Abstract
Background and Purpose
Previously, we showed that persons with Parkinson’s disease (PD) were unable to modify their postural responses, which associated with cortical preparatory activity for anticipated postural perturbations.1 Here, we asked if participants with PD could modify their postural responses and cortical preparatory activity when cued to focus on increasing movement amplitude prior to a series of predictable postural perturbations.
Methods
Twelve participants with PD performed postural responses to 30 identical backward surface translations. We examined the effects of cueing them to focus on increasing movement amplitude by measuring postural responses (center-of-pressure (CoP) initial rate of change, automatic postural response (APR) stability, peak trunk flexion, peak ankle extension) and preparatory cortical activity (electroencephalographic (EEG) measures of contingent negative variation (CNV), alpha and beta event-related desynchronization (ERD)).
Results
Participants with PD modified their postural responses during the amplitude trials by increasing trunk flexion, slowing CoP initial rate of change and decreasing APR stability. However, no significant differences in CNV amplitude or alpha or beta ERD were observed with versus without amplitude cueing.
Discussion and Conclusions
Persons with PD were able to modify their feet-in-place postural responses with amplitude cueing. These changes were not associated with changes in cortical preparation during amplitude cue trials, suggesting that other regions or measures of brain function were responsible for changes in postural responses. Future studies are needed to determine the effects of long-term amplitude-cueing practice on cortical preparation and postural stability.
Keywords: Parkinson’s disease, cueing, postural responses, electroencephalography, contingent negative variation, event related desynchronization
Introduction
People with Parkinson’s disease (PD) demonstrate impaired postural responses. Impairments include hypometric force production, difficulty modifying and scaling postural responses based on initial context and poor use of the hip strategy.2–7 Abnormal proprioceptive-motor integration likely also contributes to impaired feet-in-place (feet are stationary, no step is performed) postural responses. People with PD tend to over-estimate the size and strength of their movements; although they may perceive that they are moving with appropriate size and strength, they actually demonstrate hypometric movements and do not reach far enough, walk far enough, or step far enough to a target when performing without visual feedback.8–11
Our laboratory previously observed that participants with PD had difficulty modifying the amplitude of their automatic postural response (APR) when asked to actively ‘resist’ or ‘give in’ to surface translations.4 Postural stepping responses and voluntary control of the center of mass (CoM) of the body can improve with training or external cueing in people with PD,11–13 however whether feet-in-place, APRs can be improved with training or external cueing is uncertain. Rehabilitation programs have addressed impaired, small movement patterns and abnormal proprioceptive-motor integration by encouraging patients to make larger, faster, more forceful voluntary body movements.14–18 Similarly, our intervention strategy here was to cue participants to think about making a larger postural response in an effort to address impaired, hypometric APRs in persons with PD.
Previous studies have examined the effects of encouraging large-amplitude movements and focusing attention on the sensory awareness of “movement bigness” (often referred to as “Think Big”) to recalibrate the patient’s perception of movement effort and increase movement amplitude.16,19,20 Existing studies include: 1) a randomized trial of 60 participants with PD19 that reported improved general motor performance and walking velocity, and 2) a non-controlled trial with 18 participants with PD16 that showed improved arm movement velocity for reaching and increased walking velocity. Although it has not been empirically tested, the underlying assumption of these rehabilitation programs is that practice of larger movements will create permanent behavioral change, presumably by changing underlying neural function. Although effective at improving immediate movement patterns, improvements are not maintained six17 or twelve21 weeks later.
In order to better understand the neural mechanisms of effect associated with amplitude cueing interventions, we measured how attentional cueing to make larger amplitude postural responses affects preparatory neural function in PD. We also wanted to know the extent to which attentional cueing altered feet-in-place postural responses. Previously, we showed that persons with PD were unable to scale the size of their postural responses under conditions of predictable perturbation amplitudes, and that diminished scaling associated with increased preparatory cortical activity.1 Here, we asked if participants with PD could modify their postural responses and cortical preparatory activity when cued to increase movement amplitude prior to a series of anticipated postural perturbations. We measured postural responses using initial rate of change of the center of pressure (CoP: represents central set and planning of response), APR stability (a measure of the CoM relative to the CoP), maximal trunk flexion and maximal ankle extension. We measured cortical preparatory activity using electroencephalography (EEG), specifically contingent negative variation (CNV) and alpha and beta event-related desynchronization (ERD).
Methods
Participants
Participants were 12 adults with moderate PD (Hoehn and Yahr Stage II or III, also see Table 1). They were sensitive to dopamine replacement medication and did not have history or evidence of significant health conditions not associated with PD. They were without pain or conditions that would prevent standing independently for 30 minutes and had vision corrected to 20/40 or better. Participants were not taking medications known to affect balance or attention (other than medications for PD) and were without cognitive impairment (score >= 26 on the Montreal Cognitive Assessment).22 Participants were recruited from the Parkinson’s Center of Oregon and community support groups. In our preceding paper,1 we found the group of 12 participants with PD demonstrated significant differences in beta ERD between conditions of 30 trials each (similar to our protocol here), and a power analysis of these specific results indicated that a sample size of 10 would be sufficient to detect a similar difference here.
Table 1.
