Abstract
Objective
To report the frequency, severity, and types of comorbidities in people with Parkinson’s disease (PD) using a validated self-report comorbidity-screening tool and to determine the relationship between comorbidity and functional mobility.
Design
A secondary analysis and cross-sectional observational study design.
Setting
University hospital; outpatient Balance Disorders Laboratory.
Participants
Seventy-six persons with mild to moderate idiopathic PD.
Intervention
Not Applicable.
Main outcome measures
The Cumulative Illness Rating Scale-Geriatric (CIRS-G) and a comprehensive mobility assessment including gait (distance walked in 3 minutes), balance (Mini-BESTest), and physical function (Physical Performance Test).
Results
All participants reported comorbidities in addition to their diagnosed PD. The average number of comorbidities was 6.96 ± 2.0 (range 2–11) and total CIRS-G score was 12.7 (±4.8). The most commonly reported organ systems with comorbidity were Eyes & Ears (89%), Psychiatric (68%), Musculoskeletal (64%), Lower GI (62%), Respiratory (60.5%), Upper GI (59.2%), and Genitourinary (53.9%). The total CIRS-G score was significantly related to functional mobility: gait (r=−0.53; p=0.0001), balance (r=−0.43; p=0.0003) and Physical Performance (r=−0.36; p=0.0041). Of the original 14 organ systems measured, there were 7 systems that, when combined, best predicted gait performance; 6 systems combined best-predicted balance performance and 4 systems combined that predicted functional performance.
Conclusion
This study reports high frequency of multiple medical system comorbidity in people with mild to moderate PD. Furthermore, comorbidity scores were associated with mobility disability: gait, balance and physical function. Early intervention is important to delay mobility disability in PD and we recommend that people with PD found to have comorbidities should be screened for balance and gait deficits.
Keywords: Parkinson’s disease, comorbidity, comorbidities, CIRS-G, mobility
An estimated 1 million Americans and 10 million people worldwide are currently living with Parkinson’s disease (PD)1. It is expected that this number will double worldwide by 2030 2. PD is a progressive neurological disease that increases in prevalence with age. Elderly persons are susceptible to other chronic and debilitating conditions associated with aging, such as age-related changes in brain,, musculoskeletal, visual, auditory, digestive, and urogenital function. An estimated 80% of older Americans are living with at least one chronic condition and 50% are living with at least two comorbidities 3. Many common conditions of aging have been tied to population risk factors for falls, including muscle weakness, visual impairment, psychiatric illness, neurocardiovascular instability, and polypharmacy4. Persons with PD, regardless of comorbidity status, are at a higher risk than the general population for balance impairment, falls, hip fractures, and increased hospital admission5–8. Some commonly reported comorbidities for persons with PD, such as decreased cognition, depression, peripheral neuropathy, and weakness may further increase fall risk and mobility decline.
While PD is primarily characterized by motor symptoms, non-motor symptoms such as constipation, sleep disorders, orthostatic hypertension, urinary incontinence, apathy, depression, and fatigue may also impair mobility. A recent study concluded that non-motor symptom development in persons with PD is associated with a significant negative impact on quality-of-life and does not follow motor deterioration9. Furthermore, age-related chronic conditions may be exacerbated when occurring concurrently with PD. For example, age-related muscle strength decline may be even more problematic for a person with PD who may also have centrally-impaired muscle activation and reduced activity levels 10,11.
While high rates of individual comorbidities in people with PD have been reported, few studies have systematically examined the overall presence of multiple-system comorbidity in relation to mobility. In other populations, comorbidity has been predictive of negative outcomes after hip fracture and acute myocardial infarction and is predictive of mortality in the elderly12–14. Studies suggest that comorbidities contribute to increased mortality in persons with PD but the relationship between type and severity of comorbidities and mobility disability in persons with PD has not been reported. The purpose of this study was to report the frequency, severity, and types of comorbidity reported by people with PD and to determine the relationship of comorbidity with important measures of functional mobility. This study was a secondary analysis of a larger exercise intervention study not reported here.
