensitive molecular markers to be used for population screening. This could lead to earlier diagnosis and improve outcome. The functions of those biomarkers are also needed to be further investigated in order to better understand the tumor biology and use them as targets for future therapeutic agents.
Acknowledgments
Grant support was received from Khon Kaen University, the Hilton Foundation and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health cluster (SHeP-GMS).
Footnotes
Conflict of interest None declared.
Author contributions Conceived and planned the manuscript: GJR and CW. Performed the review and wrote the manuscript: AS and KS. Shared and edited the manuscript: GJR and CW. Approved the final version: AS, KS, GJR and CW.
Contributor Information
Atit Silsirivanit, Department of Biochemistry, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Road, Khon Kaen 40002, Thailand; Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
Kanlayanee Sawanyawisuth, Department of Biochemistry, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Road, Khon Kaen 40002, Thailand; Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; Department of Neurosurgery, Johns Hopkins University, Baltimore, MD, USA.
Gregory J. Riggins, Department of Neurosurgery, Johns Hopkins University, Baltimore, MD, USA
Chaisiri Wongkham, Department of Biochemistry, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Road, Khon Kaen 40002, Thailand; Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology (Baltimore, Md.) 2008;48:308–21. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhea JM, Molinaro RJ. Cancer biomarkers: surviving the journey from bench to bedside. MLO Med Lab Obs. 2011;43:10–2. 6, 8. quiz 20, 2. [PubMed] [Google Scholar]
- 3.Wongkham S, Silsirivanit A. State of serum markers for detection of cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):17–27. [PubMed] [Google Scholar]
- 4.Darby IA, Vuillier-Devillers K, Pinault E, Sarrazy V, Lepreux S, Balabaud C, et al. Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron. 2011;4:73–91. doi: 10.1007/s12307-010-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Deng X, Zhan Q, Shen B, Jin X, Zhu Z, et al. A prospective proteomic-based study for identifying potential biomarkers for the diagnosis of cholangiocarcinoma. J Gastrointest Surg. 2013;17:1584–91. doi: 10.1007/s11605-013-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, et al. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat. 2003;77:245–52. doi: 10.1023/a:1021899904332. [DOI] [PubMed] [Google Scholar]
- 7.Chavez-Munoz C, Morse J, Kilani R, Ghahary A. Primary human keratinocytes externalize stratifin protein via exosomes. J Cell Biochem. 2008;104:2165–73. doi: 10.1002/jcb.21774. [DOI] [PubMed] [Google Scholar]
- 8.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, et al. Gene expression profiling of cholangio-carcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawase H, Fujii K, Miyamoto M, Kubota KC, Hirano S, Kondo S, et al. Differential LC-MS-based proteomics of surgical human cholangiocarcinoma tissues. J Proteome Res. 2009;8:4092–103. doi: 10.1021/pr900468k. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen TZ, Harsha HC, Gronborg M, Maitra A, Pandey A. Differential membrane proteomics using 18O-labeling to identify biomarkers for cholangiocarcinoma. J Proteome Res. 2008;7:4670–7. doi: 10.1021/pr800215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Santos A, Court M, Thiers V, Sar S, Guettier C, Samuel D, et al. Identification of cellular targets in human intrahepatic cholangiocarcinoma using laser microdissection and accurate mass and time tag proteomics. Mol Cell Proteomics. 2010;9:1991–2004. doi: 10.1074/mcp.M110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonglitthipagon P, Pairojkul C, Bhudhisawasdi V, Mulvenna J, Loukas A, Sripa B. Proteomics-based identification of alpha-enolase as a potential prognostic marker in cholangiocarcinoma. Clin Biochem. 2012;45:827–34. doi: 10.1016/j.clinbiochem.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Yonglitthipagon P, Pairojkul C, Chamgramol Y, Loukas A, Mulvenna J, Bethony J, et al. Prognostic significance of peroxiredoxin 1 and ezrin-radixin-moesin-binding phosphoprotein 50 in cholangiocarcinoma. Hum Pathol. 2012;43:1719–30. doi: 10.1016/j.humpath.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonglitthipagon P, Pairojkul C, Chamgramol Y, Mulvenna J, Sripa B. Up-regulation of annexin A2 in cholangiocarcinoma caused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol. 2010;40:1203–12. doi: 10.1016/j.ijpara.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisomsap C, Sawangareetrakul P, Subhasitanont P, Panichakul T, Keeratichamroen S, Lirdprapamongkol K, et al. Proteomic analysis of cholangiocarcinoma cell line. Proteomics. 2004;4:1135–44. doi: 10.1002/pmic.200300651. [DOI] [PubMed] [Google Scholar]