Descriptive Characteristics: Mean (95% Confidence Interval of the Mean) of Participants
| Weight (kg) | 81.2 (76.5–85.8) |
| Height (m) | 1.78 (1.74–1.82) |
| Age (yrs) | 66.3 (62.6–70.0) |
| Duration of PD (yrs) | 7.3 (5.0–9.6) |
| UPDRS Motor Score* | 17.3 (13.4–21.1) |
| Gender | 11m, 1f |
on dopamine replacement medications
Procedures
The rights of human subjects were protected and the Oregon Health & Science University Institutional Review Board approved all procedures. Participants came into the laboratory for one 2–2.5-hour visit. We explained all procedures and answered any questions before they signed an informed consent document. We administered a health history survey, the Montreal Cognitive Assessment and Part III (Motor section) of the Unified Parkinson’s Disease Rating Scale (original version). Participants were tested during their self-described optimal “On” medication state, although some reported a lack of fluctuation in state (no “Off” times). All participants were tested 1–2.5 hours after taking a morning dose.
We placed an Advanced Neuro Technology (ANT; Enschede, the Netherlands) Waveguard 32-channel EEG cap (sintered silver/silver-chloride electrodes; standard 10/20 system placement) on participants and used gentle abrasion of the scalp and conductive electrode gel (Electro-gel; Electro-Cap International; Eaton, OH, USA) to obtain impedances below 5 kΩ. To identify eye movement artifacts, electrooculographic (EOG) recordings were measured by silver/silver-chloride electromyography electrodes placed above and below the right orbit and lateral to the right and left orbits. EEG and EOG data were collected at 512 Hz using an ANT high-density ASA-Lab amplifier and software. Electrodes were referenced to linked mastoid electrodes.
We used a 3-dimensional motion analysis system with eight Falcon video cameras (Motion Analysis Corporation, Santa Rosa, CA, USA) to collect body position data at 60 Hz. Reflective markers were placed bilaterally on the fifth metatarsophalangeal joint, bony prominence of the calcaneus, lateral malleoli, lateral knee joint center, greater trochanter, acromion process, lateral epicondyle, wrist joint center, mandible and temple. Anthropometric measures of the body segments were collected for estimation of the position of the CoM. Forceplate data were collected at 480 Hz with a custom system (QNX Software Systems, Ottowa, Ontario, CA). Motion analysis and force plate data recordings were started for each trial by a signal from the forceplate system, which also placed marks in the EEG data (recorded continuously) at the start of each trial and at the time of perturbation onset.
Participants stood with one foot on each force plate, their feet at a comfortable, self-selected, approximately hip-distance width. We used tape outlines to provide consistent foot placement between trials. Participants wore a harness attached without tension to the ceiling and had a spotter standing on their left side. They were instructed to stand quietly, with their arms resting at their sides just in front of their hips and their vision focused on a target area located 1.25 m high on a board 3.5 m in front of them.
Participants knew that they would see a visual warning stimulus 2 s prior to receiving a balance perturbation. A 12 cm posterior perturbation was delivered using a translating force plate at 15 cm/s, resulting in forward-body disequilibrium. This perturbation was small enough so that the participant could maintain balance without stepping.1,2 We allowed them to experience the perturbation once before we began data collection, so that they were familiar with it.
There were 90 trials total (30 perturbation trials for each of 3 conditions, performed in sets of 10 trials). This is consistent with our previous study and is expected to yield a minimum of 25 trials of usable EEG data per condition to average in order to obtain the dependent variables.1 For the first condition (Baseline), no specific instructions were provided on how to respond other than requesting a feet-in-place response. The second condition (Amplitude) was composed of trials where participants were instructed to perform a “maximally big” postural response, larger than they thought they needed, while still keeping their feet in place. We reminded them after every third trial to “think about making a big response”. In addition, we asked them about perceptual feedback (”How big did that feel?”) after 10 trials to ensure they were considering how their movement felt. For the third condition (Baseline 2), we told participants to stop thinking about making a big response and respond as they did during Baseline trials. The third condition was included with the intention of examining whether changes in the second condition of amplitude cueing resulted from general practice effects. If present, an amplitude specific effect would be present in the Amplitude trials only, whereas a general practice effect would be present in the Amplitude and Baseline 2 trials. Trials were performed in blocks of 10, followed by a rest period. The experimenters did not otherwise speak to the participant during the block of 10 trials, except to confirm that the trial was complete and the force plates were resetting to their original position or to remind them to “move big” during the Amplitude condition. After the force plates reset, the experimenter waited to observe the participant standing quietly with a stable baseline EEG signal before sending the visual warning cue and subsequent perturbation. Each set of 10 trials lasted approximately 3–5 minutes. There were 10–20 seconds between trials.
Data Analysis
Postural Responses
To identify changes in the planned activity of the cortex (central set) to prepare for the anticipated perturbation amplitudes, we measured changes in the initial rate of change of the CoP response. Preparation of postural responses involves the cortex, based on knowledge of the upcoming perturbation.23,24 Central set is defined as a state of readiness to receive a stimulus or make a movement, represented by a task-dependent preparatory neural discharge within the central nervous system.25 We calculated CoP from the force plate data26 and low-pass filtered it at 10 Hz. We calculated the initial rate of change, which is the slope (change in position/change in time) of the anterior-posterior position of the CoP between 100 and 200 ms after the perturbation (see Figure 1a). This time period was chosen to represent the initial active postural response, after the passive response and before long loop feedback correction,2,27,28 thus reflecting preparation of the postural response before feedback adjustments have been made. We also identified the peak anterior displacement of the CoP in response to the perturbation (see Figure 1a).
