Abstract
Background
Data to guide the management of advanced pulmonary carcinoid (APC) come from retrospective reports and subgroup analyses of trials that included mainly extrapulmonary carcinoid tumors. We report the largest series to date of 49 patients with locally advanced or metastatic pulmonary carcinoid.
Methods
The Johns Hopkins Pathology Database was reviewed for APC patients treated between January 1992 and December 2012. Data on time to recurrence, progression-free survival, and overall survival were estimated by using the Kaplan–Meier method.
Results
Forty-nine patients were treated for APC in the specified time period. Median time to recurrence after surgical resection was 2.5 years (atypical carcinoid [AC] versus typical carcinoid [TC], 2.5 versus 6.3 years; p = 0.063). Median survival with advanced disease was 7.1 years and significantly longer for TC compared with AC (10.2 versus 4 years; p = 0.009). Among the diverse systemic therapies used, responses occurred in four of 17 patients (23.5%) who received platinum/ etoposide with a median progression-free survival of 7 months.
Conclusion
Although systemic chemotherapy has moderate activity for APC, novel approaches are required. TC and AC, although both classified as pulmonary carcinoid, are clearly different clinical and molecular entities and require separate treatment paradigms in the advanced/metastatic setting.
Keywords: Pulmonary carcinoid, Metastatic carcinoid, Chemotherapy pulmonary carcinoid, Chemotherapy bronchial carcinoid, Therapy carcinoid, Atypical carcinoid, Typical carcinoid
The incidence of pulmonary carcinoid (PC) tumors has increased during the last 30 years in part because of a combination of asymptomatic tumors being incidentally noted on chest imaging and the use of more rigorous histological diagnostic criteria.1–3 PC tumors represent 1% to 2% of all lung malignancies and approximately 25% of all carcinoid tumors originate in the lung.3,4
PC tumors are divided into typical carcinoid (TC) and atypical carcinoid (AC) and are classified as foregut neuroendocrine tumors (Table 1).1,2 Although the majority of TCs are cured by surgical resection, AC represents a more aggressive phenotype with frequent recurrences being reported.
TABLE 1.
WHO/IASLC Classification of Pulmonary Neuroendocrine Tumors
| Grade | Nomenclature | Histopathologic Characteristics |
|---|---|---|
| Low | Typical carcinoid | Carcinoid morphology, less than two mitoses/10 HPFs, no necrosis, >0.5 cm diameter |
| Intermediate | Atypical carcinoid | Carcinoid morphology, two to 10 mitoses/10 HPFs or foci of necrosis |
| High | Large-cell neuroendocrine carcinoma | Neuroendocrine structure, >10 mitoses/10 HPFs, necrosis (may be extensive), cytology resembling NSCLC, IHC positive for NE markers |
| Small-cell carcinoma | Small cell size, scant cytoplasm, nuclei with finely granular chromatin and faint nucleoli, >11 mitoses/10 HPFs, extensive necrosis |
WHO, World Health Organization; IASLC, International Association for the Study of Lung Cancer; NSCLC, non–small-cell lung cancer; IHC, immunohistochemistry; NE, neuroendocrine; HPF, high powered fields.
METHODS
This retrospective cohort study included all patients referred to Johns Hopkins for the management of advanced (unresectable, locally advanced, or metastatic) PC between January 1, 1992 and December 31, 2012. All patients’ tumor specimens were reviewed by the Johns Hopkins pathology department and confirmed as PC. Data on time to recurrence, progression-free survival (PFS), and overall survival were estimated by using the Kaplan–Meier method and log-rank test comparing TC and AC.
