Summary
In this issue of Cancer Cell, Yang et al. describe a causal relationship between gene body methylation and gene expression and a role for genic methylation in response to clinical DNA methylation inhibitors, which suggests that the mechanism of action of these inhibitors includes gene body hypomethylation-induced downregulation of cancer-associated genes.
DNA cytosine methylation is an ancient regulatory mechanism present in diverse phylogenies, including vertebrates, plants, and some fungi (Jones, 2012). Cytosine methylation of CG dinucleotides by DNA methyltransferases (DNMTs) in promoters is associated with gene silencing, e.g., in X-chromosome inactivation and imprinting (Jones, 2012), and promoter hypermethylation is thought to contribute to aberrant regulatory programs in cancer (Shenker and Flanagan, 2012). Within the last decade, so-called “epigenetic” drugs have come to the fore with US Food and Drug Administration approval of cytidine analog DNMT inhibitors such as 5-Aza-2-deoxycytidine (5-Aza-CdR) for myelodysplastic syndrome and acute myeloid leukemia. Excitingly, studies have also hinted at the efficacy of this mode of intervention in solid tumors (Shenker and Flanagan, 2012), expanding the utility of these chemotherapeutics beyond hematologic malignancies.
Although promoter methylation, which maintains a “closed” chromatin state that impairs transcriptional initiation (Jones, 2012), has been well studied, less is known about the role of methylation in gene bodies. Intriguingly, unlike at promoters where methylation is associated with gene repression, genic methylation is positively correlated with gene expression (Fig. 1A) (Maunakea et al., 2010; Varley et al., 2013). It is thought that DNA methylation inhibitors cause promoter hypomethylation and subsequent gene reactivation (Shenker and Flanagan, 2012). However, the effect of these drugs on gene body methylation has not been extensively studied.
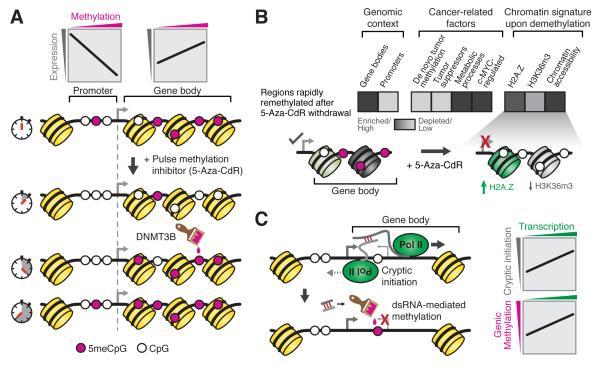
Figure 1. Gene body methylation, gene expression, and DNA methylation inhibitors.
(A) Promoter methylation is associated with gene silencing, while gene body methylation is correlated with gene expression. Measurement of methylation at various time points after treatment with the DNA methyltransferase (DNMT) inhibitor 5-Aza-2-deoxycytidine (5-Aza-CdR) reveals that promoters and gene bodies can be differentially re-methylated. Rapid re-methylation of genic regions is dependent on DNMT3B. (B) Rapidly remethylated regions tend to be located in gene bodies and are enriched for c-MYC-regulated genes. Upon demethylation, rapidly remethylated gene bodies acquire a chromatin signature that may modulate transcription by altering nucleosome stability. (C) Methylation of gene bodies may attenuate cryptic initiation from alternative promoters.
The work of Yang, et al. suggests a causal relationship between gene body methylation and gene expression and expands the understanding of the mechanism of action of cytidine analogue cancer chemotherapeutics (Yang, et al., 2014). They assayed genome-wide methylation at various time points after short treatment of a dividing colon cancer cell line with 5-Aza-CdR, allowing interrogation of remethylation kinetics and the durability of methylation changes upon drug withdrawal (Fig. 1A). A clustering approach used to classify genomic regions into groups with high and low remethylation rates revealed that gene bodies were rapidly remethylated compared to promoters (Fig. 1B). Furthermore, gene body remethylation was correlated with gene expression. Exploiting DNMT knockout cell lines, the authors determined that DNMT3B is required for rapid remethylation of gene bodies following 5-Aza-CdR treatment (Fig. 1A). Both DNA methylation and gene expression were attenuated in DNMT3B deficient cells following 5-Aza-CdR treatment, suggesting that DNMT inhibitor-induced gene body demethylation downregulates gene expression.
This causal relationship between 5-Aza-CdR-induced genic hypomethylation and downregulation of gene expression is particularly important in the context of the observation that c-MYC-regulated genes were overrepresented in the rapidly remethylated gene set (Fig. 1B). Slowly remethylated regions tended to be de novo methylated in primary tumors and associated with tumor suppressor genes. This raises the possibility that methylation inhibitors buffer against c-MYC-associated gene expression changes characteristic of many cancers. Moreover, the observed attenuation of ostensible housekeeping functions upon 5-Aza-CdR treatment might manifest as inhibition of cell growth, consistent with an association between genic methylation and constitutive expression (Coleman-Derr and Zilberman, 2012).