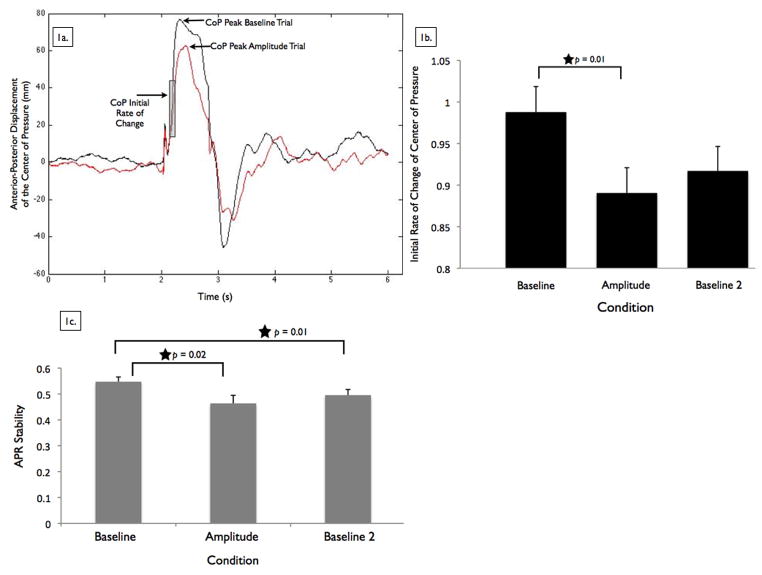
Figure 1. Center of pressure and automatic postural response stability.
Figure 1a shows an example from one participant’s center of pressure (CoP) anterior-posterior displacement data from a trial of Baseline and Amplitude conditions. The peaks of each CoP trace are identified, while the shaded area indicates the time period from 100 to 200 ms after the perturbation, where we calculated the initial rate of change of the CoP. Figure 1b shows group means for the initial rate of change of the CoP, by condition. Error bars are 95% confidence intervals. The star indicates conditions that were significantly different from each other in follow up analyses. Figure 1c shows group means for automatic postural response (APR) stability, by condition. APR stability is calculated from the anterior-posterior center of pressure position relative to the center of mass position, as described in the text. A higher score reflects a fast and strong, more stable, APR. Error bars are 95% confidence intervals. The stars indicate conditions that were significantly different from each other in follow up analyses.
We used custom software programs (MATLAB, MathWorks, Natick, MA) to identify the peak trunk flexion and ankle joint extension angles in response to the perturbation. Trunk flexion was calculated from the angle created between the upper leg (line between the lateral knee joint center and greater trochanter) and trunk (line between the greater trochanter and acromion process) markers. Trunk flexion is a non-specific, overall description of flexion behavior as it included flexion occurring at the hips, trunk, and/or knees. Ankle joint extension was calculated from the angle created by the right 5th metatarsophalangeal joint, bony prominence of the calcaneus and lateral knee joint center.
APR Stability
CoM was calculated from the weighted sum of the CoM locations of individual body segments.29 APR stability, a measure of the active control generated through the feet to arrest the falling CoM, is calculated as the difference between the CoP and CoM areas under the curve over 300 ms (normalized to each person’s height) with a higher score reflecting a fast and strong, more stable, APR.30,31 A higher score reflects a more stable APR because it represents a larger difference between the CoP and CoM in the direction of the induced CoM displacement, which leads to faster recovery of equilibrium due to the CoP reversing the induced displacement of the CoM. 30,31
EEG Data: Cortical Preparation
Raw EEG data were imported into custom programs (EEGLAB;32 MATLAB, MathWorks, Natick, MA) for analysis. We analyzed 90 trials (30 for each of 3 conditions) for most participants. For 3 out of the 12 participants, the total number of trials was 83, 87 and 88 due to missing data or technical difficulties. Missing trials were evenly distributed across the 3 conditions.
EEG data were resampled to 480 Hz to match the other data sources and then detrended to remove the direct current (DC) mean from our DC amplifier. Data were low-pass filtered at 40 Hz; this was 1) consistent with previous work,1,33,34 and 2) based on our upper frequency of interest being 29 Hz. We did not high-pass filter the data due to the low frequency of the CNV; we did not want to filter out our signal of interest. We extracted epochs from 3 s before to 0.5 s after the perturbation. From −3 to −2 s the participant was standing quietly. The visual cue was at −2 s and the platform started moving at 0 s. We baseline corrected the EEG signals of each trial’s recording epoch by subtracting the mean signal amplitude of the interval from −3 to −2 s prior to perturbation onset. We used Independent Components Analysis to remove eye blinks and other obvious noise from the EEG epochs.32 Components were rejected if they represented eye blinks or muscle activity or had a highly unstable baseline. Generally, 4–7 components were rejected per participant.
