Abstract
Purpose
Myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) play a key role in the progression of head and neck squamous cell carcinoma (HNSCC). On the basis of our preclinical data demonstrating that phosphodiesterase-5 (PDE5) inhibition can modulate these cell populations, we evaluated whether the PDE5 inhibitor tadalafil can revert tumor-induced immunosuppression and promote tumor immunity in patients with HNSCC.
Experimental Design
First, we functionally and phenotypically characterized MDSCs in HNSCCs and determined, retrospectively, whether their presence at the tumor site correlates with recurrence. Then, we performed a prospective single-center, double-blinded, randomized, three-arm study in which patients with HNSCC undergoing definitive surgical resection of oral and oropharyngeal tumors were treated with tadalafil 10 μg/day, 20 μg/day, or placebo for at least 20 days preoperatively. Blood and tumor MDSC and Treg presence and CD8+ T-cell reactivity to tumor antigens were evaluated before and after treatment.
Results
MDSCs were characterized in HNSCC and their intratumoral presence significantly correlates with recurrence. Tadalafil treatment was well tolerated and significantly reduced both MDSCs and Treg concentrations in the blood and in the tumor (P < 0.05). In addition, the concentration of blood CD8+ T cells reactive to autologous tumor antigens significantly increased after treatment (P < 0.05). Tadalafil immunomodulatory activity was maximized at an intermediate dose but not at higher doses. Mechanistic analysis suggests a possible off-target effect on PDE11 at high dosages that, by increasing intracellular cAMP, may negatively affect antitumor immunity.
Conclusions
Tadalafil seems to beneficially modulate the tumor micro- and macro-environment in patients with HNSCC by lowering MDSCs and Tregs and increasing tumor-specific CD8+ T cells in a dose-dependent fashion.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a deadly disease with significant social and economic impact. Despite advances in multimodality treatment and improvements in mortality rates, loco-regional recurrence rates remain high (1). Although many factors contribute to treatment failure in HNSCC, some of the most important are the profound immune defects found in these patients. Such defects include generalized T-cell anergy and increased concentration of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg; ref. 2).
Since the mid-1990s, it has been known that MDSC (at that time called natural suppressors cells; ref. 3) recruitment at the tumor site is a negative prognostic factor and is associated with an increased rate of metastasis and recurrence in HNSCC (4). Furthermore, the increased frequency of CD34+ MDSC in the peripheral blood mononuclear cells (PBMC) of patients with HNSCC has also been correlated with suppression of amnestic responses to recall antigens (5). Human MDSCs were later described as CD34+CD33+CD13+CD15− HLADR− immature cells (6). More recently two additional CD33+CD11b+MDSC subsets have been included: the CD15+CD14− granulocytic-MDSCs (g-MDSC) and the CD14+CD15− monocytic MDSC (m-MDSC; refs. 7–11). Both subsets correlate with HNSCC staging; however, functional studies on g-MDSC are logistically complicated by their cryosensitive nature (12, 13). Interestingly, recent data indicate these subsets might represent diverse differentiation states of the same population (14).
CD4+CD25+FoxP3+ Tregs in circulation have been associated with poor prognosis in HNSCC (15–18). Although FoxP3 expression is generally sufficient to define human Treg in nonactivated PBMCs, their identification in the tumor is complicated by the fact that activated conventional T cells (c-T cells) can also express this marker. Nevertheless, c-T cells and Tregs can be discriminated by the subcellular localization of FoxP3, residing respectively in the cytoplasm (c-T cells) or in the nucleus (Treg; ref. 19). The Treg:c-T cell ratio at the tumor site predicts recurrence in HNSCC (20).
Reducing MDSCs and Treg accumulation and activity are thus desirable therapeutic goals. In preclinical models, we demonstrated that these goals can be achieved by phosphodiesterase-5 (PDE5) inhibition that is able to reverse MDSC suppression and Treg accumulation, promote antitumor immunity, and induce a measurable antitumor effect (21, 22). The current study seeks to determine whether tadalafil, a PDE5 inhibitor, with favorable toxicity profile and long-acting pharmacodynamics, can modulate the host–tumor immune response in HNSCC.
Patients and Methods
Retrospective study
Paraffin-embedded tumor specimens from patients with human papillomavirus (HPV)–negative oral SCC (OSCC) T1 or T2 who underwent surgical resection without prior treatment were evaluated (same specimens used in ref. 20). Patient characteristics are described in Supplementary Table S1. Forty-nine patients were classified as cases or controls based on whether or not medical records included evidence of disease recurrence within 36 months after surgery (allowing a 2.5-month buffer). This resulted in 19 cases of recurrence at a median time of 12.3 months (range, 3.7–38.5) and 30 nonrecurrent controls with a median follow-up of 59.7 months (range, 36.9–103).
Prospective study
Patients with a biopsy-proven SCC of the oral cavity or oropharynx undergoing curative surgical resection of the tumor were eligible. Patient characteristics are described in Supplementary Table S2. Enrolled patients were randomized in a ratio of 3:3:1 to arm A (10-μg tadalafil), arm B (20-mg tadalafil), or arm C (placebo), using a permuted block design with block size 7 (six blocks). Patient recruitment procedures, randomization, inclusion and exclusion criteria, and the statistical considerations in trial design are detailed in the Supplementary Data.
Specimen collection
Blood (30 mL) was drawn before treatment (t1) and at the day of the surgery (after treatment, t2) in EDTA-containing tubes. At least 6 weeks after surgery (t3), blood (60 mL) was drawn for dendritic cell (DC) preparation and additional analyses. White blood cells were purified from the blood using Ficoll-Hypaque, recovering both the lymphocytes interphase and most of the neutrophil-containing Ficoll phase but discarding the RBC pellet. Fresh tumor specimen (at least 14 mm3) was collected at t2 for tumor lysate preparation and was processed within 1 hour of harvesting. Additional specimens from the pretreatment biopsy and surgery were paraffin-embedded for immunofluorescence studies.
