Abstract
Efforts to reduce the ever-increasing rates of osteoarthritis (OA) in the developed world require the ability to non-invasively detect the degradation of joint tissues before advanced damage has occurred. This is particularly relevant for damage to articular cartilage because this soft tissue lacks the capacity to repair itself following major damage and is essential to proper joint function. While conventional magnetic resonance imaging (MRI) provides sufficient contrast to visualize articular cartilage morphology, more advanced imaging strategies are necessary for understanding the underlying biochemical composition of cartilage that begins to break down in the earliest stages of OA. This review discusses the biochemical basis and the advantages and disadvantages associated with each of these techniques. Recent implementations for these techniques are touched upon, and future considerations for improving the research and clinical power of these imaging technologies are also discussed.
Keywords: Osteoarthritis, Cartilage, Magnetic resonance imaging (MRI), Computed tomography (CT), Imaging, Quantitative
Introduction
Osteoarthritis (OA) is a chronic, degenerative disease of the joint that affects over 26 million people in the United States alone [1]. The effect of OA on joint tissues causes a high degree of morbidity, including loss of mobility and pain. Because of this, OA places a large burden on society, with annual medical costs totaling over $185 billion in the US [2]. Moreover, the prevalence and cost of this disease are expected to increase in the coming years due to rising rates of obesity and aging populations across the world. Such alarming numbers have sparked efforts within the scientific and medical communities to better understand the disease and to develop and assess strategies for prevention and treatment. While OA is understood to affect many joint tissues, degeneration of articular cartilage is of primary concern in all stages of the disease [3].
Various imaging strategies are available to observe the biochemical and morphological degradation of cartilage that occurs as the disease progresses. Conventional radiography can indirectly detect gross cartilage loss by revealing a narrowing of the space between two bones [4]. Osteophytes can also be observed, although the diagnostic accuracy with radiography is low relative to other imaging modalities [5]. Alternatively, magnetic resonance imaging (MRI) provides optimal soft tissue contrast for assessing the health of articular cartilage [6]. Conventional use of MRI allows for the visualization of morphological degradation of articular cartilage that is characteristic of acute trauma to the joint and within later stages of OA. However, biochemical changes to the cartilage ultrastructure are known to precede gross morphological changes, and thus more advanced imaging techniques are required for assessing these more subtle changes.
Advanced quantitative imaging technologies provide information relating to cartilage composition that is useful for detecting and monitoring the early stages of OA. The most common and well-validated techniques utilize MRI, and include delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T2 relaxation time mapping, T1ρ relaxation time mapping, and sodium MRI. Other MRI techniques include ultrashort echo time (UTE) imaging, chemical exchange saturation transfer sensitive to glycosaminoglycans (gagCEST), and diffusion-weighted imaging (DWI). Lastly, quantitative computed tomography arthrography (CTa) provides an alternative to MRI for capturing biochemical information relating to cartilage.
These imaging methods are employed in clinical research studies to better understand OA as it relates to degradation of cartilage and other joint tissues. Quantitative imaging lends itself best to studying these questions by comparing control to study groups. Within this design, changes in cartilage quality over time can be studied. Typical research objectives include understanding effects of factors such as age, gender, and obesity on cartilage health. Damage after traumatic injury, such as anterior cruciate ligament rupture or meniscal tear, can also be observed. Finally, efficacy of treatments intended to slow down or reverse the progression of OA are often studied with these techniques. In order to appreciate the role of these techniques in current research and practice, it is first important to understand the biochemical composition of articular cartilage and the changes that occur as it begins to break down.
Cartilage Composition
The three components of articular cartilage that are most relevant to imaging the ultrastructure within the extracellular matrix are water, a network of collagen composed predominantly of type II collagen fibers, and the surrounding proteoglycan (PG) macromolecules. The collagen matrix provides structural support to resist stress at all depths within the cartilage layer, from the articular surface to the chondral plate [7]. Collagen fibrils in superficial regions, closest to the synovial fluid, are oriented parallel to the articular surface to reduce friction and shear stress. In contrast, collagen in deep regions of cartilage is oriented perpendicular to the bone surface in order to anchor itself to the subchondral bone.
Proteoglycans consist of a protein core with many covalently attached glycosaminoglycan (GAG) side-chains that are rich in negatively charged carboxyl and sulfate groups. These create a fixed charged density (FCD) that attracts cations, such as sodium, and generates an osmotic pressure that draws water into the cartilage [8]. Because of this osmotic pull, water constitutes 65–85 % of the total weight of healthy cartilage [9].
In the earliest stages of OA, prior to gross cartilage loss, the biochemical composition of cartilage breaks down. The concentration of PGs and GAGs decreases, leading to decreased FCD. Additionally, the structure of the collagen matrix breaks down, leading to an influx of water into these areas [10]. In summary, cartilage in the early stages of OA has reduced PG and GAG content, reduced FCD, and increased water content compared to physiologically normal cartilage. Each of the following imaging techniques relies on one or more of these compositional changes to detect and track OA in its earliest stages.