By examining histone modifications and distribution of the H2A.Z histone variant in regions exhibiting rapid and slow remethylation kinetics, Yang, et al. (2014) defined the interplay between 5-Aza-CdR treatment, gene body methylation, and chromatin signatures. Histone H3 lysine 36 trimethylation (H3K36m3) is enriched in actively transcribed genic regions where it is thought to suppress histone turnover to maintain transcriptional fidelity, whereas H3K27m3 is a mark of Polycomb-associated silencing. Additionally, the distribution of H2A.Z, which is thought to destabilize nucleosomes, is strikingly anti-correlated with DNA methylation at promoters in vertebrates and plants (Conerly et al., 2010; Zilberman et al., 2008). Upon DNMT depletion, gene bodies of rapidly remethylated genes became slightly enriched for H2A.Z, while H3K36m3 was enriched independent of methylation level, possibly implicating this mark in rapid remethylation. Slowly remethylated regions were strongly enriched for H3K27m3 and H2A.Z, consistent with antagonism between these chromatin features and DNA methylation. Additionally, analysis of selected genes showed that gene body demethylation caused by 5-Aza-CdR was associated with H2A.Z deposition, a modest depletion of H3K36m3, and increased chromatin accessibility (Fig. 1B). Collectively, these results suggest that genic demethylation by cytidine analogs may influence transcription by modulating nucleosome stability (Fig. 1B) and that remethylation after drug withdrawal is influenced by chromatin context.
There are many hypotheses regarding the function of gene body methylation. For example, genic methylation might regulate alternative promoters (Maunakea et al., 2010). Alternatively, gene body methylation might inhibit cryptic transcription initiation events. In plants, cryptic initiation was proposed to generate anti-sense transcripts that base pair with mRNA transcribed from the canonical TSS, leading to dsRNA-mediated methylation and silencing of the cryptic TSS (Tran et al., 2005) (Fig. 1C). Consistent with this model, genic methylation is anti-correlated with transcriptional noise in vertebrates (Huh et al., 2013). It is attractive to hypothesize that an increase in cryptic initiation in active genes upon nucleosome destabilization by methylation inhibitors leads to DNMT3B recruitment and suppression of cryptic transcription by rapid remethylation.
This work by Yang, et al. (2014) also provides important insights into the pharmacology of DNA methylation inhibitors. Despite the apparent lack of specificity of methylation inhibitors, remethylation kinetics after drug withdrawal are dependent on genomic and chromatin contexts and driven by DNMT3B. Promoter demethylation appears to be sustained after drug withdrawal, while genic demethylation is comparatively short-lived, which may have implications for understanding clinical responses to methylation inhibitors. Mechanistically, DNA methylation inhibitors may normalize gene expression in cancer by reactivating silenced tumor suppressors through their action at promoters and buffer overexpression of oncogenes and metabolic genes by hypomethylating gene bodies.
Acknowledgements
We thank Gabriel E. Zentner for comments on this manuscript. SK is supported by NIH grant 1F30CA186458 and the Micki & Robert Flowers ARCS Endowment from the Seattle Chapter of the ARCS Foundation. SH is supported by the Howard Hughes Medical Institute.
References
- Coleman-Derr D, Zilberman D. DNA methylation, H2A. Z, and the regulation of constitutive expression. Cold Spring Harbor Symposia on Quantitative Biology. 2012;77:147–154. doi: 10.1101/sqb.2012.77.014944. [DOI] [PubMed] [Google Scholar]
- Conerly ML, Teves SS, Diolaiti D, Ulrich M, Eisenman RN, Henikoff S. Changes in H2A. Z occupancy and DNA methylation during B-cell lymphomagenesis. Genome Research. 2010;20:1383–1390. doi: 10.1101/gr.106542.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh I, Zeng J, Park T, Yi SV. DNA methylation and transcriptional noise. Epigenetics & Chromatin. 2013;6:9. doi: 10.1186/1756-8935-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature Reviews Genetics. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. British Journal of Cancer. 2012;106:248–253. doi: 10.1038/bjc.2011.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran RK, Henikoff JG, Zilberman D, Ditt RF, Jacobsen SE, Henikoff S. DNA methylation profiling identifies CG methylation clusters in Arabidopsis genes. Current Biology : CB. 2005;15:154–159. doi: 10.1016/j.cub.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Cross MK, Williams BA, Stamatoyannopoulos JA, Crawford GE, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Research. 2013;23:555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S. Histone H2A. Z and DNA methylation are mutually antagonistic chromatin marks. Nature. 2008;456:125–129. doi: 10.1038/nature07324. [DOI] [PMC free article] [PubMed] [Google Scholar]