Preparatory cortical activity was quantified by CNV and upper alpha and beta ERD amplitudes. These measures represent distinct preparatory functions of the cerebral cortex, and we have previously observed them in people with PD prior to experiencing balance perturbations.1 The CNV represents both non-motor processes related to stimulus anticipation and sensorimotor processes related to preparation of movement and is typically measured at the Cz electrode located in the region of the supplementary motor area.35,36 ERD measures desynchronization of brain activity in defined brain areas across specific frequency ranges, representing differential preparation of these areas preceding movement. Upper alpha ERD represents enhanced cortical excitability or readiness for an impending movement and is located over sensory-motor regions specific to the planned movement.37 Upper beta ERD is thought to represent changes in synchronization of circuits involving the supplementary motor area and is localized at mesial central electrodes.38,39 Both upper alpha and beta bands appear to represent synchrony of distinct circuits among the cortex, basal ganglia, and thalamus.38,40 Measuring both alpha and beta ERD allowed us to determine whether amplitude cueing modifies response preparation through sensory-motor cortico-thalamic circuits (alpha ERD) or through motor cortico-striatal circuits (beta ERD).
For quantifying CNV amplitudes, we analyzed data from the Cz electrode, where we found the largest CNV amplitude (see Table Supplemental Data 1). EEG signals were averaged by participant and condition. The CNV amplitude was then determined as the mean amplitude of each condition’s average EEG signal during the final 100 ms prior to perturbation onset.
For quantifying alpha and beta ERD,41 the EEG signals from each trial were digitally re-referenced to a common average reference of the cephalic electrodes. The reference-free analysis allows identification of a more focal spatial pattern of ERD.41 For each of the artifact-free trials, we performed continuous morlet wavelet transforms (a specific type of basic mathematical signal analysis) in the upper alpha (10–12 Hz) and upper beta (20–29 Hz) frequencies. We rectified and averaged the morlet coefficients by condition and participant, then averaged across the alpha or beta bands. We normalized the time-varying alpha or beta coefficients to a percentage change in alpha or beta activity from a baseline average calculated from the 500 ms before the warning cue. Mean percentage changes in coefficients were binned over the four subsequent 500-ms intervals that corresponded to the time between the warning cue and perturbation onset.
To examine the effects of amplitude cueing on the amplitude of ERD, we chose to analyze data from the electrode that demonstrated the most alpha or beta ERD averaged over the final 1000 ms preceding the perturbation. For alpha ERD, the largest amount of desynchronization occurred at the CP1 electrode (see Table Supplemental Data 2). The largest amount of beta ERD occurred at the CZ electrode (see Table Supplemental Data 2).
Statistical Analysis
We used linear mixed models to test for differences across conditions. Dependent variables for postural responses were CoP initial rate of change, peak ankle extension, peak trunk flexion and APR stability. Dependent variables for cortical activity measures were CNV amplitude, alpha ERD and beta ERD. We used 3-condition (Baseline, Amplitude, Baseline 2) linear mixed models with condition as a repeated measure. We tested for a condition fixed main effect, used an unstructured covariance structure and adjusted for multiple comparisons with the Bonferroni correction. We used follow-up pairwise comparisons to determine significant differences between conditions when a main effect was present. We used SPSS predictive analytics software (IBM, Chicago, IL) version 19 for statistical analysis and set our alpha level of significance at 0.05.
Results
Participants were 12 adults with moderate PD (Hoehn and Yahr Stage II or III). Descriptive data further summarizing participants’ anthropometrics and PD status are provided in Table 1.
Postural Responses
Figure 1b shows that participants demonstrated a significant difference in initial rate of change of CoP response across conditions (F[2,11]= 7.07, p = 0.01). Follow-up pairwise comparisons of the condition main effect revealed significantly slower initial rate of change of CoP in the Amplitude condition compared to Baseline (p = 0.01). There were no significant differences in initial rate of change of CoP between the Amplitude and Baseline 2 conditions (p = 0.09) or between the Baseline and Baseline 2 conditions (p = 0.53).
Participants demonstrated a significant difference in peak trunk flexion across conditions (F[2,11]= 5.71, p = 0.02; Table 2). Follow-up pairwise comparisons of the condition main effect revealed significantly greater trunk flexion in the Amplitude condition compared to the Baseline (p = 0.02) or Baseline 2 (p = 0.02) conditions, consistent with a change from an ankle to a hip strategy for postural correction. There were no significant differences in trunk flexion between the Baseline and Baseline 2 conditions (p = 0.31). Timings of peak trunk flexion and ankle extension angles are presented in Table 2. Participants did not demonstrate a significant difference in peak ankle extension angle across conditions (F[2,11]= 1.42, p = 0.28; Table 2).
Table 2.
Amplitude and timing of postural response measures, by condition. Values are M (95% confidence interval)
| Condition | Baseline | Amplitude | Baseline 2 |
|---|---|---|---|
| Peak center of pressure (CoP) displacement (cm) | 7.05 (0.14) | 6.86 (0.24) | 6.58 (0.14) |
| Peak center of mass (CoM) displacement (cm) | 4.38 (0.14) | 4.98 (0.35) | 4.44 (0.21) |
| Peak trunk flexion angle (degrees) | 4.12 (0.37) | 17.19 (1.60) * | 5.02 (0.64) |
| Peak ankle extension angle (degrees) | −0.09 (0.24) | −0.05 (0.98) | 0.15 (0.28) |
| Time of peak CoP displacement (ms) | 361.04 (55.05) | 406.92 (94.55) | 390.55 (76.74) |
| Time of peak CoM displacement (ms) | 691.09 (67.91) | 720.06 (101.04) | 712.48 (65.27) |
| Time of peak trunk flexion (ms) | 627.49 (145.84) | 796.89 (149.75) | 614.26 (145.72) |
| Time of peak ankle extension (ms) | 539.11 (135.39) | 688.63 (153.55) | 571.62 (139.14) |
significantly greater trunk flexion in the Amplitude condition compared to Baseline (p = 0.02) or Baseline 2 (p = 0.02).