Imaging Strategies
Delayed Gadolinium-Enhanced MRI of Articular Cartilage (dGEMRIC)
dGEMRIC provides an assessment of GAG concentration through the use of the intravenous contrast agent Gd(DTPA)2−. Diffusion of the contrast agent into the cartilage is expedited by exercise and a 90-min delay prior to imaging. Within cartilage, Gd(DTPA)2− molecules encounter repulsive electrostatic forces from the negatively charged GAGs. This causes the contrast agent to distribute in an inverse relationship with GAG content [11]. The paramagnetic properties of Gd(DTPA)2− cause nearby protons to relax more quickly following a radiofrequency (RF) pulse, resulting in shorter T1 relaxation times. The outcome measure for dGEMRIC is therefore T1 relaxation time, with higher T1 relaxation times indicating higher GAG concentration and healthier cartilage.
This relationship between dGEMRIC T1 relaxation times and GAG content has been validated both in vitro [12] and in vivo [13]. Of note, however, is recent evidence suggesting that the diffusion of Gd(DTPA)2− into the cartilage is not only dependent on GAG distribution but also collagen content and diffusion direction [14, 15]. As is the case with other imaging techniques discussed below, there may not be one precise biochemical correlate that accounts for the entire variation in quantitative results. However, the most important correlate for each technique is clear, and the relationship between dGEMRIC and GAG content is well established.
The dosage of intravenous Gd(DTPA)2− typically ranges from 0.1 to 0.3 mm/kg [16, 17], and intra-articular, rather than intravenous, injection has been used for dGEMRIC in the hip [18]. The suggested delay time between administration of contrast agent and image acquisition varies between joints [17]. The optimal dosage and delay time may also vary when other tissues, such as the meniscus, are being studied along with cartilage [19]. T1 relaxation time mapping with dGEMRIC can be achieved with several 2D and 3D MR pulse sequences that generally involve multiple acquisitions with variable flip angles or variable inversion times [20, 21]. Some sequences, however, have demonstrated superior reproducibility compared to others [22]. Post-processing techniques such as 3D image registration can improve the reproducibility of dGEMRIC T1 relaxation time measurements, allowing this to be a more reliable technique for tracking change over time [23, 24].
Being a well-validated technique for assessing GAG content, dGEMRIC has been used widely in OA research. It has been used to distinguish between healthy and diseased cartilage in the hip [25, 26], finger [27], and knee joints [28]. dGEMRIC T1 relaxation times have been used as outcome measures following surgical intervention in both the knee and ankle [29–31] and are predictive of progression to late-stage OA [32].
While dGEMRIC is a widely used and well-validated tool for assessment of in vivo GAG content, it has important drawbacks (Table 1). The injection of a contrast agent is an invasive aspect of dGEMRIC that is not required by other quantitative MR techniques. The use of gadolinium contrast agent poses health risks particularly for individuals with renal impairment [33]. Also, the added time necessary to allow the contrast to diffuse into the joint makes the full examination time longer than times needed for other techniques.
Table 1.
Techniques for imaging cartilage composition and their pros/cons
| Technique | Outcome measure | Biochemical correlate |
Advantages | Disadvantages |
|---|---|---|---|---|
| dGEMRIC | T1 relaxation time | GAG content | Well validated | Long exam time Requires use of contrast agent |
| T2 relaxation time mapping |
T2 relaxation time | Collagen content/ orientation |
Assessment of collagen content without contrast Widely available |
Magic angle effect May not capture initial biochemical changes to cartilage |
| T1ρ relaxation time mapping |
T1ρ relaxation time | GAG content | Assessment of GAG content without contrast |
SAR limits due to high RF power |
| Sodium MRI |
23Na signal intensity/ concentration |
GAG content | High specificity to GAG content without contrast |
Requires specialized MRI hardware Long scan time Low SNR Better results at 7 T |
| UTE | T1, T2, T2*, and T1ρ
relaxation times |
Variable | Assessment of deep regions of cartilage |
Long scan time Requires specialized MRI sequences |
| gagCEST | CEST asymmetry | GAG content | High specificity to GAG content without contrast |
Difficult to perform at 3 T and below Requires advanced post-processing tools |
| DWI | ADC | Collagen content/ orientation |
Assessment of collagen content without contrast Widely available |
Low SNR Limited evaluation of deep cartilage regions |
| CTa | X-ray attenuation | GAG content | Useful for patients who cannot undergo MRI Short scan times |
Ionizing radiation Requires use of contrast agent |
T2 Relaxation Time Mapping
T2 relaxation time mapping provides an indirect assessment of collagen structure and orientation, as it relates to free water content. The presence of unbound water molecules slows down the loss of transverse magnetization following an RF pulse, such that regions of cartilage with more free water have higher T2 relaxation times. In healthy cartilage, the collagen matrix traps and immobilizes water molecules. When this structured matrix breaks down, the extra space is filled with free, unbound water. Therefore, elevated T2 relaxation times are generally indicative of cartilage degeneration.