Interim monitoring
An adverse event (AE) questionnaire was administered to each patient on day 5 of treatment with study drug. Criteria for discontinuation of therapy were defined and are included in the Supplementary Data for both efficacy and toxicity. No dose modifications were allowed. The study was reviewed quarterly by the Data Safety and Monitoring Committee of the University of Miami Sylvester Comprehensive Cancer Center (Miami, FL). Stopping guidelines were defined for grade 2 or higher treatment-related episodes of toxicities associated with tadalafil use (headache, dyspepsia, back pain, myalgia, nasal congestion, flushing, and limb pain). Development of more significant toxicities of priapism, visual symptoms, or hearing loss in 2 patients on a single treatment arm would result in the stopping of that arm.
Flow cytometry
Flow cytometry was performed on cryoconserved ficolled specimens. The used of cryoconserved specimen was initially chosen to minimize inter-assay variation, and to allow for inclusion of additional antibodies in the analysis if new MDSC-specific markers were discovered during the trial. Data acquisition was performed at the SCCC flow cytometry core on a BD LSR-Fortessa SORP equipped with the following wavelengths lasers: 355 nm (60 mW), 404 nm (100 mW), 488 nm (50mW), 561 nm (50 mW), and 639 nm (40 mW). MDSC phenotype analysis was performed using LIVE/DEAD Fixable Yellow Dead Cell (Invitrogen) and the following anti-human Abs: CD13-APC (clone Wm15; BD), CD15-PercP (clone W6D3; BioLegend), CD33-APC (clone WM53; BD), CD34− PECy7 (clone 8G12; BD), HLA-DR brilliant violet 711 (clone L243; BioLegend), CD14-APC-H7 (clone MjP9; BD), CD11b-Pacific Blue (clone ICRF44; BD). DC analysis was performed using LIVE/DEAD Fixable Yellow Dead Cell (Invitrogen) with the following antibodies: CD11c-APC (clone B-ly6; BD), CD80-PE (clone L307.4; BD), CD86-FITC (clone 2331; BD), CD40-V450 (clone 5C3), IL4Rα-PE (clone 25463; R&D Systems). T-cell analysis was performed using LIVE/ DEAD Fixable Yellow Dead Cell (Invitrogen) with the following antibodies: CD3-percp (clone SP34-2; BD), CD8-PE-Cy7 (clone RPA-T8), CD4-Pacific Blue (clone RPA-T4;BD), CD25-PE (clone M-A251; BD), CD69-APC-Cy7 (clone FN50; BD), Foxp3-APC (clone PCH101; e-Bioscience). Proliferation was evaluated using CD3-percp (clone SP34-2; BD), CD8-PE-Cy7 (clone RPA-T8), CD4-Alexa Fluor 700 (clone RPA-T4; BD), CFSE (Invitrogen), and DAPI as vital dye staining. Staining was performed by 5 × 105 ficolled PBMCs, blocked for 10 minutes with fc blocking peptide (Innovex Bioscience) at 4− C, stained with the optimized concentration of surface antibodies in 100 mL of PBS-BSA-EDTA (1 × - 0.5%-2 nmol/L) for 15 minutes at 4°C and washed with PBS. LIVE/DEAD staining and eventual permeabilization and fixation were performed following the manufacturer's instructions. Foxp3 staining was performed using IC Fixation Buffer and Permeabilization Buffer (e-Bioscience) following the manufacturer's instructions. Samples were read in the cytofluorimeter within 3 hours of staining. At least 105 events were collected. Compensation was performed using compi-beads (BD) after data collection. FMO were used as negative controls. Data were analyzed using the FCS vs3 (denovo software). Gating strategy for MDSCs quantification is summarized in Supplementary Fig. S1. For Treg quantification, the percentage of CD3+CD4+CD25+Foxp3+ T cells among “Live” cells was evaluated. The CD4:CD8 ratio was evaluated among the CD3+ Live T cells.
FACS sorting
For the suppressive assay, cryoconserved PBMCs were thawed and stained with Percp-Cy5.5–conjugated anti-human HLADR, FITC-conjugated anti-human CD33 (BD) and PE-conjugated anti-human IL4Rα (R&D Systems). For the cGMP and cAMP analysis, cryoconserved PBMCs from patients treated with “high” or “intermediate” dose of tadalafil, cells were labeled with: BV421-conjugated anti-human CD3 (e-Bioscience), FITC-conjugated anti-human CD33 (BD), PE-conjugated anti-human IL4Rα (R&D Systems), and brilliant violet 711–conjugated anti-human HLADR. Cells were sorted at the Diabetes Research Institute (DRI) flow cytometry core at the University of Miami, Miami, FL, using a BD-FACS-ARIA.
Suppressive assays
Ficoll-purified, FACS-sorted, CD33+IL4Rα+HLADR+, CD33+ IL4Rα+HLADR− , or CD33+IL4Rα− cells were isolated by FACS from HNSCC patients' ficoll-purified PBMCs. Cells (5 × 104) of each cell type were then incubated with 105 CFSE-labeled, magnetically purified CD3+ T cells stimulated with 7.5 × 104 anti-CD3/anti-CD8-conjugated beads (Life Technologies). CD3+ CD8+ T-cell proliferation was evaluated by FACS 3 days later.
Dendritic cells preparation
Monocytes from freshly drawn PBMCs were isolated by adherence in a T75 flask (BD) for 2 hours in RPMI-1640 containing 1% heat-inactivated human AB serum. Following washing to remove nonadherent cells, the adherent monocytes were differentiated into DC with RPMI-1640 1% AB serum containing 800 U/mL GM-CSF and 500 U/mL IL4 (Peprotech) for 5 days. Fresh GM-CSF and IL4 was added on day 3. On day 5, immature DC were transferred into 24-well plates and pulsed with autologous tumor lysate (50 μg/mL) in RPMI-1640 1%AB serum supplemented with GM-CSF and IL4. Two hours later, pulsed immature DC were induce to mature by the addition of Mimic cytokine mix [5 ng/mL TNFα (Peprotech), 5 ng/mL IL1β (Peprotech), 750 ng/mL IL6 (Peprotech), and 1 μg/mL PGE2 (Sigma)].