APR Stability
Figure 1c shows that participants demonstrated a significant difference in APR stability (CoP-CoM) across conditions (F[2,11]= 8.15, p = 0.01). Follow-up pairwise comparisons of the condition main effect revealed significantly less APR stability in the Amplitude condition (p = 0.02) and Baseline 2 condition (p = 0.01) compared to the Baseline condition. There was no significant difference in APR stability between the Amplitude and Baseline 2 conditions (p = 0.35). Mean amplitude and timing of peak CoP and CoM displacements are provided in Table 2.
Cortical Preparation: CNV
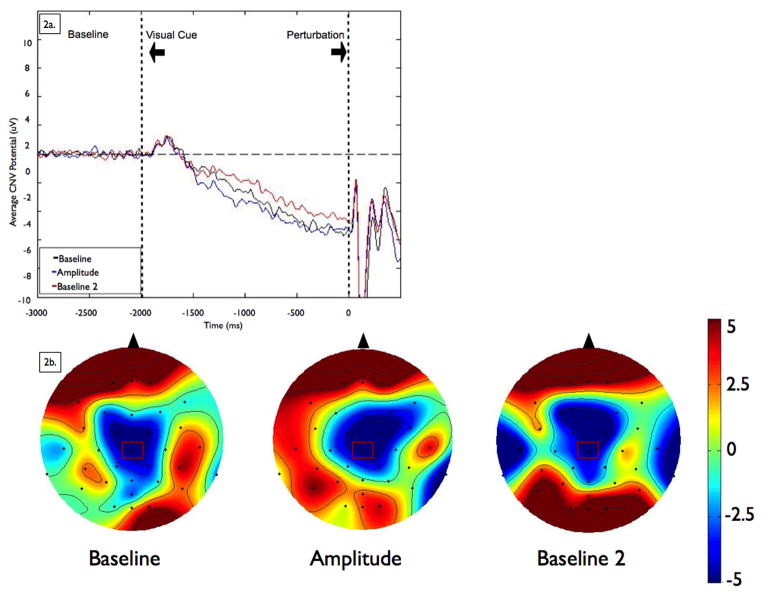
As Figure 2 shows, there was no difference in CNV amplitude across the conditions (F[2,21]= 0.26, p = 0.77).
Figure 2. Contingent Negative Variation.
Figure 2a shows grand averages of contingent negative variation (CNV) amplitudes at the CZ electrode by condition. Data were low-pass filtered at 15 Hz for display purposes only (not during analysis). Baseline is −3000 to −2000 ms, followed by the visual warning cue at −2000 ms and perturbation onset at 0 ms. Figure 2b shows scalp topographies represent the average voltage of the signal for each condition across all electrodes at 100 ms before the imperative signal (postural perturbation). Scalp topographies were observed to be consistent across the last 500 ms so a representative time point is shown here. The view is of the head, from above, with the nose toward the top of the figure. To examine the effects of amplitude cueing on the size of the CNV, we chose to analyze data from the electrode that demonstrated the largest amplitude CNV (CZ), which is marked in rectangles on the scalp topographies.
Cortical Preparation: Alpha ERD
There was no difference in alpha ERD at CP1 (the most active electrode) among the conditions (F[2,11]= 1.07, p = 0.38). Topographies for alpha ERD by condition are shown in Figure 3a.
Figure 3. Event-Related Desynchronization.