Methods
Ethical Review
Oregon Health & Science University (OHSU) Institutional Review Board approved this study. All work was conducted in accordance with the declaration of Helsinki (1964). Informed consent was obtained from all of the participants in the study.
Participant Recruitment
In a convenience sample, participants of either gender with idiopathic PD were recruited for an exercise study from OHSU’s Movement Disorders Clinic and the community. To be included, participants had to: 1) be between the ages of 40–80; 2) have PD as diagnosed by a movement disorder neurologist; 3) walk unassisted (use of assistive device allowed); and 4) give informed consent to participate in an exercise program (part of another study not reported in this paper). Participants were excluded if they: 1) needed assistance with activities of daily living; 2) did not speak or read English; 3) engaged in more than 10 hours of exercise per week; 4) were enrolled in an exercise study in the last year; 5) had cognitive impairment that would prevent informed consent or cooperation with the study; and 6) lacked transportation to come to OHSU for testing.
One-hundred persons were screened over the phone and 24 were ineligible based on inclusion/exclusion criteria. Seventy-six people participated in the comorbidity phone interview to obtain frequency of comorbidity measures. However, 12 participants dropped out after completing the phone interview and before mobility testing leaving sixty-four participants for all correlation and measures of gait, balance and functional performance. Subject characteristics are presented in Table 1.
Table I.
| Variables N=76 |
Mean±SD |
|---|---|
| Age (y) | 63.9±7.79 |
| UPDRS (n=64) | 36.9±12.8 |
| H & Y (n=64) | 2.45±0.53 |
| PO Duration (y) | 6.44±6.11 |
| Height (em) | 174±9.8 |
| Weight (kg) | 82.4±17.4 |
| Body Mass Index | 27.1±4.94 |
| Falls in last 6 months | 1.75±5.00 |
| No Falls | N=51 |
| 1 Fall | N=6 |
| Frequent Falls (>1) | N=19 |
Study Design
This cross-sectional study presents data from the Cumulative Illness Rating Scale-Geriatric (CIRS-G) and an in-person mobility assessment15. The CIRS-G screening, a self-report based screening tool, consisted of a telephone interview regarding current and past history of pathologies for each of the 14 organ systems covered in the scale. After the CIRS-G assessment, participants came in to OHSU’s Oregon Clinical Translational Research Institute for a performance-based assessment of their, gait, balance, functional mobility and disease severity. Testing was performed in the same order and rest breaks were given, as needed, to avoid fatigue. All participants took their anti-Parkinson medication as normally indicated and each person was tested approximately 1 hour later. The testing was administered by a trained examiner and overseen by a physical therapist.
Measures
Comorbidity Assessment
Comorbidities were assessed using the CIRS-G. This test was developed for measuring disease burden in the geriatric population and has good inter-rater reliability, face validity, and validation for use over the phone 16. The CIRS-G, via self-report, evaluates 14 organ systems: 1) Heart, 2) Vascular, 3) Hematopoietic, 4) Respiratory, 5) Eyes, Ears, Nose, Throat, Larynx, 6) Upper Gastrointestinal Tract, 7) Lower Gastrointestinal Tract, 8) Liver, 9) Renal, 10) Genitourinary, 11) Musculoskeletal and Integument, 12) Neurological, 13) Endocrine, Metabolic, Breast, and 14) Psychiatric Illness. The severity of comorbidity in each organ system is based on a scale from 0–4 (0: No problem; 1:Current mild problem or past significant problem; 2:Moderate disability, requiring “first line” therapy; 3:Severe, constant, or significant disability, or “uncontrollable” chronic problems; 4:Extremely severe, immediate treatment required, end organ failure). We followed the scoring manual published by the authors of the scale and each category score is based on self-report 15. The total score is calculated by adding together all of the categories. The severity index is calculated by the total score divided by the number of categories endorsed. In this study, we report both frequency and severity of organ-specific comorbidities, as the number of organ-specific categories endorsed and total score (sum) of all organ systems and each organ-specific category.