Tumor lysate preparation
Fresh tumor specimens were processed within 1 hour of resection, washed twice with PBS, and incubated for 20 minutes at 37°C with five volumes of PBS containing Clostridium histolyticum collagenase type IV (10 μg/mL; Sigma), MgCl2 (100 μmol/L), and CaCl2 (100 mmol/L). Cells were filtered, washed with PBS, and frozen in RPMI at 80− C until the day in which DC needed to be pulsed. Before use, cells were lysed by three additional snap-freeze (dry-ice þ ethanol) and thaw (37°C) cycle, and cellular debris removed by cell centrifugation and filtration through a 0.4-μm filter. Protein concentration was quantified by the BCA Protein Assay Kit (Thermo Scientific).
Magnetic sorting
CD3+ T cells were purified by negative selection using the human Pan T Cell Isolation Kit II (Miltenyi Biotec) in combination with the LS column and following the manufacturer's instruction. Purity was evaluated by FACS and was generally higher than 90%.
Functional assays
Magnetically purified, CFSE-labeled T cells (105) from T1, T2, or T3 were incubated with 3 × 105 autologous, monocyte-derived, DC pulsed with the autologous tumor. CD3+CD8+ T-cell proliferation was evaluated by FACS 4 days later.
Immunofluorescence staining and analysis
Quantification of the tumor leukocyte infiltration was performed by standard techniques (Supplementary Data) and analyzed using the previously described computer-aided method (20).
cGMP and cAMP evaluation
cGMP and cAMP concentration was measured on the acetylatedlysate of sorted cells (at least 4 × 105) by competitive ELISA (Enzo Life Science) following the manufacturer's instructions. Results were normalized by the number of sorted cells used in each assay.
Statistical analysis
The study was designed to enroll at least 42 patients with an estimated postenrollment exclusion rate of 20% and was designed to analyze most data using paired tests to minimize the influence of inter-individual variation. The analysis of intratumoral responses was performed by one-side ANOVA at t2, because preoperative biopsy tissues were generally too small for sufficient sampling. Time comparison within a treatment arm was assessed by one-sample t test or the Wilcoxon signed-rank test. Comparisons between treatment arms were done by two-sample t tests or ANOVA, or by nonparametric methods, the Mann–Whitney or Kruskal–Wallis test. All tests were two-sided with 5% significance. Statistical considerations on study design are detailed in the Supplementary Data.
Results
Clinical trial design and protocol adherence
We performed a single-center, double-blinded, randomized, three-arm study (Clinicaltrials.gov: NCT00843635) in which patients undergoing definitive surgical resection of oral and oropharyngeal HNSCC were treated with tadalafil 10 mg/day, 20mg/day, or placebo (3:3:1 ratio) for at least 20 days preoperatively. Study drug was discontinued approximately 36 hours before surgery, and not given postoperatively. Exclusion and inclusion criteria and enrollment procedures are detailed in the Supplementary Data. Tadalafil was studied as an investigational new drug for a non-FDA-approved indication (IND102495; D.T. Weed, IND holder and sponsor). All subjects received appropriate standard of care adjuvant therapy postoperatively. The primary endpoints of the trial were: (i) to determine the effect of PDE5 inhibition on MDSC and Treg, (ii) to evaluate the effect of PDE5 inhibition on tumor T-cell immunity, and (iii) to evaluate whether dose response was present.
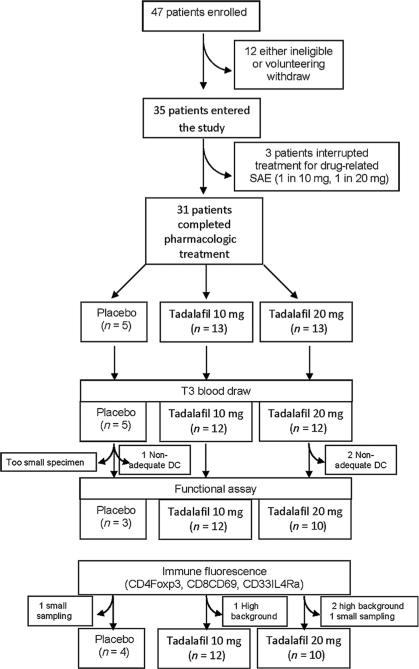
Forty-seven patients consented and enrolled in the trial. Twelve were subsequently found to have exclusion criteria or voluntarily withdrew. Of the 35 patients who were randomized and started on study drug, one was subsequently found to be taking an exclusionary medication and was withdrawn from the study after 2 days of treatment. Three patients voluntarily withdrew from the study after experiencing grade 3 side effects (back pain and myalgia; see below). Of the 31 patients who completed therapy, 29 took 20 doses of study drug; 1 patient took only 18 doses due to inadvertent noncompliance with study protocol but did take last dose 2 days before surgery; and 1 patient took a total of 32 doses of study drug due to a delay in the surgical date that occurred after initiation of study drug.
Patients' characteristics and drug toxicity
Of the 35 patients who were randomized, the mean age was 60 years with 27 males (77%) and 8 females (23%; Supplementary Table S2). The majority of randomized patients had oral cavity tumors (31, 88.6%); 4 patients (11.4%) had oropharyngeal tumors. Twenty-six patients (74.3%) were previously untreated, 4 (11.4%) received prior chemotherapy and radiotherapy, and 5 (14.3%) received prior radiotherapy. Four patients had HPV-positive tumors. All three treatment groups had similar distributions of age, sex, site, and T stage. Although differences were not statistically significant at P = 0.05, the 10-mg tadalafil group had a higher percentage of white non-hispanics, the 20-mg tadalafil group had a higher percentage of previously untreated patients, and the control group had a higher percentage of N0 tumors. Patient demographics are detailed in Supplementary Table S2.