RESULTS
Patient and Disease Characteristics
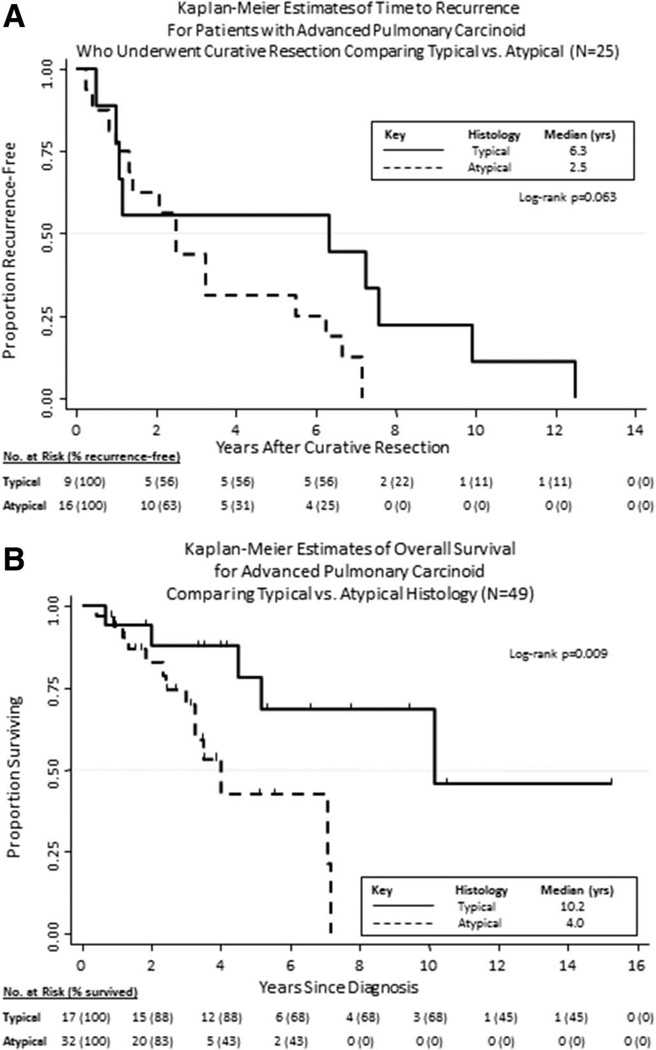
Patient characteristics are listed by histologic subtype in Table 2. Forty-nine patients were included in this series; of these, 32 had AC and 17 had TC. The median age at diagnosis of advanced PC (APC) was 56 years (range, 28–79), and 61% of patients were women. At a minimum, all patients were initially staged and followed with regular computed tomography scans of chest and abdomen. The majority of patients (53%) were never smokers. Median follow-up was 54 months (range, 10–183+). Most patients had metastatic disease (83.7% versus 16.3% with locally advanced disease only), and the commonest sites of metastases throughout the course of the disease were liver (59.2% of patients), mediastinum or contralateral lung (36.7%), bone (36.7%), and central nervous system (12.2%). All central nervous system metastases occurred in patients with AC. Only eight patients (16.3%; 6 TC and 2 AC), all with liver metastases, had symptoms consistent with the carcinoid syndrome. Median survival from diagnosis of locally advanced unresectable or metastatic PC was 7.1 years and was significantly longer for TC-histology patients compared with AC-histology patients (10.2 versus 4 years; p = 0.009) (Fig. 1B).
TABLE 2.