Functional Mobility
Physical Performance was assessed using the Physical Performance Test (PPT) 17. The PPT simulates tasks encountered in daily life (e.g., donning a jacket). The 7-item version of the test was used and scoring is from 0–4 for each item. Gait was assessed using a 3-minute walk test. This test measures the distance covered in 3 minutes as an indicator of endurance. Balance was assessed using the Mini-BESTest18. This test is a 14-item test of dynamic balance, measured on a scale of 0–2 (2 = normal) with a maximum score of 28. The Mini-BESTest is a sensitive measure for discerning balance deficits in the PD population19.
Cognition was assessed using the Montreal Cognitive Assessment (MoCA)20. This cognitive test measures several cognitive domains and has good sensitivity for persons with PD21.
Severity of PD was measured using the commonly reported gold-standards: The Unified Parkinson’s Disease Rating Scale (UPDRS) Part III (motor) and the Hoehn & Yahr (H&Y) Scale22,23.
Data Analysis
Data from the CIRS-G is reported as the number of organ-specific categories endorsed, the total score (sum) of all organ systems and each organ-specific category (percentages). Organ-specific endorsement along with severity was used for reporting frequency scores while the total comorbidity score was used for correlations with function. Spearman correlations were used to determine the relationship among comorbidities and the balance, gait and physical performance measures. STATA (12.0) was used for summary statistics, correlations, and correlation plots and Microsoft Excel was used to create the frequency graph. To explore which combinations of comorbidities best predicted mobility, we fitted 3 separate predictive linear regression models for the 3 mobility endpoints: gait, balance and functional performance. Stepwise variable selection was used to pick the systems that jointly were most predictive for each of the 3 endpoints. Spearman and Pearson correlations were used to assess the association between the total scores (both total 14-item scale and subsections) and the mobility endpoints.
Results
Frequency, severity and types of comorbidities in patients with PD
Participant characteristics are detailed in Table 1. Every participant reported comorbidities in addition to PD. The average number of comorbidities was 6.96 ± 2.0 (range 2–11). The most commonly reported organ systems with comorbidities, with at least half of participants reporting complaints were Eyes & Ears (89%), Psychiatric (68%), Musculoskeletal (64%), Lower GI (62%), Respiratory (60.5%), Upper GI (59.2%), and Genitourinary (53.9%).
Table 2 details the frequency of each organ-system’s comorbidity and breaks the reporting by severity. While Eyes & Ears was the most commonly reported organ system, we found that close to 60% of the complaints were mild; primarily vision impairment with corrected vision (98%), mild hearing loss (34%), and vertigo, lightheadedness, dizziness (34%). Over half of the participants (54.4%) reported more than 1 condition in this category.
Table II.
Score Distribution: the percentage of participants with PD reporting mild, moderate or severe comorbidity per organ system (listed in order of most to least common).