Correlation between T2 relaxation time mapping and collagen content has been validated both in vitro [34] and in vivo [35]. As with dGEMRIC, however, there is evidence to suggest that T2 relaxation may also be dependent on other factors, such as GAG content [36, 37]. Still, this technique is thought to be more sensitive to collagen content than other techniques that are sensitive to loss of GAGs, and it is used in many clinical studies to track early OA.
T2 relaxation time mapping usually involves imaging at several echo times along the T2 decay curve [38]. A mono-exponential fit is then typically applied to obtain the desired relaxation time constants. This can be achieved with 2D multi-echo spin and fast spin echo sequences. 3D pulse sequences are also available and achieve shorter scan times [39, 40]. Additionally, the 3D double echo steady state (DESS) sequence has been modified to provide simultaneous estimation of T1, T2, and apparent diffusion coefficient (ADC) [41••].
T2 relaxation times are widely used as quantitative measures in clinical OA research to track cartilage degradation in the knee [42–44], ankle [45, 46], and hip joints [47]. Natural spatial variation along the range of depths from the articular surface has been observed with T2 relaxation times [48, 49]. Because T2 can theoretically increase or decrease in disease states, some investigators have looked into the heterogeneity of relaxation times within regions of cartilage [34, 50]. 3D datasets have demonstrated the enhanced ability to detect site-specific variation in cartilage biochemistry that would other-wise be unavailable with 2D sequence [51]. On a larger scale, the Osteoarthritis Initiative uses this technique to assess in vivo cartilage composition in many types of patients [52–54].
An understood drawback to T2 relaxation time mapping is its susceptibility to the magic angle effect. When collagen fibers are aligned at certain angular orientations to the main magnetic field (B0), estimations of T2 relaxation times become elevated and inaccurate [55]. Additionally, this technique may not capture biochemical changes in cartilage as early as GAG-sensitive imaging methods because PG depletion may occur prior to breakdown of the collagen matrix [56].
T1ρ Relaxation Time Mapping
T1ρ relaxation time mapping is an additional quantitative MR technique that is sensitive to GAG content within cartilage. With this technique, a spin-lock RF pulse follows the initial RF pulse to lock protons in phase. Protons then relax in the presence of a B1 field with the time constant T1ρ, and this decay can be sampled similar to that of T2 decay to obtain quantitative measurements. Water protons that are associated with large macromolecules such as PGs dissipate energy faster than free water protons. Thus, regions of cartilage with more free water as a result of GAG depletion have longer T1ρ relaxation compared to physiologically normal regions [57].
Studies validating the inverse relationship between GAG content and T1ρ relaxation times have been performed both in vitro [58, 59] and ex vivo [36, 60]. MR pulse sequences for T1ρ relaxation time mapping use a T1ρ magnetization preparation pulse [61] followed by either a spin echo [62] or gradient echo readout [63, 64].
This noninvasive assessment of GAG content is used in many research studies. It has been applied primarily to imaging of the knee [65–67] and the hip [68]. As with T2 relaxation time mapping, findings suggest that increased heterogeneity of T1ρ relaxation times within regions of cartilage is indicative of degenerative changes [69]. T1ρ relaxation is commonly measured along with T2 relaxation in cartilage studies to obtain information regarding collagen and PG biochemistry [37, 70, 71]. Recently, T1ρ relaxation time mapping has been applied at 7 T to provide higher special resolution [72].
T1ρ relaxation time mapping has the benefit that it provides an assessment of GAG content without the need for invasive contrast agent or specialized hardware. However, this method is not as specific for GAG content as other MR methods. Additionally, the spin-lock RF pulse results in large amounts of energy deposited to tissue, making longer scan times necessary to conform to FDA-regulated specific absorption rates (SAR) [73].
Sodium MRI
Sodium MR imaging captures signal from 23Na ions, rather than the standard 1H protons [74]. Positively charged sodium ions exist in association with the negatively charged GAG side-chains, making sodium MRI an excellent measure of GAG concentration. Cartilage with healthy GAG concentrations, therefore, has high sodium signal relative to cartilage experiencing depletion of GAGs and loss of FCD.
Correlation of sodium MRI with GAG content in cartilage has been validated in vitro [75, 76]. Sodium imaging is typically performed at 7 T, rather than 3 T, in order to obtain sufficient signal from 23Na ions. While this makes the technique less clinically relevant, research studies applying sodium imaging have been able to successfully distinguish between OA and healthy cartilage [77, 78].
Compared to other quantitative MR techniques discussed above, sodium imaging is not as common due to several limitations. Firstly, the concentration of 23Na ions in cartilage is significantly lower than that of 1H protons, making it difficult to obtain sufficient SNR. Longer scan times and higher field strengths are two approaches for counteracting the reduced sodium signal. Lowering the spatial resolution with sodium imaging can also help provide sufficient signal within each voxel of cartilage. This may, however, result in partial volume effects with surrounding tissues and lead to artificially decreased measurements of GAG concentration. Lastly, transmit–receive coils made especially for sodium imaging are generally required to capture the sodium signal [79].
Other Imaging Techniques: UTE, gagCEST, DWI, and CT Arthrography
Several other techniques are available for imaging cartilage composition. These are less commonly used for cartilage imaging compared to the techniques discussed above, but are important to note, nonetheless.