Patient and Disease Characteristics by Histologic Subtype (N = 49)
| Typical (n = 17) | Atypical (n = 32) | ||||
|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | pa |
| Age at diagnosis (yr) | 0.117 | ||||
| Median and (IQR) | 50 | (47–57) | 58 | (47–64) | |
| Sex | 0.715 | ||||
| Male | 6 | 35.3 | 13 | 40.6 | |
| Female | 11 | 64.7 | 19 | 59.4 | |
| Smoking status | 0.234 | ||||
| Ever smoked | 6 | 35.3 | 17 | 53.1 | |
| Never smoked | 11 | 64.7 | 15 | 46.9 | |
| Prior curative resection | 0.845 | ||||
| Yes | 9 | 52.9 | 16 | 50 | |
| No | 8 | 47.1 | 16 | 50 | |
| Time to recurrence (mo) | 0.008b | ||||
| Median | 119 | 66 | |||
| Advanced disease stage (IASLC Seventh Edition) | 0.855 | ||||
| Locally advanced | 3 | 17.7 | 5 | 15.6 | |
| IIIA | 1 | 2 | |||
| IIIB | 2 | 3 | |||
| Metastatic (stage IV) | 14 | 82.3 | 27 | 84.4 | |
| Stage at initial presentation (TNM) | |||||
| T1A/B N0/X M0 | 2 | 1 | |||
| T1A N2 M0 | 0 | 1 | |||
| T2A/B N0 M0 | 1 | 2 | |||
| T2A/B N1 M0 | 2 | 0 | |||
| T2A/B N2 M0 | 1 | 7 | |||
| T3 N0 M0 | 1 | 0 | |||
| T3 N2 M0 | 0 | 2 | |||
| T3 N3 M0 | 0 | 1 | |||
| T4 N1 M0 | 1 | 0 | |||
| T4 N2/3 M0 | 2 | 2 | |||
| T2B N0 M1A | 2 | 0 | |||
| T4 N1 M1A | 1 | 2 | |||
| T2A/B N0/X M1B | 2 | 4 | |||
| T3 N1/X M1B | 1 | 6 | |||
| T4 NX M1B | 2 | 4 | |||
| Metastatic disease sites | |||||
| Liver | 10 | 58.8 | 19 | 59.4 | |
| Pleura/mediastinum/contralateral lung | 8 | 47.1 | 10 | 31.3 | |
| Bone | 7 | 41.2 | 11 | 34.4 | |
| Brain/meninges | 0 | 0 | 6 | 18.9 | |
| Other | 2 | 11.8 | 5 | 15.6 | |
| Carcinoid syndrome | 0.015 | ||||
| Yes | 6 | 35.3 | 2 | 6.3 | |
| No | 11 | 64.7 | 30 | 93.7 | |
| Chromogranin A level (serum) | 0.025 | ||||
| Increased | 9 | 52.9 | 6 | 18.8 | |
| Normal | 6 | 35.3 | 12 | 37.5 | |
| Result not available | 2 | 11.8 | 14 | 43.7 | |
χ2 test for homogeneity or Fisher’s exact test for binary and categorical variables and nonparametric Mann–Whitney U test for continuous variables.
Log-rank test.
IQR, interquartile range; IASLC, International Association for the Study of Lung Cancer; TNM, tumor, node, metastasis.
FIGURE 1.
Kaplan–Meier Curves of (A) time to recurrence after surgical resection and (B) overall survival from diagnosis of locally advanced or metastatic pulmonary carcinoid by histologic subtype.
Therapy Administered
More than half of the patients (25 of 49 patients) had previously undergone lung resections for primary PC. Median time to recurrence for patients who underwent curative resection was 2.5 years, and a trend toward prolonged time to recurrence was noted for TC compared with AC (6.3 versus 2.5 years; p = 0.063) (Fig. 1A).
Six patients (3 AC and 3 TC patients) with locally advanced PC received definitive radiation ± concurrent chemotherapy as primary therapy. This group included three patients who received definitive radiation alone (2 TC and 1 AC patients; radiation dose range, 45 to 67 Gy) and three who received concurrent chemotherapy with cisplatin/etoposide or carboplatin/paclitaxel (2 AC and 1 TC patients). Overall, from this group, five patients had stable disease as best response and one patient had progressive disease. Median PFS after definitive radiation or chemoradiation was 10 months (range, 2–117). All six patients who received radiation ± chemotherapy for locally advanced disease eventually experienced progressive disease; however, four patients (2 AC and 2 TC patients) had prolonged stable disease lasting between 2 and almost 10 years.
In total, 39 of 49 patients received systemic anti-cancer therapy. Etoposide with either cisplatin or carboplatin was administered to 17 patients (any line of therapy), of whom, four patients (2 AC and 2 TC patients) (23.5%) had a radiologic response. Median PFS for all patients who received platinum/ etoposide was 7 months. Long-acting somatostatin analogue therapy was administered to 17 patients with only one tumor response being noted; however, prolonged stable disease was seen in 13 patients (range, 6–25 months). In two patients who received everolimus with sandostatin LAR, one pretreated patient has had an ongoing partial response for more than 8 months. Other therapies used comprised a variety of chemotherapies and targeted therapies administered mainly on phase I clinical trials; however, no tumor responses were seen.