| CIRS-G Organ System | No Problem 0 | Mild Problem 1 | Moderate Severity 2 | Severe/Constant Disability 3 | Extremely Severe 4 |
|---|---|---|---|---|---|
| Eyes & Ears | 10.5% | 59.2% | 26.3% | 3.9% | 0% |
| Psychiatric | 31.6% | 14.5% | 9.2% | 43.4% | 1.3% |
| Musculoskeletal | 35.5% | 25.0% | 31.6% | 7.9% | 0% |
| Lower GI | 38.2% | 35.5% | 23.7% | 1.3% | 1.3% |
| Respiratory | 39.5% | 27.6% | 23.7% | 7.9% | 1.3% |
| Upper GI | 40.8% | 42.1% | 14.5% | 2.6% | 0% |
| Genitourinary | 46.1% | 18.4% | 22.4% | 10.5% | 2.6% |
| Endocr & Breast | 59.2% | 25% | 13.2% | 1.3% | 1.3% |
| Vascular | 63.2% | 13.2% | 13.2% | 9.2% | 1.3% |
| Heart | 69.7% | 11.8% | 5.3% | 13.2% | 0% |
| Liver | 84.2% | 13.2% | 2.6% | 0% | 0% |
| Hematopoietic | 92.1% | 2.6% | 2.6% | 1.3% | 1.3% |
| Renal | 93.4% | 2.6% | 1.3% | 1.3% | 1.3% |
Psychiatric comorbidity was rated as much more severe than eye and ear function with 43% of complaints being severe. Most commonly, people reported depression (77%), anxiety (55%), cognitive impairment (15%) and substance abuse (13%). Approximately half had multiple psychiatric conditions (49%). Of the participants reporting depression, 53% reported current use of a pharmacological intervention. Pharmacological intervention for anxiety (11%) was much lower than for depression.
For participants with Musculoskeletal comorbidities, most (31%) reported moderate severity. The most commonly reported conditions were arthritis (54%), neuropathy (32%), joint replacement (28%), osteoporosis (16%), and low back pain (24%). Approximately half of the participants (46%) had more than 1 musculoskeletal condition.
Most Lower and Upper GI complaints were mild to moderate (35.5 % and 23.7% Lower GI; 42.1% and 14.5% Upper GI). The most common Lower GI comorbidities were constipation (81%), inguinal hernias (26%), and diverticulosis (15%). Twenty-three percent of participants had more than 1 condition in this organ system. The most common Upper GI complaints were heartburn (67%), dysphagia (44%), and hiatal hernia (31%). Almost half of the participants, 44%, had more than 1 condition in this organ system.
People with Respiratory comorbidities reported mild (27.6%) and moderate (23.7%) severity. The most commonly reported conditions were past smoking (65.2%), pneumonia (34.8%), sleep apnea (26.1%), and chronic bronchitis, asthma, emphysema (21.7%). Almost half of the participants (43.5%) reported more than one condition in this organ system. Similarly Genitourinary complaints were of mild (18.4%) or moderate severity (22.4%). The most common genitourinary conditions were urinary complaints regarding incontinence and urgency (53.7%), and prostate problems (36.6%).
The least commonly reported comorbidities were Metabolic & Breast (40.8%), Vascular (36.8%), Heart (30.3%), Liver (15.8%), Hematopoetic (7.9%), and Renal (6.6%). In addition to their PD, 24% of participants reported other Neurological conditions such as headaches, seizure disorders, sleep issues, pituitary tumor, TIAs, CVA, Bell’s palsy, and polio.
The total CIRS-G score for our sample was 12.7 (±4.8). Previously reported healthy normative values (aged 71.1) scored 4.5 (±2.5); close to 3 times less than the observed PD score15. The average number of comorbidity categories endorsed for our sample was 7.0 (±2.0) verses 3.7 (±1.9) for the previously reported control subjects15. Figure 1 shows that every organ-specific severity score from our PD cohort exceeds previously conducted scores from health adults15. It should be noted that the published normative values exclude the psychiatric category since that category was added after the cited study.
Figure 1.
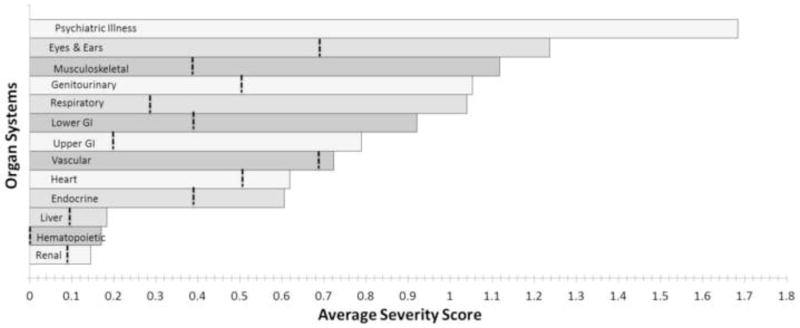
Average severity score (0–4) of each individual organ system. The dotted vertical line represents means for previously reported healthy normal subjects [10].