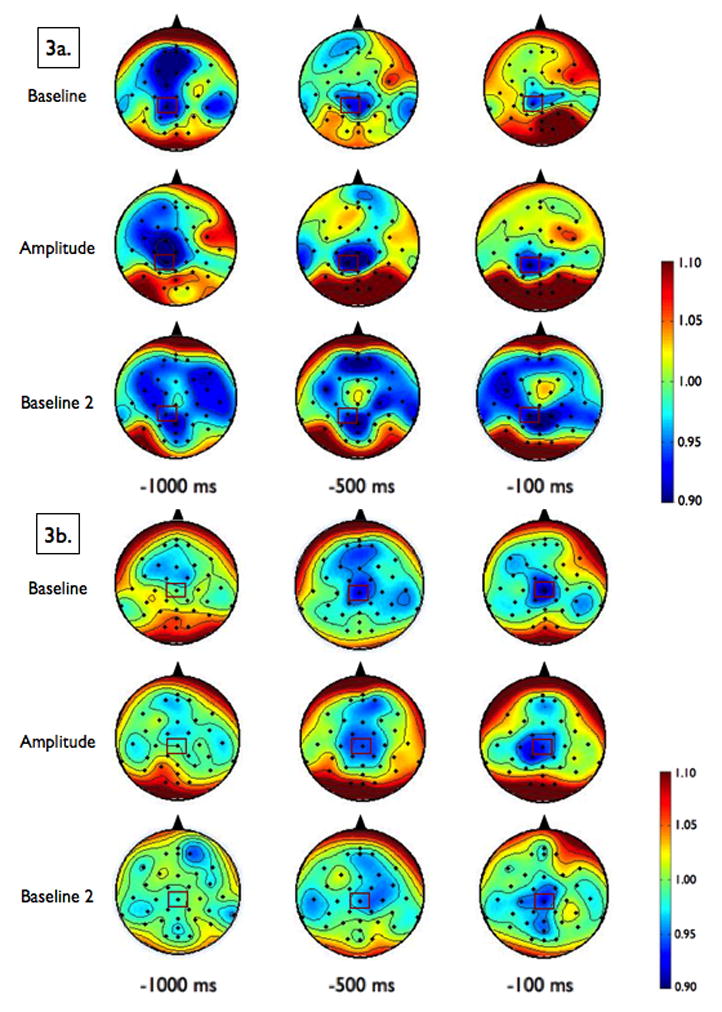
Scalp topographies of the upper alpha-band (10–12 Hz, Figure 3a) and upper beta-band (20–29 Hz, Figure 3b) event-related desynchronization (ERD), by condition, at 1000 ms, 500 ms and 100 ms preceding the perturbation. Smaller values represent greater ERD, referenced to a baseline value of 1. To examine the effects of amplitude cueing on ERD, we chose to analyze data across the 1000 ms preceding the perturbation from the electrode that demonstrated the most desynchronization, CP1 for alpha and CZ for beta, which are marked in rectangles on the scalp topographies.
Cortical Preparation: Beta ERD
There was no difference in beta ERD at CZ (the most active electrode) among the conditions (F[2,11]= 0.41, p = 0.67). Topographies for beta ERD by condition are shown in Figure 3b.
Discussion
Amplitude cueing resulted in a different postural response strategy for a consistent postural perturbation from participants with PD. When they were thinking about making a large amplitude movement (trials 31–60), they slowed down their initial rate of change of CoP and used more trunk flexion compared to the Baseline condition (trials 1–30). This change in postural strategy resulted in a worse APR stability score, as the resulting behavior (slower CoP displacement and more trunk flexion) brought the CoM closer to the CoP and closer to theoretical limits of stability. Despite a worse APR score, however, participants did not fall or step; they were still successful at the feet-in-place postural response task. After the Amplitude condition, during the Baseline 2 condition (trials 61–90), they continued to demonstrate significantly lower APR stability scores, but changes in initial rate of change of CoP and trunk flexion during the Amplitude condition were not maintained.
The significant difference in the initial rate of change of the CoP during Amplitude trials indicates that participants were preparing to make a different response to the upcoming perturbation; they demonstrated a difference in central set as they prepared to make a bigger postural response to the consistent perturbation. However, this difference in central set was not captured by our EEG measures. CNV amplitude or beta ERD at CZ or alpha ERD at CP1 did not differ across conditions.
In our previous paper,1 we compared predictable small magnitude perturbations, predictable large magnitude perturbations, and unpredictable small or large magnitude perturbations (all with a preceding visual cue) with the same timing and feedback schedule as performed here. There was not an amplitude-focused condition in the previous study. Participants with PD did not scale their postural responses to the magnitude of the anticipated postural perturbations. However, they did modify their beta ERD by condition; they demonstrated more beta ERD before predictable small perturbations compared to predictable large perturbations. They also demonstrated overall greater beta ERD than the control participants. In contrast, here we found that participants with PD were able to modify their postural responses, but this was not accompanied by differences in beta ERD. In our previous paper,1 we also found that control participants demonstrated larger amplitude CNV before unpredictable-magnitude, compared to predictable-magnitude, perturbations. We attributed this finding to the possibility that not knowing the amplitude of the perturbation increased task difficulty and/or heightened anticipation of and attention to perturbation timing, thereby eliciting a larger CNV. Thus we thought that participants with PD might demonstrate larger CNV amplitudes when focusing on and paying more attention to making a larger amplitude movement, which was not observed.
We did, however, note what appears to be a widespread surround increase in alpha ERD with a central decrease in alpha ERD after the Amplitude condition, during the Baseline 2 condition (see Figure 3a). This topographical shift in alpha ERD could represent active processing by the brain to inhibit the previous amplitude-focused processes; previous work supports the notion that increased power in alpha inhibits task-irrelevant regions to focus resources to task-relevant regions.42 During the Baseline 2 condition, we asked participants to stop thinking about making a bigger movement and try to return to their baseline behavior, and it is certainly possible that to achieve this they had to actively inhibit the networks they were using while thinking about larger amplitude movements. Patients with PD are known to have difficulty changing set quickly or inhibiting one response for another, so this increased cortical activity could be related to attempts to unlearn a learned response.4
Recent functional magnetic resonance imaging studies support the idea that persons with PD require more brain activity to achieve certain outcomes. Compared to controls, participants with PD showed less activation in the basal ganglia and supplementary motor area and more activation in the primary motor cortex, premotor cortex, inferior frontal gyrus, precuneus, and cerebellum when performing anti-phase finger movements. The basal ganglia and dorsolateral prefrontal cortex were less connected with the supplementary motor area, while the primary motor cortex, parietal cortex, precuneus and cerebellum were more strongly connected with the supplementary motor area..43–45
Our results show that persons with PD are capable of modifying feet-in-place automatic postural responses by changing cortical planning (central set) by focusing on making larger amplitude movements. They did not, however, simply produce a larger ankle strategy (this would have been characterized by faster rate of change of COP and larger stability margin). Instead, they produced much greater trunk flexion. We did not observe an immediate effect of cueing on cortical preparation at the CZ and CP1 electrodes, which are located approximately above the supplementary and sensory-motor areas. Taken together, these findings support the idea that amplitude cueing is a voluntary, attention-based strategy that is not affecting the more automatic aspect of postural responses.