Among the 35 patients who received study drug, no patient experienced priapism, visual changes, or hearing loss (the most serious rare side effects of tadalafil). A total of four serious AEs (SAE) occurred, all determined to be unrelated to the study drug. A total of 102 AEs (Supplementary Table S3) occurred with 1 AE noted at baseline. Seven grade 3 AEs were noted, four of which were unrelated to study drug and did not interfere with study drug completion. Three grade 3 AEs were felt to be related to study drug and resulted in voluntary withdrawal from the study. The 3 patients that withdrew from the study experienced severe back pain or myalgia following administration of 2, 3, and 3 doses of study drug respectively. All had complete resolution of symptoms within 24 hours of discontinuation of study medication (Fig. 1). Interestingly, grades 2 and 3 AEs were associated with tadalafil arms, whereas grade 1 AEs were distributed equally between placebo and tadalafil groups. Most grades 2 and 3 AEs were related to skeletal muscular pain (Supplementary Table S3).
Figure 1.
Consolidated standards of reporting trials (CONSORT) diagram.
Blinded interim analyses were performed after accrual of the first 15 evaluable patients, with no stopping criteria met for minimal immunologic effect in the active treatment arms (as identified by the 3:3:1 randomization ratio). No stopping criteria were met for adverse effects as determined by blinded quarterly review of AEs by the data safety and monitoring committee of the Sylvester Comprehensive Cancer Center.
MDSCs in HNSCC can be defined as CD33+ IL4Rα+ CD14+ HLADRint/neg CD11b+ cells
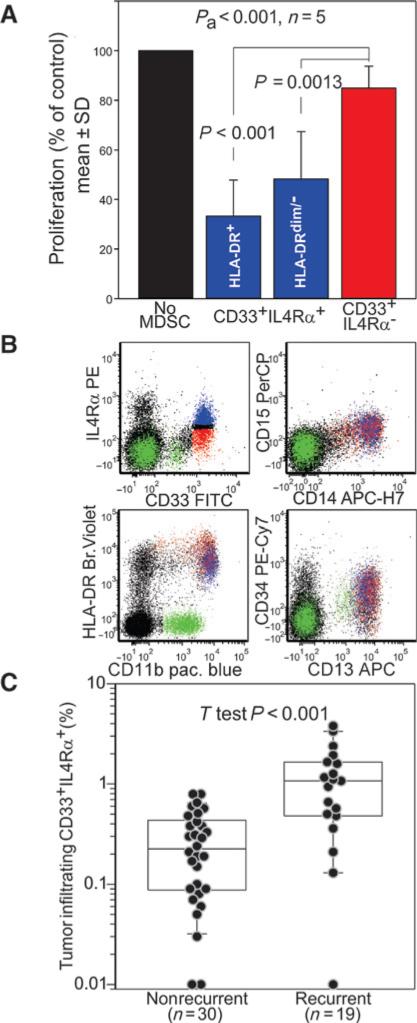
Because a consensus on human MDSC phenotype has not yet been reached (23), we first defined functionally the MDSC phenotype in HNSCC. MDSCs were sorted from PBMCs based on the expression of the pan-myeloid marker CD33, the MDSC functional marker IL4Rα (24–26), and HLADR. Suppressive activity of the sorted cells was assessed against autologous, anti-CD3/anti-CD28 stimulated, CFSE+ T cells. Although no suppression was observed using CD33+IL4Rα− cells (Fig. 2A), a significant suppressive activity was observed with the CD33+ IL4Rα+ cells regardless of their HLADR expression. This population (Fig. 2B, blue dots) has a phenotype consistent with the m-MDSCs (7, 8): it is positive for CD14, CD11b, CD13, and CD34, has intermediate expression of HLADR, and no CD15 expression.
Figure 2.
MDSCs identification. A, the indicated myeloid cell subsets were tested for suppressive activity against CFSE-labeled autologous T cells stimulated with beads coated with anti-CD3/anti-CD28 antibodies. Data normalized on the control (no MDSC) are cumulative of five independent experiments using PBMCs from 5 patients. P value for the ANOVA test (Pa) and the Tukey post hoc test are reported. B, example of multicolor FACS analysis for MDSC phenotype CD33+IL4Rα+ cells are highlighted in blue. C, intratumoral CD33+ IL4Rα+ cells were retrospectively evaluated in the tumor specimen of recurrent or nonrecurrent OSCC patients by immunofluorescence microscopy. P value for t test is reported.
The prognostic value of CD33+IL4Rα+ cells presence was evaluated through a case–control retrospective analysis of 49 patients with T1–T2 oral SCC. In particular, intratumoral concentration of CD33+IL4Rα+ MDSC was compared in 19 patients whose tumor recurred within 36 months form surgery, and 30 recurrence-free patients. This analysis (Fig. 2C) reveals that CD33+IL4Rα+ MDSCs not only suppress T-cell proliferation, but that their intratumoral accumulation correlates with tumor recurrence. This correlation is still significant in multivariate analysis with adjustment for clinical predictors (Supplementary Table S4).