Relationship between Comorbidities and Mobility
The total CIRS-G score was significantly related to functional mobility based on gait (distanced walked in 3 minutes; r=−0.53; p=0.0001), balance (Mini-BESTest; r=−0.43; p=0.0003), and the Physical Performance Test; (r=−0.36; p=0.0041). The Comorbidity Score also correlated with age (r=0.27; p=0.03) but not with PD severity, (UPDRS; r=0.13; p=0.31), disease duration (r= −0.11; p=0.39) or cognition (MOCA; r = −0.17; p=0.18). The total number of comorbidity categories reported per person also correlated with measures of functional mobility; gait (r=−0.44; p=0.0001), balance (−0.34; p=0.006), and physical performance(r=−0.28; p=0.006). The severity index had weaker correlations; gait: (r= −0.3; p = 0.018), balance: (r =−0.26; p = 0.037) and functional performance: (r=−0.20 ; p = 0.121).
Of the original 14 organ systems measured, there were 7 systems that, when combined, best predicted gait performance; 6 systems combined best-predicted balance performance and 4 systems combined that predicted functional performance (Table 3). We confirmed that the correlations with the subset of items included were consistent with the correlations with the total 14-item test and found them to be comparable: gait (r = −0.46; p = 0.0001); balance (r = −0.42; p = 0.0006) and functional performance (r = −0.34; p = 0.0052).
Table III.
Categories most predictive of gait, balance and physical performance measures.
| Gait (3 Min Walk Test) | Balance (MiniBES Test) | Functional performance (Physical Performance Test) | |
|---|---|---|---|
| Neurological | * | * | * |
| Psychiatric Illness | * | * | * |
| Musculoskeletal | * | * | |
| Respiratory | * | * | |
| Lower Gi | * | * | |
| Upper Gi | * | * | |
| Liver | * | * | |
| Renal | * |
Discussion
Our sample of persons with PD had a significantly higher frequency and severity of comorbidity than previously reported for persons with PD and higher frequency and severity of comorbidity than the data published for people without PD15,16. Furthermore, comorbidity was associated with gait, balance and physical function.
The presence of comorbidities is an important factor in rising healthcare costs and presents a challenge to our system of care. In fact, the National Institute on Aging and the Clinical Gerontology Program recently established the National Institute of Aging Task Force, focused on developing clinical and research initiatives surrounding comorbidity24. The elderly population will double from 43.1 million in 2012 to 92 million by 2060, indicating that 1 in 5 U.S. residents will be 65 or older (currently 1 in 7). Similarly, the number of people living with PD is expected to double worldwide by 20302. This rise in the numbers of people with chronic, degenerative disease has serious implications as increasing comorbidities are associated with increased hospitalizations and is predictive of mortality25,26. Consequently, recognizing and treating comorbidities may significantly improve quality of life and reduce health care utilization for persons with PD.
While many of the comorbidities reported in this study were mild, the accumulation of even mild comorbidities may affect overall wellbeing and mobility4. In our study, 89% reported at least a mild impairment in Eyes & Ear function. In a recent study surveying US Medicare beneficiaries, those with corrected cataracts had lower odds of hip fracture during a 1-year time period compared to those without cataract surgery, suggesting that uncorrected vision impairment was related to balance and falls27. More than half of the participants had complaints in Upper and Lower GI and Genitourinary function, further supporting previous evidence that even mild constipation or urinary incontinence may significantly impact lifestyle and/or mobility 28.