Almost 60% of patients (n = 29) developed liver metastases during the course of their disease and of these, nine patients (6 AC and 3 TC patients) underwent hepatic transarterial chemoembolization (TACE) with cisplatin/ doxorubicin/mitomycin (7 patients) or doxorubicin-eluting beads (2 patients). TACE had promising efficacy in this cohort with only one patient having progressive disease and eight patients (3 partial responses) experiencing prolonged stable disease in the liver lasting between 5 and 19+ months. In addition, one patient who received therasphere radio- embolization experienced a prolonged partial response lasting 24 months. TACE was overall well tolerated; however, one patient died from Gram-negative bacteremia related to a third TACE procedure.
LITERATURE REVIEW
The only well-described curative option for PC is surgical resection, and large series have described 10-year overall survival rates for resected TC of 82% to 93% and for resected AC of 56% to 64%.5,6
The Role of Adjuvant Therapy
There are currently no clear data available on the use of adjuvant systemic therapy in PC tumors. On the basis of the relatively high rate of recurrence of resected AC and reported responses to platinum/etoposide in advanced disease such as those reported in our series, the National Comprehensive Cancer Network recommends adjuvant cisplatin/etoposide for fully resected stage II AC and consideration of adjuvant chemoradiation for completely resected stage III disease (category 2B evidence).7,8 Observation is recommended at the present time for resected stage I AC and stage I to III TC.
Locally Advanced Unresectable PC
There are very limited data to support the use of definitive chemoradiation for PC; however, in the absence of surgical options, it is recommended for stage IIIB AC.8 For locally advanced unresectable TC, definitive radiation without concurrent chemotherapy should be considered.8
Systemic Therapy for Advanced Disease
Evidence to support systemic therapy for APC comes from small retrospective series and subgroup analyses of clinical trials that enrolled predominantly gastrointestinal neuroendocrine tumor patients (Table 3).9–14 Because of the rarity of APC, the majority of these studies have reported response rates across several different lines of therapy and with diverse regimens.
TABLE 3.
Studies of Chemotherapy and Targeted Agents in Locally Advanced or Metastatic Pulmonary Carcinoid
| Study and Type | No. of Patients and Histology, N |
Regimen(s) | CR + PR (%) Radiologic) |
Median PFS (mo) |
Median Survival (mo) |
|---|---|---|---|---|---|
| Moertel et al., Subgroup of a prospective study, 1979 | 17 | Streptozocin/Cy Streptozocin/5-FU |
12 17 |
Not reported | 15.1 |
| Granberg et al., Retrospective series, 2001 | 31 27 TC, 4 AC |
Interferon-based ± Octreotide LAR (N = 27) Cisplatin/etoposide (N = 8) |
3.7 25 |
Not reported Not reported |
76 |
| Wirth et al., Retrospective, 2004 | 18 8 TC, 10 AC |
Platinum/etoposide-based (N = 13) | 23.1 | Not reported | 20 |
| Ekeblad et al., Retrospective subgroup, 2007 | 13 10 TC, 3 AC |
Temozolomide (N = 13) | 30.8 | 7 | 16 |
| Fazio et al., Subgroup analysis of prospective study, 2012 | 44 33 TC, 9 AC, 2 unknown |
Octreotide LAR + Everolimus (N = 33) Octreotide LAR (N = 11) |
0 (67% had minor response) 0 (27% had minor response) |
13.6 5.6 |
Not reached Not reached |
CR, complete response; PFS, progression-free survival; TC, typical carcinoid; AC, atypical carcinoid; 5-FU, fluorouracil; PR, partial response.