Ultrashort echo time imaging (UTE) allows for the assessment of tissues with short intrinsic T2 relaxation, such as menisci, tendons, ligaments, and the deep radial and calcified layers of cartilage. These tissues have T2* relaxation times shorter than 5 ms, and do not generate sufficient signal with the standard echo times used for T2 relaxation time mapping [80]. UTE can be applied to obtain T1, T2, T2*, and T1ρ relaxation time measurements in deep regions of cartilage [81].
While applications of UTE for in vivo cartilage imaging are limited, the technique has demonstrated sufficient repeatability for T2* mapping of cartilage [82]. T2* measurements with UTE have recently been used to demonstrate biochemical changes to the deep regions of cartilage following ACL rupture and reconstruction [83•]. The technical requirements for obtaining such short echo times mean that UTE requires lengthy scan times and the use of special MR pulse sequences that are not widely available [84].
Chemical exchange saturation transfer (CEST) is a new MR contrast enhancement technique that enables the indirect detection of molecules with exchangeable protons. CEST makes MRI sensitive to the concentrations of endogenous metabolites and their environments. GAGs in cartilage exhibit a concentration-dependent CEST effect between their hydroxyl (-OH) protons and bulk water protons [85]. This technique allows for imaging of GAG distribution with high spatial resolution.
The relationship between the gagCEST effect and GAG content has been validated in vivo by comparison to sodium imaging at 7 T [86, 87]. The high specificity to GAG without the need for special hardware or intravenous contrast makes gagCEST a promising method for studying cartilage matrix composition. The major limitation of gagCEST is that it is difficult to perform at 3 T, and, instead, requires a higher B0 field strength of 7 T [88]. Advanced post-processing tools are also required to correct for field non-homogeneities and to obtain accurate quantitative results.
A final MR technique for assessing cartilage composition is diffusion-weighted imaging (DWI). The translational motion of water protons is measured by a value called the apparent diffusion coefficient (ADC). This is achieved by applying diffusion-sensitizing gradients that break down phase coherence amongst mobile protons, reducing the MR signal from regions with mobile water [79]. Higher ADC values indicate more translational movement of protons. In articular cartilage, the structure and orientation of the collagen matrix influences movement of protons. When the matrix breaks down, there is increased movement of water. Elevated ADC is thus believed to be indicative of early cartilage degeneration [89, 90].
Applications of DWI to clinical research are limited and sometimes involve the use of a semi-quantitative outcome, rather than quantification of ADC [91, 92]. While echo planar imaging is typically used for DWI [93], quantitation of ADC can be achieved simultaneously with T1 and T2 relaxation times using a modified version of the double echo steady state (DESS) pulse sequence [41••].
Quantitative CT arthrography (CTa) is an alternative to the many MRI techniques for assessing cartilage composition. CTa takes advantage of the repulsive forces between GAGs in cartilage and the negatively charged contrast agent ioxaglate. Contrast is injected intra-articularly and a delay with exercise is taken prior to scanning. In the scanner, x-ray attenuation in the cartilage is measured in Hounsfield units (HU), with higher HU indicating depletion of GAGs.
The relationship between GAG content and x-ray attenuation measured by CTa has been validated ex vivo [94]. While applications to clinical OA research are very limited, several advantages of CTa over MRI techniques make it a potentially useful tool moving forward. For instance, scan times are short and CT scanners are widely available and relatively less expensive. However, one major limitation is the ionizing radiation that comes with the use of x-ray technology [95].
Future Considerations
As imaging technologies for assessing cartilage composition continue to evolve, several broader considerations are being investigated. One question that arises is how these techniques perform between scanners and across vendors. Several studies have recently begun looking at this question. An analysis of in vivo T1ρ and T2 relaxation time mapping between study sites found that reproducibility was generally moderate to excellent, but better in some regions of cartilage than others [96]. These differences need extensive further study before large-scale clinical studies can involve imaging with equipment from different vendors.
Another question relating to quantitative imaging of cartilage is how best to process and interpret data, especially with so many 3D sequences becoming available. Data are conventionally collected from one or more slices within the joint of interest, but the selection of slices can have a substantial impact on the results [22]. New methods for better utilizing 3D data sets are being developed and tested [97], and will likely provide better sensitivity to detect regional changes in cartilage quality.
Finally, multiple imaging techniques within single studies may provide assessments of both the collagen matrix and the concentration of GAG molecules to yield a better understanding of early changes in OA (Fig. 1). For instance, T2 and T1ρ relaxation time mapping are commonly applied together in clinical studies [98, 99•], and sometimes provide different results among study participants [100]. An interventional study utilizing both dGEMRIC and T2 relaxation time mapping suggests that dGEMRIC may be more sensitive to the earliest biochemical changes in cartilage [101], possibly attributable to the suggestion that GAG loss precedes breakdown of the collagen matrix in OA [56]. This is further supported by a study comparing T1ρ, T2, and dGEMRIC T1 relaxation times between healthy and early arthritic cartilage [102], in which T1ρ and T1 relaxation times were more sensitive to differences between these two groups than T2 relaxation times. Results from another study that used these two techniques suggest that combining datasets from dGEMRIC and T2 relaxation time mapping provides useful information that is otherwise unavailable from only one technique [103].