Our study corroborates the notion that depression is a significant problem for people with PD; 77% of our cohort reported depression. This number is higher than other reports in the literature for PD and one explanation may be that we were recruiting people with at least one other comorbidity, which could have included depression. A systematic review conducted by Reijnders reported that the average prevalence of major depressive disorder in PD is 17%, dysthymia is 13%, and minor depression is 22%29. In contrast, major depression is reported in only 4.4% in women and 2.7% in men in the general elderly population30. The high incidence of depression and other psychiatric conditions in PD is an important consideration for fall risk as depressive symptoms are associated with falls in the elderly and PD populations31.
While much of PD clinical management for mobility disability targets dopaminergic/motor-based symptoms, our results suggest that non-motor and other comorbidities may be important to address as well. Screening for the overall number of comorbidities, however mild, could serve as an indicator of declining mobility and fall risk in a person with PD, even via phone interview in the case of rural locations. Changes in mobility due to age-related biomechanical changes can lead to an increased risk for falling in the elderly and early intervention may slow or break the vicious cycle of immobility for those with PD.
Multidisciplinary care is strongly recommended for the medical management of chronic illness and the findings from this study provide further support for collaboration among health care providers. In the US, the current model of health care delivery is often fragmented and focused on provider specialization. A neurologist, focused on clinical management of a patient with PD, may be unaware of other conditions affecting the patient, previously or currently, in other organ systems. In this case, a provider may have an incomplete perspective regarding the overall health of his or her patient. Addressing comorbidities directly could help reduce mobility problems that are not entirely resolved through medication management.
Study Limitations
This study was advertised as an exercise study and most participants went on to participate in that study also. This recruitment method may have attracted people who were relatively active, so this study sample may represent a less-impaired population. Furthermore, people unable to walk independently were excluded and the average age of our subjects was younger than reported in other comorbidity studies in PD15,16. A second possible limitation is that a phone interview may have caused an underreporting of symptoms that may be viewed as socially sensitive (e.g. Genitourinary, Psychiatric). However, the phone interview protocol of the CIRS-G has been validated16. Even if these limitations are significant, we are confident of our findings since our data represent a conservative estimation of comorbidities and mobility deficits.
Conclusions
This study reports high frequency of multiple medical system comorbidity in people with mild to moderate PD. Furthermore, comorbidity scores were associated with mobility disability: gait, balance and physical function suggesting that people with PD should be screened for comorbidities and referred for a fall-risk and mobility assessment. The presence of comorbidities in people with PD should be identified and treated, if possible, to reduce mobility disability.
Acknowledgments
Funding Sources:
Foundation for Physical Therapy (American Physical Therapy Association): Clagett Family Research Grant and Oregon Clinical Translational Research Institute (OCTRI) 1 UL1 RR024140 01 and OCTRI grant number (KL2TR000152) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study is registered at clinical trials.gov; NCT01361724
Abbreviations
- CIRS-G
Cumulative Illness Rating Scale-Geriatric
- H&Y
Hoehn & Yahr
- MoCA
Montreal Cognitive Assessment
- OHSU
Oregon Health & Science University
- PD
Parkinson’s disease
- PPT
Physical Performance Test
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
This material has been presented in poster format at the American Physical Therapy Association; Combined Sections Meeting 2013.
OHSU and Dr. Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foundation PsD. [Accessed 3–4–14, 2014];Understanding Parkinson’s. 2014 http://www.pdf.org/en/parkinson_statistics.