Daily tadalafil treatment alters the tumor macroenvironment by reducing MDSCs and Treg in HNSCC patients
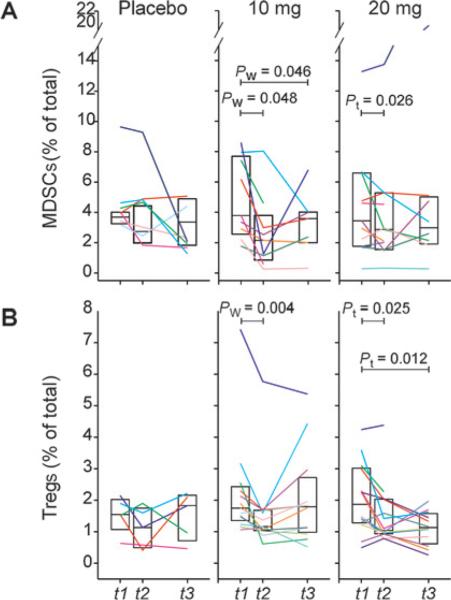
Once the MDSC phenotype was defined, the effect of preoperative tadalafil treatment was evaluated. In particular, m-MDSC and Treg concentration was evaluated in PBMCs harvested before treatment (t1), at the time of the surgery (t2), and at least 6 weeks after surgery (t3). Contrary to the placebo group (median decrease of 1.23% from baseline), a significant decrease of both m-MDSC and Treg was observed in most of the tadalafil-treated patients (Fig. 3). Discrepancies were observed at t3 among patients with some that maintained low levels of MDSC and Treg while others demonstrated an increase in either or both populations. No experimental drug was administered between t2 and t3.
Figure 3.
Tadalafil reduces MDSCs and Treg. MDSCs (A) and Treg (B) concentration was evaluated in patients PBMCs before (t1), after tadalafil treatment (t2), and 6 weeks after surgery (t3). Pw, Wilcoxon signed-rank test; Pt, paired t test.
Daily tadalafil treatment increase antitumor immunity
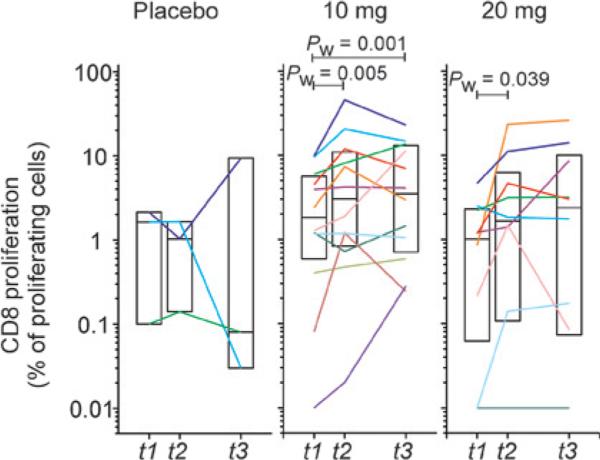
To evaluate whether PDE5 inhibition could increase antitumor immunity, magnetically purified CD3+ T cells, harvested before and after tadalafil treatment and 6 weeks after surgery, were stimulated with autologous DCs pulsed with the autologous tumor lysate. CD8+ T-cell proliferation was evaluated 4 days later by FACS. A significant increase of CD8+ T-cell proliferation was observed in both tadalafil arms, while no differences were observed in the placebo group (Fig. 4).
Figure 4.
Tadalafil increases antitumor immunity. T cells from PBMCs drawn at t1, t2, or t3 were stimulated with monocytes-derived autologous DC pulsed with autologous tumor. Four days later, CD8+ T-cell proliferation was evaluated by FACS. Background from parallel culture using unpulsed DC was subtracted. Pw, Wilcoxon signed-rank test; Pt, paired t test.
Tadalafil dosing and immune modulation
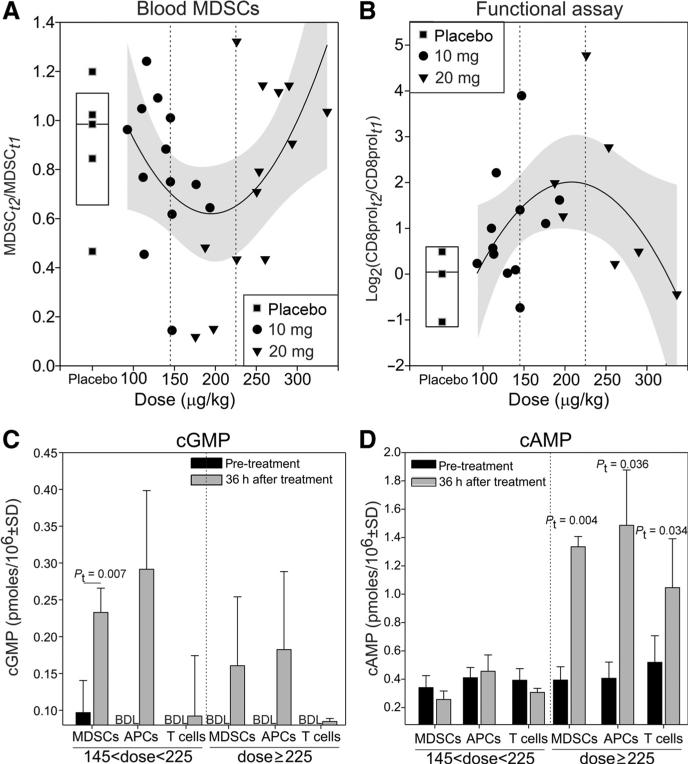
Neither tadalafil dose category (10 vs. 20mg) demonstrated clear superiority with regard to modulation of immunologic parameters (Figs. 3 and 4). Because tadalafil blood concentration negatively correlates with plasma volume that is directly dependent on body weight (27, 28), we evaluated whether a dose response was present once the dose was normalized on the body weight. Although it is important to emphasize that the trial was not designed to test the efficacy of a mg/kg dosing strategy, this analysis nevertheless yielded the surprising result that the decrease of MDSC and the increase of CD8 proliferation in response to tumor antigen following tadalafil treatment was described better by a quadratic curve than by a linear regression (Fig. 5A and B). This analysis suggests that tadalafil's maximal immunomodulatory effect is achievable between a dose of 145 and 225 μg/kg, while at higher doses, the immunomodulatory effect is significantly attenuated. Similar results were obtained when dose was normalized by body surface area, plasma volume, or body mass index (BMI; Supplementary Fig. S2). Of note, no statistically significant differences in patients’ BMI were present between study arms or dose/kg groups (data not shown).
Figure 5.