Fig. 1.
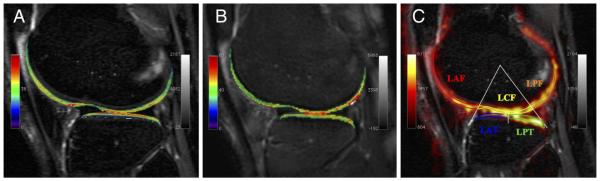
Compositional techniques in cartilage to evaluate cartilage health. Compositional markers studied here were a T2 (collagen and water), b T1ρ (glycosaminoglycan, collagen, water), and c sodium (glycosaminoglycan)
Conclusions
Imaging with MRI and CT is becoming increasingly important in studies of cartilage degeneration and in testing new therapies for prevention or delaying the progression of OA. Real progress in understanding how best to treat and manage OA has been facilitated by these techniques. While each technique provides advantages over others, several drawbacks are yet to be overcome or circumvented. Finally, new directions of research show promise in boosting the strength of these imaging techniques to be more sensitive to arthritic changes and to be used in larger-scale research studies.
Acknowledgments
Feliks Kogan and Grant W. Fong declare the receipt of travel support money, payment for manuscript review, and consulting fees, as well as an institutional grant from GE Healthcare.
Garry E. Gold declares the receipt of consulting fees from Boston Scientific, as well as institutional grants from GE Healthcare and the NIH.
Footnotes
Conflict of Interest Stephen J. Matzat declares the receipt of institutional research grants from GE Healthcare, as well as grants from the NIH.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, et al. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D662–70. doi: 10.2741/poole. [DOI] [PubMed] [Google Scholar]
- 4.Boegard T, Rudling O, Petersson IF, et al. Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Ann Rheum Dis. 1998;57(7):401–7. doi: 10.1136/ard.57.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kijowski R, Blankenbaker DG, Stanton PT, et al. Radiographic findings of osteoarthritis versus arthroscopic findings of articular cartilage degeneration in the tibiofemoral joint. Radiology. 2006;239(3):818–24. doi: 10.1148/radiol.2393050584. [DOI] [PubMed] [Google Scholar]
- 6.Hodler J, Resnick D. Current status of imaging of articular cartilage. Skelet Radiol. 1996;25(8):703–9. doi: 10.1007/s002560050165. [DOI] [PubMed] [Google Scholar]
- 7.Kaab MJ, Gwynn IA, Notzli HP. Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J Anat. 1998;193(Pt 1):23–34. doi: 10.1046/j.1469-7580.1998.19310023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maroudas A, Bayliss MT, Venn MF. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis. 1980;39(5):514–23. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36(2):121–9. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 11.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–73. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 12.Bashir A, Gray ML, Hartke J, et al. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857–65. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe A, Wada Y, Obata T, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239(1):201–8. doi: 10.1148/radiol.2383050173. [DOI] [PubMed] [Google Scholar]
- 14.Wiener E, Settles M, Weirich G, et al. The influence of collagen network integrity on the accumulation of gadolinium-based MR contrast agents in articular cartilage. Röfo. 2011;183(3):226–32. doi: 10.1055/s-0029-1245739. [DOI] [PubMed] [Google Scholar]
- 15.Salo EN, Nissi MJ, Kulmala KA, et al. Diffusion of Gd-DTPA(2)(-) into articular cartilage. Osteoarthr Cartil. 2012;20(2):117–26. doi: 10.1016/j.joca.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Tiderius CJ, Olsson LE, de Verdier H, et al. Gd-DTPA2)-enhanced MRI of femoral knee cartilage: a dose-response study in healthy volunteers. Magn Reson Med. 2001;46(6):1067–71. doi: 10.1002/mrm.1300. [DOI] [PubMed] [Google Scholar]
- 17.Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Bittersohl B, Hosalkar HS, Werlen S, et al. Intravenous versus intra-articular delayed gadolinium-enhanced magnetic resonance imaging in the hip joint: a comparative analysis. Investig Radiol. 2010;45(9):538–42. doi: 10.1097/RLI.0b013e3181ea5bb5. [DOI] [PubMed] [Google Scholar]
- 19.Sigurdsson U, Siversson C, Lammentausta E, et al. In vivo transport of Gd-DTPA2- into human meniscus and cartilage assessed with delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) BMC Musculoskelet Disord. 2014;15(1):226. doi: 10.1186/1471-2474-15-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trattnig S, Marlovits S, Gebetsroither S, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0 T: Preliminary results. J Magn Reson Imaging. 2007;26(4):974–82. doi: 10.1002/jmri.21091. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie CA, Williams A, Prasad PV, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 T and 3.0 T. J Magn Reson Imaging. 2006;24(4):928–33. doi: 10.1002/jmri.20689. [DOI] [PubMed] [Google Scholar]
- 22.Siversson C, Tiderius CJ, Neuman P, et al. Repeatability of T1-quantification in dGEMRIC for three different acquisition techniques: two-dimensional inversion recovery, three-dimensional look locker, and three-dimensional variable flip angle. J Magn Reson Imaging. 2010;31(5):1203–9. doi: 10.1002/jmri.22159. [DOI] [PubMed] [Google Scholar]