We anticipated that amplitude cueing, as a voluntary, attention-based strategy, would elicit a shift from an implicit postural response to a more explicit, cognitively-driven postural response and that this would be reflected in changes in cortical preparation as measured by ERD. Although we saw a slowing in CoP rate of displacement during the Amplitude condition (reflecting altered neural preparation for a different response) and a difference in ERD following the Amplitude trials (from Amplitude to Baseline 2 conditions), we did not see a difference in ERD from Baseline to Amplitude trials. It is possible that participants shifted from an implicit to explicit strategy but ERD did not show it. It is also possible that participants did not shift from an implicit to explicit strategy because they were paying maximal cognitive attention to the task and using an explicit strategy to maintain equilibrium from the beginning, during all trials. Lastly, we only assessed participants at one time point and do not know what effects a greater dose of practice or consolidation of learning might have.
Finally, we would like to note that we were able to elicit more trunk flexion from our participants with PD (from 4 to 17 degrees of peak trunk flexion, on average), while the hip flexion strategy is a postural response strategy they use less than controls.6,46 Although a previous study showed that participants with PD could not change their initial rate of change of CoP for postural responses when instructed alternatively to resist or give to the displacement, they had 7 alternating trials of resist or give conditions rather than 30 sequential trials within a condition.4 In this study, increasing the size of the postural response and eliciting a big hip strategy actually worsened the stability of the APR as it brought the CoM closer to the CoP and limits of stability. Instead of their tendency to be hypometric, thinking about making large amplitude movements resulted in a hypermetric trunk flexion response. Future research is needed to determine if additional practice would allow participants to improve their stability. It would also be important to determine how healthy subjects change their postural responses and EEG to the amplitude cueing instructions.
Clinical Implications
Our results show that persons with PD are able to modify their feet-in-place postural responses with attentional cueing; their intention to move with larger amplitude had an immediate effect on the postural response strategy they produced. As a result, they moved differently in response to the same perturbation. Here, for feet-in-place postural responses, eliciting more trunk flexion brought the CoM closer to the CoP and limits of stability and may not have been beneficial, however there are other cases (e.g., encouraging larger steps, training to increase limits of stability) where this would be desirable. Finally, we did not observe immediate changes in cortical preparation during Amplitude trials. Whether long-term practice would lead to more automatic control and modified cortical preparatory activity of the supplementary-motor and primary sensory-motor areas remains unknown.
Limitations
Our results are limited to persons with moderate PD (Hoehn and Yahr Stage II or III) and anticipated feet-in-place postural responses. Unanticipated perturbations, stepping responses and other gait and functional tasks were not explored. We studied the effects of immediate practice only; any learning effects of long-term practice of our paradigm on cortical preparatory activity of the supplementary-motor and primary sensory-motor areas remain unknown. Because the trial order was not randomized, effects of fatigue could be included in the later trials. Finally, we tested 12 persons with PD (11 were male) in the “On” medication state, in a laboratory setting, which may limit the ability to generalize to other contexts.
Supplementary Material
Acknowledgments
We would like to thank our participants. We would like to thank Kristin Lucas, PT, DPT, for her invaluable assistance with data collection.
Source of Funding
We would like to acknowledge our funding sources: National Institutes of Health, National Institute of Aging R37 A60006457 (FBH), National Institutes of Health, National Institute of Child Health and Human Development F32 HD070796 (BAS), Medical Research Foundation of Oregon (BAS), and the Foundation for Physical Therapy New Investigator Fellowship Training Initiative (BAS). Funding sources provided funding only and did not influence the work.
Footnotes
Conflicts of Interest
The authors declare that we have no conflict of interest.
Abstracts of this work were presented at the International Society for Posture and Gait Research 2012 Annual Conference (Trondheim, Norway) and the American Physical Therapy Association 2013 Combined Sections Meeting (San Diego, CA).
Video Abstract available
(see video, supplemental digital content) for more insights from the authors.