An intermediate tadalafil dose modulates most effectively tumor immunity. The ratio between the MDSCs (A) or the log2-ratio of the CD8 proliferation (B) after (t2) and before (t1) pharmacologic treatment was plotted against the weight-normalized tadalafil dose. Best-fitting quadratic curve and confidence interval (gray area) are reported. cGMP (C) and cAMP (D) were measured by ELISA in the following FACS-sorted cell population from patients (n = 3) treated with intermediate or high dosage of tadalafil: CD33+IL4Rα+ (MDSCs), HLADRhigh (APC), or CD3+ (T cells). Pt, paired t test; BDL, below detection limit.
In addition to its inhibitory effects on PDE5, tadalafil can also inhibit PDE11 (29–31). Although PDE5 degrades cGMP exclusively, PDE11 can hydrolyze both cAMP and cGMP (30). On the basis of the immune-inhibitory role of cAMP (32–35) and on PDE11 expression on monocytes and T cells, we hypothesized that the dose/kg efficacy findings could be explained by the off-target PDE11 inhibition. To test this hypothesis, we measured the concentration of cGMP and cAMP in CD3+ T cells, CD33− HLADRhigh antigen-presenting cells, and CD3− CD33+IL4Rα+ MDSC isolated from the PBMCs of patients treated with either high dose (>225 μg/kg) or intermediate dose (145 μg/kg < tadalafil < 225μg/kg) of tadalafil (Fig. 5C and D). Although at high doses, both cGMP and cAMP are significantly increased by tadalafil treatment, at intermediate doses only cGMP is increased. These data suggest that at high tadalafil doses, the off-target inhibition of PDE11 might be reached.
Tadalafil treatment modifies the tumor microenvironment
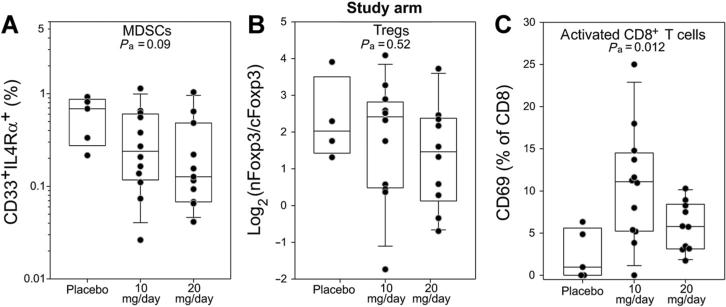
To determine whether tadalafil treatment could modify the tumor microenvironment, immunofluorescence analyses were performed on the available paraffin-embedded tumor specimens evaluating CD69+CD8+ T cells, FoxP3+CD4+ T cells, and CD33+ IL4Rα+ MDSCs concentrations. Particular attention was given to the FoxP3 intracellular localization because, as we previously demonstrated, the presence of CD4+ T cells with a cytoplasmatic FoxP3 expression correlates with a favorable prognosis, whereas an high concentration of CD4+ T cells expressing nuclear FoxP3 is strongly associated with recurrence (20). The comparison of tadalafil arms with the placebo group did not highlight any significant differences in either MDSCs or Tregs, although a higher variation is appreciable within the treatment groups and a trend (P = 0.09) toward a downregulation of MDSCs is detectable (Fig. 6A and B). Interestingly, a significant upregulation of CD69 is detectable within the CD8 T cells (Fig. 6C) with the 10-mg dose but not in the 20-mg study arm.
Figure 6.
Tadalafil modulates tumor microenvironment. CD33/IL4Rα (A), CD4/FoxP3 (B), or CD8/CD69 (C) intratumoral concentration was evaluated by immune-fluorescence microscopy. Pa, P ANOVA test.
When the same analyses are conducted dividing patients according to the dose categories described above (Supplementary Fig. S3), a significant downregulation of both MDSCs and nFoxp3:cFoxp3 ratio becomes evident, whereas a trend toward increased CD8 activation is still appreciable at lower but not higher tadalafil doses (Supplementary Fig. S3C).
Discussion
The immune system plays a key role in the progression of HNSCC as initially suggested by the numerous immunologic defects and the expansion of immunosuppressive populations (i.e., MDSCs and Treg) both at the tumor site and in the blood (2). Therapeutic manipulation of the immune system and its response, by corollary, may play an equally significant role in the treatment of HNSCC. On the basis of our preclinical data (21, 22), we evaluated the immunomodulatory impact of a relatively short course of daily tadalafil administration in HNSCC with a proof-of-concept randomized, double-blind, placebo-controlled three-arm phase II clinical trial. Tadalafil was generally well tolerated (Supplementary Table S3) with adverse side effects prompting discontinuation of study drug (severe back pain or more generalized myalgia) arising in 10.3% (3 of 29) of tadalafil-treated patients. This number is consistent with what has been previously reported (36). All 3 patients had complete resolution of symptoms without sequelae within 24 hours after treatment interruption.
Because of the heterogeneity of MDSC phenotype across malignancies, we first defined the phenotype of MDSCs in the patients with HNSCC. Because some MDSC subsets (such as other myeloid populations) are poorly resistant to freezing and thawing, and considering the logistics of the clinical trial, we focused our attention on the cryoresistant MDSC population (mostly mMDSC) that can be easily recovered by Ficoll-Hypaque gradient. It is important to note that the purification and cryopreservation procedures can alter the composition of the myeloid compartment. Nevertheless, differences induced by the pharmacologic treatment can still be determined by processing all samples in the same way and by using paired statistical analyses.
We determine for the first time in this human disease that the immunosuppressive activity within the CD33+ myeloid lineage is confined to the IL4Rα+ cells (Fig. 2A). These cells are characterized, as previously reported (7–11), as CD34+CD11b+CD14+HLADRint cells (Fig. 2B). The concentration of these cells within the tumor microenvironment correlates with the likelihood of oral SCC recurrence within 3 years (Fig. 2C), confirming an important role for MDSCs in tumor progression.