- 23.Bron EE, van Tiel J, Smit H, et al. Image registration improves human knee cartilage T1 mapping with delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) Eur Radiol. 2013;23(1):246–52. doi: 10.1007/s00330-012-2590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Tiel J, Bron EE, Tiderius CJ, et al. Reproducibility of 3D delayed gadolinium enhanced MRI of cartilage (dGEMRIC) of the knee at 3.0 T in patients with early stage osteoarthritis. Eur Radiol. 2013;23(2):496–504. doi: 10.1007/s00330-012-2616-x. [DOI] [PubMed] [Google Scholar]
- 25.Mamisch TC, Kain MS, Bittersohl B, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) in Femoacetabular impingement. J Orthop Res. 2011;29(9):1305–11. doi: 10.1002/jor.21371. [DOI] [PubMed] [Google Scholar]
- 26.Stelzeneder D, Mamisch TC, Kress I, et al. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthr Cartil. 2012;20(7):661–9. doi: 10.1016/j.joca.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Williams A, Shetty SK, Burstein D, et al. Delayed gadolinium enhanced MRI of cartilage (dGEMRIC) o f the first carpometacarpal (1CMC) joint: a feasibility study. Osteoarthr Cartil. 2008;16(4):530–2. doi: 10.1016/j.joca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuman P, Tjornstrand J, Svensson J, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury–comparison with asymptomatic volunteers. Osteoarthr Cartil. 2011;19(8):977–83. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Rutgers M, Bartels LW, Tsuchida AI, et al. dGEMRIC as a tool for measuring changes in cartilage quality following high tibial osteotomy: a feasibility study. Osteoarthr Cartil. 2012;20(10):1134–41. doi: 10.1016/j.joca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Domayer SE, Trattnig S, Stelzeneder D, et al. Delayed gadolinium-enhanced MRI of cartilage in the ankle at 3 T: feasibility and preliminary results after matrix-associated autologous chondrocyte implantation. J Magn Reson Imaging. 2010;31(3):732–9. doi: 10.1002/jmri.22093. [DOI] [PubMed] [Google Scholar]
- 31.Vasiliadis HS, Danielson B, Ljungberg M, et al. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38(5):943–9. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- 32.Owman H, Ericsson YB, Englund M, et al. Association between delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and joint space narrowing and osteophytes: a cohort study in patients with partial meniscectomy with 11 years of follow-up. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.02.929. [DOI] [PubMed] [Google Scholar]
- 33.Heverhagen JT, Krombach GA, Gizewski E. Application of extracellular gadolinium-based MRI contrast agents and the risk of nephrogenic systemic fibrosis. Röfo. 2014;186(7):661–9. doi: 10.1055/s-0033-1356403. [DOI] [PubMed] [Google Scholar]
- 34.Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith HE, Mosher TJ, Dardzinski BJ, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14(1):50–5. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 36.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosami-noglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthr Cartil. 2011;19(2):171–9. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CS, Yan CH, Gong NJ, et al. Imaging biomarker with T1rho and T2 mappings in osteoarthritis - In vivo human articular cartilage study. Eur J Radiol. 2013;82(4):647–50. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–68. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Han ET, Busse RF, et al. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Takahashi A, Han ET. 3D Quantitative Imaging of T1rho and T2; International Society for Magnetic Resonance in Medicine: Annual Meeting & Exhibition; Montréal. 2011. 19th. [Google Scholar]
- 41••.Staroswiecki E, Granlund KL, Alley MT, et al. Simultaneous estimation of T(2) and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med. 2012;67(4):1086–96. doi: 10.1002/mrm.23090. This work is of major importance because it shows that parametric information from articular cartilage (T2 and diffusion) can be obtained using a standard MR imaging method that is commonly used for cartilage thickness. Hence, one acquisition can show early changes of matrix degeneration and later cartilage loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baum T, Stehling C, Joseph GB, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging. 2012;35(2):370–8. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan J, Pialat JB, Joseph T, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507–15. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedrich KM, Shepard T, de Oliveira VS, et al. T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol. 2009;193(5):W411–5. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golditz T, Steib S, Pfeifer K, et al. Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Marik W, Apprich S, Welsch GH, et al. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2-mapping at 3 T MRI: a feasibility study. Eur J Radiol. 2012;81(5):923–7. doi: 10.1016/j.ejrad.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 47.Miese FR, Zilkens C, Holstein A, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011;52(1):106–10. doi: 10.3109/02841851.2010.516015. [DOI] [PubMed] [Google Scholar]
- 48.Dardzinski BJ, Mosher TJ, Li S, et al. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205(2):546–50. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 49.Carballido-Gamio J, Blumenkrantz G, Lynch JA, et al. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63(2):465–72. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph GB, Baum T, Alizai H, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years–data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2012;20(7):727–35. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiomi T, Nishii T, Nakata K, et al. Three-dimensional topographical variation of femoral cartilage T2 in healthy volunteer knees. Skelet Radiol. 2013;42(3):363–70. doi: 10.1007/s00256-012-1522-2. [DOI] [PubMed] [Google Scholar]