- 2.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 3.He W, Sengupta M, Velkoff VA, DeBarros KA. Current population reports. Washington DC: 2005. 65+ in the United States. [Google Scholar]
- 4.Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. The American journal of medicine. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- 5.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neruol Neurosurg Psychiatric. 2002 doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashburn A, Stack E, Pickering RM, Ward CD. Predicting Fallers in a Community-Based Sample of People with Parkinson ’ s Disease. Gerontology. 2001:277–281. doi: 10.1159/000052812. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya RK, Dubinsky RM, Lai SM, Dubinsky H. Is there an increased risk of hip fracture in Parkinson’s disease? A nationwide inpatient sample. Movement disorders : official journal of the Movement Disorder Society. 2012;27:1440–1443. doi: 10.1002/mds.25073. [DOI] [PubMed] [Google Scholar]
- 8.Temlett Ja, Thompson PD. Reasons for admission to hospital for Parkinson’s disease. Internal medicine journal. 2006;36:524–526. doi: 10.1111/j.1445-5994.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 9.Antonini A, Barone P, Marconi R, et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. Journal of neurology. 2012;259:2621–2631. doi: 10.1007/s00415-012-6557-8. [DOI] [PubMed] [Google Scholar]
- 10.Salenius S, Avikainen S, Kaakkola S, Hari R, Brown P. Defective cortical drive to muscle in Parkinson’s disease and its improvement with levodopa. Brain. 2002;125(3):491–500. doi: 10.1093/brain/awf042. [DOI] [PubMed] [Google Scholar]
- 11.Yanagawa S, Shindo M, Yanagisawa N. Muscular weakness in Parkinson’s disease. Advances in neurology. 1989;53:259–269. [PubMed] [Google Scholar]
- 12.Yang X-D, Zhao Y-S, Li Y-F, Guo X-H. Medical comorbidities at admission is predictive for 30-day in-hospital mortality in patients with acute myocardial infarction: analysis of 5161 cases. Journal of geriatric cardiology : JGC. 2011;8:31–34. doi: 10.3724/SP.J.1263.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radosavljevic N, Lazovic M, Nikolic D, Petronic I, Radosavljevic Z, Jeremic A. Influence of selective comorbidity predictors on functional recovery after hip fracture in an older population. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia. 2012;156:365–370. doi: 10.5507/bp.2012.102. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Medical care. 2005;43:607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 15.Miller MD, Parrdis CF, Houck PR, et al. Rating Chronic Medical Illness Burdgen inGeropsychiatric Practice and Research: Application of the Cumulative Illness Rating Scale. Psychiat Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 16.Visser M, Marinus J, van Hilten J, Schipper R, Stiggelbout A. Assessing Comorbidity in Patients With Parkinson’s Disease. Movement Disorders. 2004;19:824–828. doi: 10.1002/mds.20060. [DOI] [PubMed] [Google Scholar]
- 17.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 18.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation System’s Test: the mini-BESTest. Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2010;42:323. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King La, Horak FB. Lateral stepping for postural correction in Parkinson’s disease. Arch Phys Med Rehabil. 2008;89:492–499. doi: 10.1016/j.apmr.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 21.Gill DJ, Freshman A, Blender Ja, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Elton RL. Unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. 1987;2:153–163. [Google Scholar]
- 23.Hoehn MM, Yahr MD, et al. Parkinsonism: onset, progression, and mortality. Neurology. 1998;50:318. doi: 10.1212/wnl.50.2.318. [DOI] [PubMed] [Google Scholar]
- 24.Yancik R. Report of the national institute on aging task force on comorbidity. J Gerontol. 2007;62:275–280. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. Journal of the American College of Cardiology. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 26.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA : the journal of the American Medical Association. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 27.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA : the journal of the American Medical Association. 2012;308:493–501. doi: 10.1001/jama.2012.9014. [DOI] [PubMed] [Google Scholar]
- 28.Gage H, Kaye J, Kimber a, et al. Correlates of constipation in people with Parkinson’s. Parkinsonism & related disorders. 2011;17:106–111. doi: 10.1016/j.parkreldis.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:183–189. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 30.Steffens D, Skoog I, Norton M. Prevalence of depression and its treatment in an elderly population: the Cache County study. Archives of General Psychiatry. 2000;57:14–18. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- 31.Kvelde T, McVeigh C, Toson B, et al. Depressive symptomatology as a risk factor for falls in older people: systematic review and meta-analysis. Journal of the American Geriatrics Society. 2013;61:694–706. doi: 10.1111/jgs.12209. [DOI] [PubMed] [Google Scholar]