This trial confirms for the first time, in humans, the preclinical findings showing a beneficial immunomodulatory effect of PDE5 inhibition in the tumor bearer (21, 22, 37, 38): tadalafil significantly reduced MDSC and Treg numbers (Fig. 3) in the blood of patients with HNSCC while it increased the concentration of tumor-specific CD8+ T cells (Fig. 4). The reason for such a reduction of both MDSCs and Treg is still unknown. On the basis of our preclinical data, we can speculate that PDE5 inhibition can downregulate IL4Ra on MDSCs (21), reducing the survival signaling that this receptor mediates (26). Alternatively, it is possible that PDE5 blockade stops the positive loop by which MDSCs promote their own recruitment and differentiation (39).
The tadalafil-mediated reduction of circulating Treg does not seem to be related to a direct action of this drug because incubation of Treg with physiologically relevant concentrations of tadalafil does not increase their apoptosis in a small number of preliminary experiments performed (data not shown). Further study is needed to completely exclude this possibility. We have shown in a pre-clinical model that MDSCs can expand Treg in vivo and that PDE5 inhibition can block this process (22). Considering the rapid turnover of circulating Treg in the tumor-bearing host (40, 41), it is possible that MDSCs inhibition significantly decreases Treg proliferation without altering their elevated apoptotic rate, de facto reducing their frequency in the blood. Alternatively, the reduced Treg frequency in the blood and in the tumor after tadalafil treatment may be explained by altered Treg homing signaling due to changes in the tumor macroenvironment. Indeed, beneficial changes in the tumor macroenvironment are also suggested by the normalization of the CD4:CD8 T-cell ratio (Supplementary Table S5) and from the data of a similar but independent trial in which patients with HNSCC have been treated for 15 days with tadalafil before treatment (J. Califano and colleagues; submitted for publication). In that trial, it was shown that the fewer MDSCs present in the blood after tadalafil treatment were less suppressive and that the immune response against the recall antigen Candida (as measured by delayed-type hypersensitivity) was significantly increased by tadalafil treatment compared with placebo. The increase of tumor-specific CD8+ T cells observed in the clinical trial described in this report can be a consequence of these changes in the tumor macroenvironment that allows a spontaneous priming of the antitumor immune response.
Although the trial reported here was designed to compare two dosing groups of tadalafil (10 and 20 mg) with placebo, and no clear superiority of either group was identified, an analysis of the dose/response, expressed as μg/kg of drug, yielded surprising results: the most significant immunologic modulation was achieved with an intermediate dose of tadalafil (between 145 and 225 μg/kg) equal to 10.15 to 15.75 μg/day for a 70-kg patient (Fig. 5A and B). We speculate that at higher dosage tadalafil can exert an off-target effect of inhibition of PDE11. Tadalafil, in fact, can inhibit both PDE5 and PDE11 with EC50 of 9.4 and 67 nmol/L, respectively (14), yielding a specificity of tadalafil for PDE5 7.1 times that of PDE11. This off-target inhibition may be relevant for tadalafil-mediated immunomodulatory properties because PDE11 can hydrolyze both cGMP and cAMP and because it is expressed in T cells and monocytes (42, 43). Increased cAMP levels can promote Treg proliferation (35), decrease CD8+ T-cell expansion (33), and prevent DC maturation (34), thus restraining any immune activation. The inhibition of PDE11 may explain the unusual tadalafil dose–response curve: at high dosage (≥225 μg/kg/d), both cAMP and cGMP are upregulated in T cells, MDSCs, and APCs, whereas at the more effective intermediate dose, only cGMP is increased (Fig. 5C and D). Given that the trial design did not prospectively test these μg/kg dose categories, and given the small size of the trial, no firm conclusions can be stated regarding the optimal tadalafil dose strategy to use without further clinical testing of this interesting finding of a superior intermediate μg/kg dose category. Our data do add this possible negative immunomodulatory effect to the notes of caution already raised on the use of high daily doses of tadalafil because of its off-target inhibition of PDE11 thought to also be responsible for myalgic SAE and its negative impact on spermatogenesis (44).
Microscope-based immunofluorescence analysis of the tumor microenvironment seems to confirm the observed dose–response curve: (i) while a trend toward MDSC downregulation is observed in the study arms, a statistically significant reduction of both MDSCs and Tregs is seen when patients are subdivided into the identified dose categories (Supplementary Fig. S3); (ii) a significant upregulation of the activation marker CD69 is observed in the CD8+ T cells at lower but not at higher tadalafil doses. Although this trial has not measured survival or recurrence endpoints, it is important to emphasize that CD8+ T-cell activation at the tumor site has a positive prognostic value (45), whereas the accumulation of MDSCs (Fig. 1) and an increase in the nFoxP3: cFoxP3 ratio (20) has been correlated with tumor recurrence. Indeed, in our retrospective analysis, both MDSCs concentration and log2 (nFoxp3:cFoxp3) ratio at the tumor site maintained their significant predictivity for recurrence status in multivariate analysis with adjustment for clinical predictors (Supplementary Table S4). Tadalafil seems to beneficially modulate all these parameters, strongly supporting the necessity to perform a larger randomized trial with clinical endpoints to evaluate whether PDE5 inhibition should be incorporated with standard treatment of HNSCC or with other immunologic therapeutic interventions.
Supplementary Material
Translational Relevance.
The immune system of patients with head and neck squamous cell carcinoma (HNSCC) is suppressed by the accumulation of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) whose presence in many malignancies has been associated with a poor prognosis. Preclinical models have shown that the immunosuppressive action of MDSCs and Treg can be overcome by the use of phosphodiesterase-5 (PDE5) inhibitors. Here, for the first time, we demonstrate by a double-blinded, placebo-controlled clinical trial in patients with HNSCC that these preclinical findings hold true in humans. Specifically, a short course of daily tadalafil treatment is sufficient to significantly (i) reduce MDSCs and Treg systemically and at the tumor site, (ii) increase the percentage of tumor-specific CD8+ T cells in circulation, and (iii) promote the activation of CD8+ T cells at the tumor site. This study provides the rationale for new therapeutic strategies in human malignancies.