- 16.Srisomsap C, Subhasitanont P, Sawangareetrakul P, Chokchaichamnankit D, Ngiwsara L, Chiablaem K, et al. Comparison of membrane-associated proteins in human cholangiocarcinoma and hepatocellular carcinoma cell lines. Proteomics Clin Appl. 2007;1:89–106. doi: 10.1002/prca.200600168. [DOI] [PubMed] [Google Scholar]
- 17.Srisomsap C, Sawangareetrakul P, Subhasitanont P, Chokchaichamnankit D, Chiablaem K, Bhudhisawasdi V, et al. Proteomic studies of cholangiocarcinoma and hepatocellular carcinoma cell secretomes. J Biomed Biotechnol. 2010;2010:437143. doi: 10.1155/2010/437143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Wang J, Liu B, Dai S, Wang X, Chen J, et al. Serum levels of variants of transthyretin down-regulation in cholangio-carcinoma. J Cell Biochem. 2008;104:745–55. doi: 10.1002/jcb.21661. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Dai S, Zhang Z, Liu L, Wang J, Xiao X, et al. Characterization of apolipoprotein A-I as a potential biomarker for cholangiocarcinoma. Eur J Cancer Care (Engl) 2009;18:625–35. doi: 10.1111/j.1365-2354.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 20.Tolek A, Wongkham C, Proungvitaya S, Silsirivanit A, Roytrakul S, Khuntikeo N, et al. Serum alpha1beta-glycoprotein and afamin ratio as potential diagnostic and prognostic markers in cholangio-carcinoma. Exp Biol Med (Maywood) 2012;237:1142–9. doi: 10.1258/ebm.2012.012215. [DOI] [PubMed] [Google Scholar]
- 21.Sriwanitchrak P, Viyanant V, Chaijaroenkul W, Srivatanakul P, Gram HR, Eursiddhichai V, et al. Proteomics analysis and evaluation of biomarkers for detection of cholangiocarcinoma. Asian Pac J Cancer Prev. 2011;12:1503–10. [PubMed] [Google Scholar]
- 22.Rucksaken R, Khoontawad J, Roytrakul S, Pinlaor P, Hiraku Y, Wongkham C, et al. Proteomic analysis to identify plasma orosomucoid 2 and kinesin 18A as potential biomarkers of cholangiocarcinoma. Cancer Biomark. 2012;12:81–95. doi: 10.3233/CBM-130296. [DOI] [PubMed] [Google Scholar]
- 23.Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279–84. doi: 10.1097/mog.0b013e328325a894. [DOI] [PubMed] [Google Scholar]
- 24.Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, et al. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3:715–28. doi: 10.1074/mcp.M400015-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Koopmann J, Thuluvath PJ, Zahurak ML, Kristiansen TZ, Pandey A, Schulick R, et al. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer. 2004;101:1609–15. doi: 10.1002/cncr.20469. [DOI] [PubMed] [Google Scholar]
- 26.Farid SG, Craven RA, Peng J, Bonney GK, Perkins DN, Selby PJ, et al. Shotgun proteomics of human bile in hilar cholangio-carcinoma. Proteomics. 2011;11:2134–8. doi: 10.1002/pmic.201000653. [DOI] [PubMed] [Google Scholar]
- 27.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SY, Roh SJ, Kim YN, Kim SZ, Park HS, Jang KY, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep. 2009;22:649–57. doi: 10.3892/or_00000485. [DOI] [PubMed] [Google Scholar]
- 29.Korita PV, Wakai T, Ajioka Y, Inoue M, Takamura M, Shirai Y, et al. Aberrant expression of vimentin correlates with dedifferentiation and poor prognosis in patients with intrahepatic cholangiocarcinoma. Anticancer Res. 2010;30:2279–85. [PubMed] [Google Scholar]
- 30.Shen J, Wang W, Wu J, Feng B, Chen W, Wang M, et al. Comparative proteomic profiling of human bile reveals SSP411 as a novel biomarker of cholangiocarcinoma. PLoS ONE. 2012;7:e47476. doi: 10.1371/journal.pone.0047476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaub S, Wilkins J, Weiler T, Sangster K, Rush D, Nickerson P. Urine protein profiling with surface-enhanced laser-desorption/ ionization time-of-flight mass spectrometry. Kidney Int. 2004;65:323–32. doi: 10.1111/j.1523-1755.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 32.Hortin GL, Sviridov D. Diagnostic potential for urinary proteomics. Pharmacogenomics. 2007;8:237–55. doi: 10.2217/14622416.8.3.237. [DOI] [PubMed] [Google Scholar]
- 33.Metzger J, Negm AA, Plentz RR, Weismuller TJ, Wedemeyer J, Karlsen TH, et al. Urine proteomic analysis differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Gut. 2013;62:122–30. doi: 10.1136/gutjnl-2012-302047. [DOI] [PubMed] [Google Scholar]