- 52.Eckstein F, Kwoh CK, Link TM, et al. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014;73(7):1289–300. doi: 10.1136/annrheumdis-2014-205310. [DOI] [PubMed] [Google Scholar]
- 53.Hovis KK, Alizai H, Tham SC, et al. Non-traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the Osteoarthritis Initiative (OAI) Skelet Radiol. 2012;41(11):1435–43. doi: 10.1007/s00256-012-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baum T, Joseph GB, Arulanandan A, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med. 2002;48(3):460–9. doi: 10.1002/mrm.10216. [DOI] [PubMed] [Google Scholar]
- 56.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1):1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Duvvuri U, Reddy R, Patel SD, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–7. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 58.Duvvuri U, Kudchodkar S, Reddy R, et al. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthr Cartil. 2002;10(11):838–44. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 59.Regatte RR, Akella SV, Borthakur A, et al. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17(1):114–21. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Cheng J, Lin K, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witschey WR, 2nd, Borthakur A, Elliott MA, et al. Artifacts in T1 rho-weighted imaging: compensation for B(1) and B(0) field imperfections. J Magn Reson. 2007;186(1):75–85. doi: 10.1016/j.jmr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duvvuri U, Charagundla SR, Kudchodkar SB, et al. Human knee: in vivo T1(rho)-weighted MR imaging at 1.5 T–preliminary experience. Radiology. 2001;220(3):822–6. doi: 10.1148/radiol.2203001662. [DOI] [PubMed] [Google Scholar]
- 63.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11(7):741–9. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 64.Witschey WR, Borthakur A, Elliott MA, et al. T1rho-prepared balanced gradient echo for rapid 3D T1rho MRI. J Magn Reson Imaging. 2008;28(3):744–54. doi: 10.1002/jmri.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Theologis AA, Haughom B, Liang F, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsushima H, Okazaki K, Takayama Y, et al. Evaluation of cartislage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int. 2012;32(9):2867–75. doi: 10.1007/s00296-011-2140-3. [DOI] [PubMed] [Google Scholar]
- 67.Bolbos RI, Ma CB, Link TM, et al. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Investig Radiol. 2008;43(11):782–8. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakhra KS, Lattanzio PJ, Cardenas-Blanco A, et al. Can T1-rho MRI detect acetabular cartilage degeneration in femoroacetabular impingement?: a pilot study. J Bone Joint Surg (Br) 2012;94(9):1187–92. doi: 10.1302/0301-620X.94B9.29981. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Ma BC, Bolbos RI, et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28(2):453–61. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Souza RB, Kumar D, Calixto N, et al. Response of knee cartilage T and T relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr Cartil. 2014 doi: 10.1016/j.joca.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishioka H, Hirose J, Nakamura E, et al. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35(1):147–55. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 72.Singh A, Haris M, Cai K, et al. High Resolution T1rho Mapping of In Vivo Human Knee Cartilage at 7 T. PLoS One. 2014;9(5):e97486. doi: 10.1371/journal.pone.0097486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pedersen DR, Klocke NF, Thedens DR, et al. Integrating carthage-specific T1rho MRI into knee clinic diagnostic imaging. Iowa Orthop J. 2011;31:99–109. [PMC free article] [PubMed] [Google Scholar]
- 74.Granot J. Sodium imaging of human body organs and extremities in vivo. Radiology. 1988;167(2):547–50. doi: 10.1148/radiology.167.2.3357970. [DOI] [PubMed] [Google Scholar]
- 75.Insko EK, Kaufman JH, Leigh JS, et al. Sodium NMR evaluation of articular cartilage degradation. Magn Reson Med. 1999;41(1):30–4. doi: 10.1002/(sici)1522-2594(199901)41:1<30::aid-mrm6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 76.Borthakur A, Shapiro EM, Beers J, et al. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthr Cartil. 2000;8(4):288–93. doi: 10.1053/joca.1999.0303. [DOI] [PubMed] [Google Scholar]
- 77.Madelin G, Babb J, Xia D, et al. Articular Cartilage: Evaluation with Fluid-suppressed 7.0-T Sodium MR Imaging in Subjects with and Subjects without Osteoarthritis. Radiology. 2013 doi: 10.1148/radiol.13121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang G, Madelin G, Sherman OH, et al. Improved assessment of cartilage repair tissue using fluid-suppressed (2)(3)Na inversion recovery MRI at 7 Tesla: preliminary results. Eur Radiol. 2012;22(6):1341–9. doi: 10.1007/s00330-012-2383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gold GE, Hargreaves BA, Stevens KJ, et al. Advanced magnetic resonance imaging of articular cartilage. Orthop Clin N Am. 2006;37(3):331–47. doi: 10.1016/j.ocl.2006.04.006. vi. [DOI] [PubMed] [Google Scholar]