Acknowledgments
The authors thank Oliver Umland (DRI) for assistance with the cell-sorting, the imaging core at the DRI, the flow-cytometry core at the SCCC, Gail Walker for statistical help with the initial Trial design and interim analysis, and Mark Lippman for the critical reading of the article.
Grant Support
This work was entirely supported by the Flight Attendant Medical Research foundation YCSA award.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
Conception and design: D.T. Weed, I. Reis, P. Serafini
Development of methodology: D.T. Weed, I. Reis, R. Nazarian, P. Serafini
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): D.T. Weed, J.L. Vella, A.C. De la fuente, C. Gomez, Z. Sargi, R. Nazarian, P. Serafini
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): D.T. Weed, I. Reis, C. Gomez, J. Califano, P. Serafini Writing, review, and/or revision of the manuscript: D.T. Weed, J.L. Vella, I. Reis, Z. Sargi, J. Califano, I. Borrello, P. Serafini
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): D.T. Weed, A.C. De la fuente, R. Nazarian, P. Serafini
Study supervision: D.T. Weed, P. Serafini
Disclosure of Potential Conflicts of Interest
P. Serafini and I. Borrello are inventors on a patent, which is owned by Johns Hopkins University, regarding the use of PDE5 inhibitors as immune-modulators. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Howell GM, Grandis JR. Molecular mediators of metastasis in head and neck squamous cell carcinoma. Head Neck. 2005;27:710–7. doi: 10.1002/hed.20222. [DOI] [PubMed] [Google Scholar]
- 2.Freiser ME, Serafini P, Weed DT. The immune system and head and neck squamous cell carcinoma: from carcinogenesis to new therapeutic opportunities. Immunol Res. 2013;57:52–69. doi: 10.1007/s12026-013-8462-3. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JA, Rao VS, Mitchell MS. Systemic bacillus Calmette-Guerin (BCG) activates natural suppressor cells. Proc Natl Acad Sci U S A. 1978;75:5142–4. doi: 10.1073/pnas.75.10.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MRI. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 6.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 7.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 8.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–63. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 11.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–39. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Trellakis S, Bruderek K, Hutte J, Elian M, Hoffmann TK, Lang S, et al. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. 2013;19:328–36. doi: 10.1177/1753425912463618. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–20. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–20. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drennan S, Stafford ND, Greenman J, Green VL. Increased frequency and suppressive activity of CD127(low/−) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunology. 2013;140:335–43. doi: 10.1111/imm.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott AK, Pries R, Wollenberg B. Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. Int J Mol Med. 2010;26:67–75. doi: 10.3892/ijmm_00000436. [DOI] [PubMed] [Google Scholar]
- 18.Alhamarneh O, Agada F, Madden L, Stafford N, Greenman J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2011;33:415–23. doi: 10.1002/hed.21464. [DOI] [PubMed] [Google Scholar]
- 19.Magg T, Mannert J, Ellwart JW, Schmid I, Albert MH. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur J Immunol. 2012;42:1627–38. doi: 10.1002/eji.201141838. [DOI] [PubMed] [Google Scholar]
- 20.Weed DT, Walker G, De La Fuente AC, Nazarian R, Vella JL, Gomez-Fernandez CR, et al. FOXP3 subcellular localization predicts recurrence in oral squamous cell carcinoma. PLoS ONE. 2013;8:e71908. doi: 10.1371/journal.pone.0071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–8. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 25.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–83. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 27.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–8. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632–6. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 29.Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16::S11–4. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- 31.Reffelmann T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation. 2003;108:239–44. doi: 10.1161/01.CIR.0000081166.87607.E2. [DOI] [PubMed] [Google Scholar]
- 32.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation—overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–16. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Kambayashi T, Wallin RP, Ljunggren HG. cAMP-elevating agents suppress dendritic cell function. J Leukoc Biol. 2001;70:903–10. [PubMed] [Google Scholar]
- 34.Challier J, Bruniquel D, Sewell AK, Laugel B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology. 2013;138:402–10. doi: 10.1111/imm.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011;23:282–92. doi: 10.1016/j.smim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brock G, Glina S, Moncada I, Watts S, Xu L, Wolka A, et al. Likelihood of tadalafil-associated adverse events in integrated multiclinical trial database: classification tree analysis in men with erectile dysfunction. Urology. 2009;73:756–61. doi: 10.1016/j.urology.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 37.Karakhanova S, Yang Y, Link J, Soltek S, vonAhn K, Umansky V, et al. Gender-specific immunological effects of the phosphodiesterase 5 inhibitor sildenafil in healthy mice. Mol Immunol. 2013;56:649–59. doi: 10.1016/j.molimm.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–6. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molon B, Viola A, Bronte V. Smoothing T cell roads to the tumor: chemokine post-translational regulation. Oncoimmunology. 2012;1:390–2. doi: 10.4161/onci.19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–65. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 42.D'Andrea MR, Qiu Y, Haynes-Johnson D, Bhattacharjee S, Kraft P, Lundeen S. Expression of PDE11A in normal and malignant human tissues. J Histochem Cytochem. 2005;53:895–903. doi: 10.1369/jhc.5A6625.2005. [DOI] [PubMed] [Google Scholar]
- 43.Bazhin AV, Kahnert S, Kimpfler S, Schadendorf D, Umansky V. Distinct metabolism of cyclic adenosine monophosphate in regulatory and helper CD4+ T cells. Mol Immunol. 2010;47:678–84. doi: 10.1016/j.molimm.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Pomara G, Morelli G. Inhibition of phosphodiesterase 11 (PDE11) impacts on sperm quality. Int J Impot Res. 2005;17:385–6. doi: 10.1038/sj.ijir.3901304. [DOI] [PubMed] [Google Scholar]
- 45.Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71:659–65. doi: 10.1016/j.humimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.