DISCUSSION
Despite surgical resection being the recognized first-line treatment of choice, recurrence is not unusual in PC tumors. Because of their rarity, thoracic oncologists lack prospective data on the optimal management of recurrent or unresectable disease. To date, this study is the most comprehensive series describing the presentation, disease course, and treatment outcomes of 49 APC patients. In our series, patients with TC had a prolonged disease course with a median time to recurrence for surgically resected cases of more than 6 years compared with 2.5 years for AC although this difference was not statistically significant (p = 0.063) given relatively small patient numbers. Median survival from time of diagnosis of advanced disease was 7.1 years and ranged from 4 years for AC patients to more than 10 years for patients with TC (p = 0.03). Responses to systemic therapy reported in our study demonstrate that platinum/etoposide chemotherapy is a moderately effective regimen for both advanced TC and AC with 23.5% of patients demonstrating a radiological response. This is consistent with the few other published reports in PC and confirms the perception of APC as a much less chemoresponsive disease than small-cell lung cancer. Somatostatin analogue therapy is a recognized therapy in carcinoid tumors and, although in our experience, it has limited activity in terms of tumor radiologic response, several patients did maintain prolonged stable disease with this treatment.
Our study has several limitations primarily related to being retrospective in nature. Because of small patient numbers, it is not possible to reliably describe prognostic factors for our cohort other than histology and indeed for rare cancers such as APC, the establishment of a prospectively maintained multi-institutional database would be very useful in this regard. Although 52% of patients analyzed in this cohort had previously undergone surgical resection, thus providing ample tumor for analysis, the remainder of diagnoses were made using tumor biopsies of varying quantity and quality. Given the challenges involved in differentiating AC from TC (Table 1), it is conceivable that some patients may have been incorrectly categorized. Response rate and PFS for each therapy were estimated using both radiology and clinical notes and thus reflect real-life practice; however, as a consequence have a degree of associated uncertainty.
To our knowledge, this study confirms for the first time, the longstanding perception that advanced AC patients have a significantly poorer prognosis when compared with patients with advanced TC. Given the long natural history of this disease uncertainty exists regarding optimal therapy and the potential survival benefit associated with systemic therapy, both TC and AC tumors demonstrate response to platinum/ etoposide chemotherapy and we consider this to be a standard first-line option at our institution. A better understanding of the biology of these tumors through advanced technologies, such as next-generation sequencing, may help identify potential markers for prognosis and treatment of advanced cases. The rarity of APC prevents us from performing large randomized clinical trials specifically for this subgroup of patients. Rare diseases may, however, lend themselves perfectly to investigation using molecular profiling–directed therapies.
Acknowledgments
David S. Ettinger, MD, has served in a consultant or advisory role for Boehringer-Ingelheimm GmbH, Eli-Lilly & Co, and Roche/Genentech.
Footnotes
Disclosure: The other authors have no relevant disclosures.
This study was presented, in part, at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31 to June 4, 2013.
REFERENCES
- 1.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology & Genetics: Tumours of the Lung, Pleura, Thymus and Heart. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2004. The concept of pulmonary neuroendocrine tumours; pp. 19–20. [Google Scholar]
- 2.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 4.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 5.Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001;119:1647–1651. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 6.Rea F, Rizzardi G, Zuin A, et al. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur J Cardiothorac Surg. 2007;31:186–191. doi: 10.1016/j.ejcts.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001;92:1101–1107. doi: 10.1002/1097-0142(20010901)92:5<1101::aid-cncr1426>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Kalemkerian GP. Small Cell Lung Cancer—Lung Neuroendocrine Tumors. [Accessed 2009];NCCN Clinical Practice Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/PDF/sclc.pdf. [Google Scholar]
- 9.Wirth LJ, Carter MR, Jänne PA, Johnson BE. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer. 2004;44:213–220. doi: 10.1016/j.lungcan.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Granberg D, Eriksson B, Wilander E, et al. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann Oncol. 2001;12:1383–1391. doi: 10.1023/a:1012569909313. [DOI] [PubMed] [Google Scholar]
- 11.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 12.Pavel ME, Hainsworth JD, Baudin E, et al. RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 13.Fazio N, Granberg D, Grossman A, et al. Everolimus plus octreotide long- acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143:955–962. doi: 10.1378/chest.12-1108. [DOI] [PubMed] [Google Scholar]
- 14.Moertel CG, Hanley JA. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials. 1979;2:327–334. [PubMed] [Google Scholar]