- 80.Du J, Takahashi AM, Chung CB. Ultrashort TE spectroscopic imaging (UTESI): application to the imaging of short T2 relaxation tissues in the musculoskeletal system. J Magn Reson Imaging. 2009;29(2):412–21. doi: 10.1002/jmri.21465. [DOI] [PubMed] [Google Scholar]
- 81.Du J, Carl M, Bae WC, et al. Dual inversion recovery ultrashort echo time (DIR-UTE) imaging and quantification of the zone of calcified cartilage (ZCC) Osteoarthr Cartil. 2013;21(1):77–85. doi: 10.1016/j.joca.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthr Cartil. 2011;19(1):84–8. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Chu CR, Williams AA, West RV, et al. Quantitative Magnetic Resonance Imaging UTE-T2* Mapping of Cartilage and Meniscus Healing After Anatomic Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014 doi: 10.1177/0363546514532227. This work shows that compositional MRI can detect important changes in cartilage and meniscus after joint injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyler DJ, Robson MD, Henkelman RM, et al. Magnetic resonance imaging with ultrashort TE (UTE) PULSE sequences: technical considerations. J Magn Reson Imaging. 2007;25(2):279–89. doi: 10.1002/jmri.20851. [DOI] [PubMed] [Google Scholar]
- 85.Ling W, Regatte RR, Navon G, et al. Assessment of glycosami-noglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–70. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmitt B, Zbyn S, Stelzeneder D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260(1):257–64. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 87.Krusche-Mandl I, Schmitt B, Zak L, et al. Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthr Cartil. 2012;20(5):357–63. doi: 10.1016/j.joca.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 88.Singh A, Haris M, Cai K, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012;68(2):588–94. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mlynarik V, Sulzbacher I, Bittsansky M, et al. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J Magn Reson Imaging. 2003;17(4):440–4. doi: 10.1002/jmri.10276. [DOI] [PubMed] [Google Scholar]
- 90.Xia Y, Farquhar T, Burton-Wurster N, et al. Self-diffusion monitors degraded cartilage. Arch Biochem Biophys. 1995;323(2):323–8. doi: 10.1006/abbi.1995.9958. [DOI] [PubMed] [Google Scholar]
- 91.Welsch GH, Trattnig S, Domayer S, et al. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthr Cartil. 2009;17(9):1219–27. doi: 10.1016/j.joca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Friedrich KM, Mamisch TC, Plank C, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. 2010;73(3):622–8. doi: 10.1016/j.ejrad.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Zhu SC, Shi DP, Xuan A. Human patellar cartilage: echo planar diffusion-weighted MR imaging findings at 3.0 T. Clin Imaging. 2012;36(3):199–202. doi: 10.1016/j.clinimag.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Siebelt M, van Tiel J, Waarsing JH, et al. Clinically applied CT arthrography to measure the sulphated glycosaminoglycan content of cartilage. Osteoarthr Cartil. 2011;19(10):1183–9. doi: 10.1016/j.joca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Biswas D, Bible JE, Bohan M, et al. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882–9. doi: 10.2106/JBJS.H.01199. [DOI] [PubMed] [Google Scholar]
- 96.Mosher TJ, Zhang Z, Reddy R, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258(3):832–42. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monu UD, McWalter EJ, Jordan CD, et al. 3D Visualization of Quantitative T2 Relaxation Times in the Femoral Condylar Cartilage in Healthy and ACL-injured Individuals; International Society for Magnetic Resonance In Medicine: Annual Meeting & Exhibition; Milan, Italy. 2014. [Google Scholar]
- 98.Nishioka H, Hirose J, Nakamura E, et al. Detecting ICRS grade 1 cartilage lesions in anterior cruciate ligament injury using T1rho and T2 mapping. Eur J Radiol. 2013 doi: 10.1016/j.ejrad.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 99•.Prasad AP, Nardo L, Schooler J, et al. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthr Cartil. 2013;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. This work shows that compositional mapping in articular cartilage predicts disease progression in osteoarthritis. These methods may be useful to the selection of subjects who are likely to show rapid disease progression for future studies of OA drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goto H, Iwama Y, Fujii M, et al. A preliminary study of the T1rho values of normal knee cartilage using 3 T-MRI. Eur J Radiol. 2012;81(7):e796–803. doi: 10.1016/j.ejrad.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 101.McAlindon TE, Nuite M, Krishnan N, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthr Cartil. 2011;19(4):399–405. doi: 10.1016/j.joca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Wang L, Regatte RR. Quantitative mapping of human cartilage at 3.0 T: parallel changes in T(2), T(1)rho, and dGEMRIC. Acad Radiol. 2014;21(4):463–71. doi: 10.1016/j.acra.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurkijarvi JE, Mattila L, Ojala RO, et al. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthr Cartil. 2007;15(4):372–8. doi: 10.1016/j.joca.2006.10.001. [DOI] [PubMed] [Google Scholar]