References
- 1.Smith BA, Jacobs JV, Horak FB. Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Exp Brain Res. 2012;222(4):455–470. doi: 10.1007/s00221-012-3232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75(6):2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 3.Bloem BR. Postural instability in Parkinson’s disease. Clin Neurol Neurosurg. 1992;94:41–45. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- 4.Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci. 2000;175(1):57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 5.Chong RK, Jones CL, Horak FB. Postural set for balance control is normal in Alzheimer”s but not in Parkinson”s disease. J Gerontol A Biol Sci Med Sci. 1999;54(3):M129–35. doi: 10.1093/gerona/54.3.m129. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs JV, Dimitrova DM, Nutt JG, Horak FB. Can stooped posture explain multidirectional postural instability in patients with Parkinson’s disease? Exp Brain Res. 2005;166(1):78–88. doi: 10.1007/s00221-005-2346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 8.Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience. 2001;104(4):1027–1041. doi: 10.1016/s0306-4522(01)00099-9. [DOI] [PubMed] [Google Scholar]
- 9.Almeida QJ, Frank JS, Roy EA, et al. An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience. 2005;134(1):283–293. doi: 10.1016/j.neuroscience.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Keijsers NLW, Admiraal MA, Cools AR, Bloem BR, Gielen CCAM. Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. Eur J Neurosci. 2005;21:239–48. doi: 10.1111/j.1460-9568.2004.03840.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience. 2006;141(2):999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Jobges M, Heuschkel G, Pretzel C, Illhardt C, Renner C, Hummelsheim H. Repetitive training of compensatory steps: a therapeutic approach for postural instability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(12):1682–1687. doi: 10.1136/jnnp.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119 (Pt 2):551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 15.King LA, Horak FB. Delaying Mobility Disability in People With Parkinson Disease Using a Sensorimotor Agility Exercise Program. Phys Ther. 2009;89(4):384–393. doi: 10.2522/ptj.20080214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp Brain Res. 2005;167(3):462–467. doi: 10.1007/s00221-005-0179-7. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim I, van Wegen E, de Goede C, et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil. 2005;19(7):695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- 19.Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease-the Berlin BIG Study. Mov Disord. 2010;25(12):1902–1908. doi: 10.1002/mds.23212. [DOI] [PubMed] [Google Scholar]
- 20.Fox C, Ebersbach G, Ramig L, Sapir S. LSVT LOUD and LSVT BIG: Behavioral Treatment Programs for Speech and Body Movement in Parkinson Disease. Parkinsons Dis. 2012;2012(2):1–12. doi: 10.1155/2012/391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieuwboer A, De Weerdt W, Dom R, Truyen M, Janssens L, Kamsma Y. The effect of a home physiotherapy program for persons with Parkinson’s disease. J Rehabil Med. 2001;33(6):266–272. doi: 10.1080/165019701753236455. [DOI] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs JV, Fujiwara K, Tomita H, Furune N, Kunita K, Horak FB. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol. 2008;119(6):1431–1442. doi: 10.1016/j.clinph.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33(4):281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- 26.Henry SM, Fung J, Horak FB. Control of stance during lateral and anterior/posterior surface translations. IEEE Trans Rehabil Eng. 1998;6(1):32–42. doi: 10.1109/86.662618. [DOI] [PubMed] [Google Scholar]
- 27.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62(4):841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 28.Horak F, MacPherson J. Postural Orientation and Equilibrium. In: Rowell L, Sheperd J, editors. Handbook of Physiology. Oxford: Oxford Univ Press; 1996. pp. 255–288. [Google Scholar]
- 29.Vaughan CL, Davis B, O’Connor J. Dynamics of human gait. Champaign, IL: Human Kinetics; 1991. [Google Scholar]
- 30.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80(3):1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- 31.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J Neurosurg. 2012;116(6):1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Oishi M, Mochizuki Y, Du C, Takasu T. Contingent negative variation and movement-related cortical potentials in parkinsonism. Electroencephalogr Clin Neurophysiol. 1995;95(5):346–349. doi: 10.1016/0013-4694(95)00084-c. [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki G, Sibley KM, Cheung HJ, McIlroy WE. Cortical activity prior to predictable postural instability: is there a difference between self-initiated and externally-initiated perturbations? Brain Res. 2009;1279:29–36. doi: 10.1016/j.brainres.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 35.van Boxtel GJ, Brunia CH. Motor and non-motor aspects of slow brain potentials. Biol Psychol. 1994;38(1):37–51. doi: 10.1016/0301-0511(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 36.Fischer T, Langner R, Diers K, Brocke B, Birbaumer N. Temporo-Spatial Dynamics of Event-Related EEG Beta Activity during the Initial Contingent Negative Variation. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfurtscheller G. Spatiotemporal ERD/ERS patterns during voluntary movement and motor imagery. Suppl Clin Neurophysiol. 2000;53:196–198. doi: 10.1016/s1567-424x(09)70157-6. [DOI] [PubMed] [Google Scholar]
- 38.Fogelson N. Different Functional Loops between Cerebral Cortex and the Subthalmic Area in Parkinson’s Disease. Cereb Cortex. 2005;16(1):64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- 39.Wheaton LA, Carpenter M, Mizelle JC, Forrester L. Preparatory band specific premotor cortical activity differentiates upper and lower extremity movement. Exp Brain Res. 2007;184(1):121–126. doi: 10.1007/s00221-007-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klostermann F, Nikulin VV, Kühn AA, et al. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur J Neurosci. 2007;25(5):1604–1615. doi: 10.1111/j.1460-9568.2007.05417.x. [DOI] [PubMed] [Google Scholar]
- 41.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 42.Jensen O, Mazaheri A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front Hum Neurosci. 2010;4:1–8. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128(10):2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 44.Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010;133:2394–2409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T, Long X, Wang L, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;193(2):504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.