- 34.Gomes AM, Stelling MP, Pavao MS. Heparan sulfate and heparanase as modulators of breast cancer progression. Biomed Res Int. 2013;2013:852093. doi: 10.1155/2013/852093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyalwidhe JO, Betesh LR, Powers TW, Jones EE, White KY, Burch TC, et al. Increased bisecting N-acetylglucosamine and decreased branched chain glycans of N-linked glycoproteins in expressed prostatic secretions associated with prostate cancer progression. Proteomics Clin Appl. 2013 doi: 10.1002/prca.201200134. doi:10.1002/prca .201200134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silsirivanit A, Araki N, Wongkham C, Vaeteewoottacharn K, Pairojkul C, Kuwahara K, et al. CA-S27: a novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013;104:1278–84. doi: 10.1111/cas.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-David T, Sagi-Assif O, Meshel T, Lifshitz V, Yron I, Witz IP. The involvement of the sLe-a selectin ligand in the extravasation of human colorectal carcinoma cells. Immunol Lett. 2008;116:218–24. doi: 10.1016/j.imlet.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Renkonen J, Makitie A, Paavonen T, Renkonen R. Sialyl-Lewis(x/a)-decorated selectin ligands in head and neck tumours. J Cancer Res Clin Oncol. 1999;125:569–76. doi: 10.1007/s004320050318. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y, Shimosegawa T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098–111. doi: 10.1111/j.1872-034X.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda A, Kuno A, Kawamoto T, Matsuzaki H, Irimura T, Ikehara Y, et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology. 2010;52:174–82. doi: 10.1002/hep.23654. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda A, Kuno A, Matsuzaki H, Kawamoto T, Shikanai T, Nakanuma Y, et al. Glycoproteomics-based cancer marker discovery adopting dual enrichment with Wisteria floribunda agglutinin for high specific glyco-diagnosis of cholangiocarcinoma. J Proteomics. 2013;85:1–11. doi: 10.1016/j.jprot.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Indramanee S, Silsirivanit A, Pairojkul C, Wongkham C, Wongkham S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac J Cancer Prev. 2012;13(Suppl):119–24. [PubMed] [Google Scholar]
- 43.Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, et al. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006;44:1025–38. doi: 10.1002/hep.21330. [DOI] [PubMed] [Google Scholar]
- 44.Luengpailin S, Wongkham S, Wongkham C, Sripa B, Sirijaichingkul S, Chauin S, et al. Demonstration of a biliary-associated glycoprotein in human serum. Clin Chim Acta. 1996;244:237–40. doi: 10.1016/0009-8981(95)06211-4. [DOI] [PubMed] [Google Scholar]
- 45.Bamrungphon W, Prempracha N, Bunchu N, Rangdaeng S, Sandhu T, Srisukho S, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301–8. doi: 10.1016/j.canlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Silsirivanit A, Araki N, Wongkham C, Pairojkul C, Narimatsu Y, Kuwahara K, et al. A novel serum carbohydrate marker on mucin 5AC: values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer. 2011;117:3393–403. doi: 10.1002/cncr.25912. [DOI] [PubMed] [Google Scholar]
- 47.Lv H, Yu G, Sun L, Zhang Z, Zhao X, Chai W. Elevate level of glycosaminoglycans and altered sulfation pattern of chondroitin sulfate are associated with differentiation status and histological type of human primary hepatic carcinoma. Oncology. 2007;72:347–56. doi: 10.1159/000113145. [DOI] [PubMed] [Google Scholar]
- 48.Batmunkh E, Tatrai P, Szabo E, Lodi C, Holczbauer A, Paska C, et al. Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangio-carcinoma and hepatocellular carcinoma. Hum Pathol. 2007;38:1508–15. doi: 10.1016/j.humpath.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Harada K, Masuda S, Hirano M, Nakanuma Y. Reduced expression of syndecan-1 correlates with histologic dedifferentiation, lymph node metastasis, and poor prognosis in intrahepatic cholangiocarcinoma. Hum Pathol. 2003;34:857–63. doi: 10.1016/s0046-8177(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 50.Sabit H, Tsuneyama K, Shimonishi T, Harada K, Cheng J, Ida H, et al. Enhanced expression of basement-membrane-type heparan sulfate proteoglycan in tumor fibro-myxoid stroma of intrahepatic cholangiocarcinoma. Pathol Int. 2001;51:248–56. doi: 10.1046/j.1440-1827.2001.01201.x. [DOI] [PubMed] [Google Scholar]